Abstract
The histiocytoses are rare disorders characterized by the accumulation of macrophage, dendritic cell, or monocyte-derived cells in various tissues and organs of children and adults. More than 100 different subtypes have been described, with a wide range of clinical manifestations, presentations, and histologies. Since the first classification in 1987, a number of new findings regarding the cellular origins, molecular pathology, and clinical features of histiocytic disorders have been identified. We propose herein a revision of the classification of histiocytoses based on histology, phenotype, molecular alterations, and clinical and imaging characteristics. This revised classification system consists of 5 groups of diseases: (1) Langerhans-related, (2) cutaneous and mucocutaneous, and (3) malignant histiocytoses as well as (4) Rosai-Dorfman disease and (5) hemophagocytic lymphohistiocytosis and macrophage activation syndrome. Herein, we provide guidelines and recommendations for diagnoses of these disorders.
Introduction
The histiocytoses are rare disorders characterized by the accumulation of cells thought to be derived from dendritic cells (DCs) or macrophages. Their clinical behavior ranges from mild to disseminated and, sometimes, life-threatening forms. The first classification of histiocytosis, published in 1987 by the Working Group of the Histiocyte Society (HS),1 consisted of 3 categories: Langerhans cell (LC) or non-LC-related, and malignant histiocytoses (MH). In light of recent insights, we propose to parsimoniously gather the large number of categories of histiocytic disorders into 5 groups (Figure 1; supplemental Methods, available on the Blood Web site) based on clinical, radiographic, pathological, phenotypic, genetic, and/or molecular features.
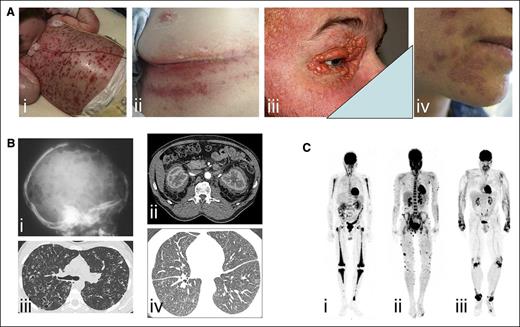
Histology and somatic mutations of histiocytoses of group L, C, R, M, and H. (A) L group: Histology of LCH (skin [i-ii] and bone [iii]) and of ECD (perirenal [iv-v]). Pie chart of relative frequencies of activating kinase mutations in LCH (vi) and ECD (vii). (B) C group: Histology of JXG (i-ii). (C) R group: Histology of RDD (meningeal with high IgG4+ plasma cell infiltration [i-ii]). (D) M group: Histology of MH (i-ii). (E) H group: Histology of inherited HLH (liver [i-ii]). Staining with CD1a (Lii in red), IgG4 (Rii in brown), CD163 (Hii in brown), or hematoxylin and eosin (all others). NOS, not otherwise specified.
Histology and somatic mutations of histiocytoses of group L, C, R, M, and H. (A) L group: Histology of LCH (skin [i-ii] and bone [iii]) and of ECD (perirenal [iv-v]). Pie chart of relative frequencies of activating kinase mutations in LCH (vi) and ECD (vii). (B) C group: Histology of JXG (i-ii). (C) R group: Histology of RDD (meningeal with high IgG4+ plasma cell infiltration [i-ii]). (D) M group: Histology of MH (i-ii). (E) H group: Histology of inherited HLH (liver [i-ii]). Staining with CD1a (Lii in red), IgG4 (Rii in brown), CD163 (Hii in brown), or hematoxylin and eosin (all others). NOS, not otherwise specified.
Histiocyte and dendritic cell lineages
DCs, monocytes, and macrophages are members of the mononuclear phagocyte system,2 whereas a histiocyte is a morphological term referring to tissue-resident macrophages. Macrophages are large ovoid cells mainly involved in the clearance of apoptotic cells, debris, and pathogens. In contrast, DCs are starry cells that present antigens on major histocompatibility complex molecules and activate naive T lymphocytes.3 Human DCs are classified into 2 main groups: plasmacytoid and myeloid (mDC). mDCs have been further subdivided into 2 subsets on the basis of their expression of CD141 (mDC1) and CD1c (mDC2). LCs are DCs localized within the epidermis, mucosae, or bronchial epithelium, expressing CD1a and containing Birbeck granules. When activated by local inflammation, LCs migrate to draining lymph nodes and differentiate into interdigitating cells (IDCs).
Blood monocytes are classified into 3 subgroups depending on CD14 and CD16 expression. In vitro, monocytes may differentiate into any cell type of the mononuclear phagocyte system, and probably also in vivo, during inflammatory conditions. However, under normal conditions, several macrophage and DC subpopulations, including LCs, are self-renewing.
The “L” (Langerhans) group
The Langerhans/non-Langerhans dichotomy noted in prior classification of histiocytoses has become questionable as nearly 20% of patients with Erdheim-Chester disease (ECD) also have LC histiocytosis (LCH) lesions.4 Furthermore, both diseases have clonal mutations involving genes of the MAPK pathway in >80% of cases.5-8 Blood monocytes harboring the same mutations as pathological histiocytes have been reported in both diseases.7,8 In addition, both conditions may be associated with similar clinical complications such as diabetes insipidus and/or neurodegenerative disease. Thus, we propose to include LCH, ECD, and extracutaneous juvenile xanthogranuloma (JXG) in a single group (Table 1).
Langerhans cell histiocytosis
LCH includes a broad spectrum of clinical manifestations in children and adults, ranging from self-healing lesions to life-threatening disseminated disease. The diagnosis of LCH is based on clinical and radiological findings in combination with histopathological analyses identifying tissue infiltration by histiocytes with ultrastructural or immunophenotypic characteristics of LCs. It is recommended that biopsy confirmation of suspected LCH be performed in all cases, especially for patients requiring therapy (grade C2).
The annual incidence of LCH in children younger than 15 years of age is around 5 to 9/106 and 1/106 in patients older than 15 years of age.9,10 Rare cases of familial LCH have been reported,11 but no genetic susceptibility has been identified to date. Lung LCH of the adult is strongly associated with smoking.12
LCH may affect any organ of the body, but those more frequently affected in children are the bones (80% of cases), skin (33%) (Figure 2), and the pituitary gland (25%), liver, spleen, hematopoietic system or lungs (15% each) (Figure 2), lymph nodes (5%-10%), or the central nervous system (CNS) (2%-4% excluding the pituitary).13 In adults, lung involvement is more frequent than in children.
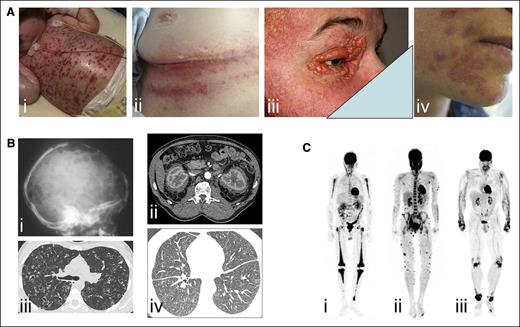
Examples of clinical involvement by histiocytoses. (A) Examples of cutaneous manifestations in (i) a child with multisystemic LCH, (ii) adult with intertrigo-like lesions, (ii) xanthelasma of ECD (ii), and (iii) skin manifestations of RDD. (B) Radiographic imaging and CT scans of (i) lytic skull bone lesions and (ii) pulmonary nodules and cysts in LCH, (iii) CT scan revealing typical “hairy kidney” lesions and (iv) micronodular ground-glass opacities and thickening of interlobular pulmonary septa in ECD. (C) 18F-labeled fluorodeoxyglucose (PET) imaging revealing (i) bilateral and symmetric signal in femurs, tibiae, and humeri in ECD, (ii) cutaneous multiple lesions in RDD, and (iii) signal over wrist, knees, and ankles of a patient with XD.
Examples of clinical involvement by histiocytoses. (A) Examples of cutaneous manifestations in (i) a child with multisystemic LCH, (ii) adult with intertrigo-like lesions, (ii) xanthelasma of ECD (ii), and (iii) skin manifestations of RDD. (B) Radiographic imaging and CT scans of (i) lytic skull bone lesions and (ii) pulmonary nodules and cysts in LCH, (iii) CT scan revealing typical “hairy kidney” lesions and (iv) micronodular ground-glass opacities and thickening of interlobular pulmonary septa in ECD. (C) 18F-labeled fluorodeoxyglucose (PET) imaging revealing (i) bilateral and symmetric signal in femurs, tibiae, and humeri in ECD, (ii) cutaneous multiple lesions in RDD, and (iii) signal over wrist, knees, and ankles of a patient with XD.
Biopsies should be fixed in buffered formalin <72 hours, to allow for histopathology, immunohistochemistry, and molecular analyses (grade B2). Pathologic histiocytes in LCH are mononucleated cells with coffee bean– or kidney-shaped nuclei. Detection of LC markers is mandatory to confirm the diagnosis. In routine practice, detection of Birbeck granules by electron microscopy has been widely replaced by detection of CD1a and CD207 expression, which can be performed on formalin-fixed samples.14 LCH cells are often associated with abundant eosinophils and multinucleated giant cells.
In the diagnostic workup of LCH, several diagnoses should be excluded, depending on the anatomic localization of the disease.15 Differential pathological diagnosis with indeterminate cell histiocytosis (ICH) is based on the absence of CD207 expression on this latter entity. ICH is a very rare entity, with similar clinical presentation, that may have BRAF mutations.16 The recent discovery of ETV3-NCOA2 translocations in 3 cases of ICH suggests that (1) this fusion may be highly specific for ICH and (2) ICH may represent a distinct clonal neoplasm.17 Thus, the diagnosis of LCH should include CD1a and CD207 immunostaining.18,19 In Rosai-Dorfman disease (RDD), S100+ histiocytes are often multinucleated with obvious emperipolesis (erythrocytes, plasma cells, and lymphocytes being engulfed by histiocytes) (Figure 1), and do not express CD1a or CD207. Mitotic activity may rarely be high in LCH, but the diagnosis of malignant histiocytosis should not be established in the absence of major nuclear atypia and clinical evidence of rapid tumor progression (grade D2). LCH has also been reported in association with other hematologic diseases including RDD,20 Hodgkin disease,21 and acute leukemia.22
Extent of disease should be assessed in all patients according to a standardized workup.15 Following the HS guidelines, 4 different groups are defined, depending on the number of organs (or systems) involved, lung involvement, and the involvement of 1 of the 3 following risk organs (ROs): liver, spleen, and bone marrow (ie, dysfunction of the hematopoietic system). In addition, disease activity score23 is a way to monitor the disease and predict outcome. Sequelae can be evaluated according to published scale.24,25
Erdheim-Chester disease
The mean age at diagnosis of ECD is 55 to 60 years of age, but rare pediatric cases have been reported.26 Male to female ratio is 3:1. Diagnosis of ECD is made with histology and phenotype of histiocytes in appropriate clinical and radiological context27 (grade C2).
Skeletal involvement occurs in >95% of ECD patients. Bilateral, symmetric cortical osteosclerosis of the diaphyseal and metaphyseal regions is highly suggestive of ECD,28 and positron emission tomography (PET) with 18F-labeled fluorodeoxyglucose (PET–computed tomography [CT]) has a high specificity29 (grade C2) (Figure 2). Cardiovascular involvement is frequently underdiagnosed, and occurs in at least 50% of patients.30 One-third of patients will develop retroperitoneal fibrosis, especially around the kidneys and ureters. CNS involvement,31 diabetes insipidus, and/or exophthalmos occur in 20% to 30% of patients. Xanthelasma, generally involving the eyelids or periorbital spaces, is the most frequent cutaneous manifestation.
Biopsy is mandatory for the diagnosis of ECD (grade C2), but histology is not specific for the disease. Histopathology is characterized by infiltration of tissues by foamy mononucleated histiocytes with small nucleus. A few multinucleated histiocytes or Touton cells are also frequently observed. Fibrosis is present in most cases and sometimes abundant. Reactive lymphocytes, plasma cells, and neutrophils are also frequent. ECD histiocytes are positive for CD68 and CD163 and negative for CD1a. Some histiocytes may be positive for S100 protein. Some biopsies may lack lipid-laden histiocytes, but the presence of fibrosis and few “peculiar” histiocytes will favor the diagnosis.
ECD can be distinguished from LCH on the basis of the immune-histopathological characteristics of the histiocytes, which are CD1a negative in ECD. However, 20% of patients with ECD also have LCH lesions, sometimes within the same biopsy.4 A few S100+ cells with emperipolesis may be present in ECD, therefore exclusion of RDD may be difficult.
An important issue concerns extracutaneous or disseminated JXG. Indeed, histopathology and phenotype are not different from ECD, and some ECD patients may not have typical bilateral and symmetric involvement of long bones. Thus, we recommend performing molecular analysis of these cases, and to consider as ECD all extracutaneous or disseminated JXG with gain-of-function mutation of BRAF, NRAS, KRAS, or MAP2K1 (grade D2). A few cases of histiocytosis for which differential diagnosis of anaplastic lymphoma was excluded have strong expression of ALK,32 some of which have translocation involving ALK.8,32 Some of these cases had histology and clinical characteristics of ECD. Thus, we recommend performing ALK immunohistochemistry screening for all clinically progressive histiocytoses lacking BRAF and MAP2K1 mutations (grade D2).
Somatic mutations or translocations and cell pathways of “L”-type histiocytoses
The detection of recurrent BRAF p.V600E mutations in LCH5,6,33-35 represents a critical recent breakthrough in understanding LCH pathogenesis. These mutations result in constitutive activation of the MAPK pathway, are known to be oncogenic in several human cancers,36 and are present in approximately half of LCH samples. Moreover, detection of BRAF mutations in CD34+ bone marrow cells of some patients with high-risk LCH provided evidence that LCH may be derived from hematopoietic progenitor cells.34 Mutations in MAP2K1 (or MEK1), the kinase just downstream of BRAF, were also identified in 19% of cases37-39 and occur in a mutually exclusive manner with BRAF mutations (Figure 1). Rare mutations in other MAPK pathway members including ARAF and MAP3K1 have also been reported.39,40 Finally, mutations affecting other signaling pathway such as PIK3CA, PICK1, and PICK3R2 have also been described in LCH.37,41
The presence of BRAF mutations has been investigated in non-LCHs. Approximately 50% of ECD patients have BRAF p.V600E mutations.6 Detection of BRAF mutants in ECD and LCH requires methods of higher sensitivity than for other neoplasia due to allele fraction <10% in some cases (grade C2).7,34 Mutations in MAP2K1, NRAS, KRAS, and ARAF have also been detected in ECD samples.7,8,42 In a recent pangenomic analysis, all cases of ECD had at least 1 mutation activating the MAPK pathway.8 In addition, in-frame fusions involving several kinases including BRAF, ALK, and NTRK1 were identified in ECD patients without BRAF, MAP2K1, or N/KRAS point mutations.8 Similarly, a few cases of histiocytosis harbor ALK translocations.32 These data highlight the possibility that structural alterations involving kinases known to activate the MAPK pathway may be recurrent in L-type histiocytic neoplasms. As in LCH, MAPK mutations have been detected in blood monocytes.7 PIK3CA mutations were also detected in ECD7 and were not exclusive of BRAF mutations. BRAF mutations were absent or only rarely detected in other histiocytoses.6,16,43-45
In our experience, the presence of BRAF mutation is useful to confirm difficult diagnoses of LCH or ECD. BRAFV600E mutations have been detected in cell-free DNA extracted from plasma in patients with disseminated LCH46 or ECD.47 Moreover, treatment with BRAF inhibitors was shown to have major antitumor effects in patients with ECD48-50 or LCH.50,51 Therefore, we recommend looking for BRAFV600E and MAP2K1 mutations in all samples with difficult diagnosis and all patients with failure of first-line treatment (grade C2).
The “C” group: cutaneous and mucocutaneous histiocytoses
Non-LCH localized to skin and/or mucosa surfaces include a wide variety of entities. Some of them may be associated with systemic involvement.52,53 In 2005, Weitzman and Jaffe proposed a practical classification based on immunophenotypic and clinical features.54 We adopted herein roughly this classification with some modifications (Table 2), although some patients display overlapping features.55,56
Xanthogranuloma family
The various diseases included in this family can be defined by the clinical setting, that is, solitary, multiple, or disseminated, the areas of the body involved and the age of the patient.57 JXG is the commonest of the non-LCH. It is a benign disorder in which 1, several, or occasionally numerous red to yellow nodules, 0.5 to 1.0 cm in diameter, are present and usually resolve spontaneously within months to years. Most lesions appear during the first years of life. Extracutaneous and disseminated JXG are discussed in L group. Adult xanthogranuloma (AXG) is usually solitary and persistent. Solitary reticulohistiocytoma (SRH) is simply a xanthogranuloma in which oncocytic macrophages and ground-glass giant cells dominate. Histologically, JXG/AXG/SRH appear as well-circumscribed dermal or dermohypodermal nodules sparing the epidermis. Mature lesions contain foamy cells, foreign-body giant cells, and Touton cells as well as macrophages, lymphocytes, and eosinophils. Older, regressing lesions show proliferation of fibroblasts and fibrosis that replace part of the infiltrate.57 Immunohistochemistry may be helpful in some cases.19 The clinical differential diagnosis, especially of the early lesions, includes Spitz nevi, mastocytomas, and dermatofibromas. Spindle cell xanthogranulomas are more likely to be misdiagnosed, usually as dermatofibromas or blue nevi.
Benign cephalic histiocytosis (BCH) is a self-healing eruption occurring during the first 3 years of life, usually limited to the head and neck. The lesions consist of red to yellow papules. BCH overlaps with generalized eruptive histiocytosis, progressive nodular histiocytosis, papular xanthomas, and multiple JXG.52
Generalized eruptive histiocytosis (GEH) is characterized by the presence of innumerable flesh-colored to red macules and papules that develop in crops, mainly occurring in adults. For some authors, GEH may represent the initial stage of a variety of macrophages disorders such as JXG, xanthoma disseminatum (XD), and progressive nodular histiocytosis (PNH).58 GEH must be distinguished from the eruptive histiocytomas associated with hyperlipidemia. Careful search for microorganisms should exclude eruptive cases of leishmaniasis, leprosy, or atypical mycobacterial infections.
PNH is a rare, clinically distinct disorder affecting elderly patients. Patients typically have hundreds of lesions of 2 distinct types: superficial xanthomatous papules to nodules of 2 to 10 mm in diameter and deep larger fibrous nodules. Conjunctival, oral, or laryngeal lesions may occur. Lesions regress rarely after many years.
XD affects young men. It characteristically has a prominent mucosal and visceral component. The eruption is composed of numerous widely disseminated, brown papules and nodules, mainly located on the flexor areas and around the eyes. Mucosa sites are affected in half of the cases. The course of the disease is chronic and persistent. Diabetes insipidus is encountered in about 40% of cases.
The non-JXG family
Skin localizations of RDD are usually unique, but may rarely be disseminated or reveal a systemic RDD. Histology is described with other RDD localizations.
Necrobiotic xanthogranuloma (NXG) is a rare disorder usually associated with paraproteinemia characterized by large, often yellow, indurated plaques, accompanied by atrophy, telangiectasia, and occasionally ulceration. The most common locations are periorbital and thoracic. Systemic involvement includes a cardiomyopathy and hematological malignancies. Most patients have a monoclonal gammopathy sometimes related to a multiple myeloma. The periorbital location and clinicopathologic correlation are helpful to distinguish NXG from necrobiosis lipoidica.
Multicentric reticulohistiocytosis (MRH) usually affects 50- to 60-year-old women, with widespread cutaneous involvement and destructive arthritis. Lesions are papules, nodules, or tumors and predominate on the extremities. “Coral bead” periungual papules are a pathognomonic sign of MRH. In about half of the patients, nodules are present also on the oral or nasal mucosa. Polyarthritis is almost always present and may destroy hand joints. MRH may be associated with a variety of malignancies or autoimmune diseases. The disease tends to wax and wane over years. The characteristic histologic feature is the presence of numerous multinucleate giant cells and oncocytic macrophages showing eosinophilic, finely granular cytoplasm. The most challenging diagnosis is fibroblastic rheumatism, which is considered by some authors as a variety of histiocytosis but by others as a fibromatosis.59
The “M” groups: malignant histiocytoses
This group includes diseases previously reported as MH, and more recently diagnosed as histiocytic, IDC, LC, or indeterminate cell sarcomas. Follicular DC sarcomas are excluded from the present review.
Primary malignant histiocytoses
MH are tumors with anaplastic histology.60 Diagnosis is mainly based on phenotypic analysis with exclusion of other tumors by negativity for keratins, EMA, Melan-A, HMB45, B and T lymphocyte markers, follicular DC markers, and expression of at least 2 of the following histiocyte/DC markers: CD68, CD163, CD4, and lysozyme. Differential diagnosis between MH relies on staining with a few antibodies, namely S100 protein, CD1a, and CD207. Some cases were shown to have different phenotype in different biopsies,61 and some cases with atypical phenotype cannot be classified. Provided that the differential diagnosis of lymphoma has been excluded, we recommend reusing the old-term “malignant histiocytosis” (grade D1), and refer to the phenotype as a subtype (Table 3).
An important diagnostic problem is the absence of precise diagnostic histologic criteria for malignancy. Both prominent mitotic activity with atypical mitoses and cellular atypia are mandatory, but MH diagnosis should only be confirmed in patients with rapidly progressing tumors (grade D2). Indeed, we have encountered a few patients with typical histology but nonprogressing or spontaneously regressive tumors. Contrasting with LCH, which generally has a normal karyotype and <5 somatic mutations,37 MH usually has frequent chromosomal gains or losses.62
Secondary malignant histiocytoses
These conditions occur after or sometimes simultaneously with another hematologic neoplasm. Malignant cells have anaplastic morphology and express markers of macrophages and/or DCs. MH have to be distinguished from myeloid sarcomas, the latter being tissue infiltration by leukemic cells with no or minor cytologic differences with blood or bone marrow cells.
Cases of MH are associated with lymphoproliferations such as follicular lymphomas,63 hairy cell leukemia,44 chronic lymphoblastic leukemia,64,65 acute lymphoblastic leukemia.66 Some were also reported after chronic myelomonocytic leukemia67 and LCH.68
Clonal relationship with primary hemopathy has been established by showing a common immunoglobulin rearangement,63,64 t(14;18) translocation,63 BRAF mutation,44 or chromosomal alteration65 in primary proliferation and MH. Thus, secondary MH could result either from anaplastic progression with aberrant expression of some histiocyte/DC markers, or from a trans-differentiation of a hemoproliferation.
The “R” group: Rosai-Dorfman disease and miscellaneous noncutaneous, non-Langerhans cell histiocytoses
Classical sporadic RDD (Table 4) involves lymph nodes.69,70 It is more common in children and young adults, and more frequent in males of African descent. The most common presentation is bilateral painless massive cervical lymphadenopathy associated with fever, night sweats, fatigue, and weight loss.69,70 Mediastinal, inguinal, and retroperitoneal nodes may also be involved.
The diagnosis requires the presence of large histiocytic cells displaying hypochromatic nuclei and pale cytoplasm, often with abundant emperipolesis with S100+, fascin+, CD68++, CD14+, HLA-DR+, CD163+ macrophages without CD1a or CD207 staining. Involved tissues usually contain abundant polyclonal plasma cells. In lymph nodes, the large S100+ histiocytes are mainly localized within the sinuses, and the cortex usually contains numerous plasma cells and activated B cells. Laboratory abnormalities are nonspecific with elevated erythrocyte sedimentation rate and leukocytosis, high ferritin, hypergammaglobulinemia, and autoimmune hemolytic anemia.71
Extranodal involvement by sporadic RDD has been documented in 43% of cases with the most frequent sites being skin, nasal cavity, bone, soft tissue, and retro-orbital tissue.71 Bone RDD may be responsible for isolated lytic lesions, or associated with other localizations.72 Intracranial RDD usually occurs without extracranial localization, and most lesions are attached to the dura.73 CNS disease can present clinically and radiologically as meningioma, but the spinal fluid is usually suggestive of RDD.71 Some patients may have pachymeningitis and differential diagnosis with IgG4 disease may be difficult.74
RDD has to be distinguished from LCH; however, negativity for CD1a, as well as the presence of large CD1a-negative histiocytes with emperipolesis are in favor of RDD. Other histiocytoses, including LC sarcomas and histiocytic sarcomas, may harbor some RDD-like cells with emperipolesis, but usually are not predominant. In skin lesions, histopathological differential diagnosis with JXG may be difficult as RDD histiocytes may have a foamy aspect and be multinucleate, and emperipolesis, which is a useful criterion is not specific for diagnosing RDD.75
Finally, RDD frequently contains abundant IgG4-positive plasma cells,76 and differential diagnosis with hyperIgG4 syndrome may be difficult. Therefore, we recommend evaluating IgG4+ plasma cell infiltration in all RDD (grade D2). A RDD pattern has been associated with autoimmune hemolytic anemia, systemic lupus erythematosus, and juvenile idiopathic arthritis.77 Sporadic RDD is often self-limited with a good outcome, especially in classical form. However, 5% to 11% of patients may die of their disease.
Inherited conditions predisposing to RDD or RDD-like lesions
H syndrome is an autosomal genetic condition characterized by hyperpigmentation, hypertrichosis, hepatosplenomegaly, hearing loss, heart anomalies, hypogonadism, low height (short stature), hyperglycemia/diabetes mellitus, and hallux valgus/flexion contractures. It is related to mutation within the SLC29A3 gene. Lymph nodes with RDD are present in 20% of patients.78 RDD lesions can also be detected in skin or nasal localizations.79
A histopathological pattern of RDD was reported in the lymph nodes of 41% of patients with autoimmune lymphoproliferative syndrome (ALPS) type Ia, with TNFRSF6 heterozygous germ line mutations affecting the gene encoding Fas.80 These patients tend to be more often males, present at an earlier age, and have more severe manifestations of ALPS. However, the RDD changes tend to be mainly nodal and self-limited in these cases, and only in a small number of patients contribute significantly to the clinical manifestations of ALPS.
Miscellaneous noncutaneous, non-Langerhans cell histiocytoses
Histological diagnosis and classification of histiocytoses may be difficult, and definitive diagnosis requires close collaboration between pathologist, clinicians, and radiologist. Despite review by panels of experts, some cases still could not be classified as a defined entity, and thus may require the term of “histiocytosis not otherwise specified”.
The “H” group: hemophagocytic lymphohistiocytosis and macrophage activation syndrome
Hemophagocytic lymphohistiocytosis (HLH) is a rare, often-fatal syndrome of intense immune activation characterized by fever, cytopenias, hepatosplenomegaly, and hyperferritinemia.81 Although HLH has various underlying causes, all subtypes of HLH are related to immune dysregulation that leads to hypercytokinemia and an accumulation of activated macrophages in organs and tissues.82 HLH is divided into primary (with known Mendelian inheritance) and secondary or reactive HLH. In cases where a molecular diagnosis is lacking, patients can be clinically diagnosed with HLH upon fulfillment of at least 5 of 8 diagnostic criteria.83 First-line treatment of both primary and secondary HLH is directed to control the hyperinflammation. For primary HLH, hematopoietic stem cell transplantation is the only available cure.84,85
Primary HLH is associated with several Mendelian inherited immune disorders, most of which induce impairment86 (Table 5; supplemental Table 1) or disturbance of the inflammasome.87,88 However, a proportion of suspected primary cases await molecular definition.89 The clinical symptoms usually become evident during the first years of life, although onset into adulthood has been reported.90 Patients may suffer from 1 or several acute episodes that may be rapidly progressive. Each acute episode may be triggered by infection (see Table 5) or vaccination. In young children, HLH-like manifestations may also occur in association with primary immunodeficiencies, especially with primary T-cell deficiencies such as MAGT1, ITK, CD27, CTPS1, MST1, Dock 8, Dock 2, ORAI1, STIM1, or Coroinin1A deficiencies. These HLH-like syndromes are not to be considered as true primary HLH, they are frequently triggered by Epstein-Barr virus infection causing B lymphoproliferation.91
Secondary HLH may occur at any age, and the first clinical symptoms are usually associated with an infectious episode, rheumatic condition, or malignancy.92 The clinical course may be severe, and the mortality is still significant.93 Recently, some cases of “secondary” HLH have been linked to mutations that confer a partial impairment of cytolytic function.92 These findings make the distinction between primary and secondary HLH increasingly difficult. Symptoms of HLH developing in a patient with an underlying rheumatic condition are historically called Macrophage Activation Syndrome (MAS). Suggestions to replace the term MAS with “secondary HLH” first appeared in the literature in 2002.94,95 We suggest using the term MAS-HLH for this subset of HLH.
Conclusion
Herein, we have provided an overview of histiocytoses with a revised proposed grouping of >100 subtypes of these rare entities in 5 groups based on clinical and/or molecular relevance. Most cases of L group diseases have clonal mutations activating MAPK pathways5-8,33-35,37 and are considered by some as inflammatory myeloid neoplasms.34 Major clinical responses have been obtained with MAPK inhibitors for patients of this group.48-51 Several patients have histiocytoses limited to skin, the hallmark of which is solitary cutaneous JXG. Pathogenesis of most cutaneous histiocytoses is still unknown. MH may be primitive cancers or secondary to other hematologic neoplasia, and may be subclassified based on primitive localization, and/or the expression of macrophage and DC markers. RDD has characteristic histologic features, but corresponds to several different inherited or sporadic conditions, often associated with IgG4+ plasma cell infiltration. Finally, histiocyte infiltration that is frequently but not always observed in HLH has been shown to be reactive to an abnormal immune activation.
Presented in part at the 31st annual meeting of the Histiocyte Society, Athens, Greece, September 28-30, 2015.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the members of the Histiocyte Society for fruitful discussions during the 31st annual meeting of the Histiocyte Society, and more specifically Robert J. Arceci and Ronald Jaffe and the members of the HLH working group: Gritta Janka, Julie-An Talano, Kai Lehmberg Milen Minkov, Maurizio Arico, and Eiichi Ishii. The authors also thank Fleur Cohen-Aubart, Philippe Maksud, and Philippe Grenier for providing pictures of patients with histiocytosis.
Authorship
Contribution: J.-F.E. coordinated the writing group; J.-F.E., O.A., S.F., A.H., J.H., J.D., L.R.-C., O.A.-W., C.E.A., F.C., R.M.E., A.F., J.-I.H., F.J., M.B.J., M.M., J.P., B.J.R., C.R.-G., A.T., R.V., and L.M.W. discussed the plan and approved it; J.-F.E., O.A., S.F., A.H., J.H., J.D., L.R.-C., and O.A.-W. wrote the manuscript; C.E.A., F.C., E.L.D., R.M.E., A.F., J.G.H., J.-I.H., F.J., M.B.J., M.M., J.P., B.J.R., C.R.-G., A.T., R.V., and L.M.W. corrected the manuscript; and all of the authors approved the manuscript.
Conflict-of-interest disclosure: J.-F.E. received honoraria from Roche and GlaxoSmithKline (GSK). J.H. received grants from Roche. C.E.A. was an unpaid consultant to Novimmune. J.G.H. received grants from Fondo de Investigacion Sanitaria (FIS). R.V. received grants from GSK. L.M.W. received fees from BioTheranostics and Genentech. The remaining authors declare no competing financial interests.
A list of the members of the Histiocyte Society appears in “Appendix.”
Correspondence: Jean-François Emile, EA4340 and Pathology Department, Ambroise Paré Hospital, 9 Av Ch de Gaulle, 92104 Boulogne, France; e-mail: jean-francois.emile@uvsq.fr.
Appendix: study group members
The Histiocyte Society includes: Executive Board: C. Rodriguez-Galindo, M. Minkov, I. Astigarraga, M.B. Jordan, A. Horne, and K. Nichols; Scientific Committee: Y. Bryceson, S. Ehl, J. Haroche, C. Hutter, R. Marsh, B.J. Rollins, J. Visser, and K. Zhang.

![Figure 1. Histology and somatic mutations of histiocytoses of group L, C, R, M, and H. (A) L group: Histology of LCH (skin [i-ii] and bone [iii]) and of ECD (perirenal [iv-v]). Pie chart of relative frequencies of activating kinase mutations in LCH (vi) and ECD (vii). (B) C group: Histology of JXG (i-ii). (C) R group: Histology of RDD (meningeal with high IgG4+ plasma cell infiltration [i-ii]). (D) M group: Histology of MH (i-ii). (E) H group: Histology of inherited HLH (liver [i-ii]). Staining with CD1a (Lii in red), IgG4 (Rii in brown), CD163 (Hii in brown), or hematoxylin and eosin (all others). NOS, not otherwise specified.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/22/10.1182_blood-2016-01-690636/4/m_2672f1.jpeg?Expires=1767743426&Signature=SEEhxTbeoHcH-5GyXP0eCD3-Jgbsz7JC0kMdtraha6i8cXul4zsAwLfwwWuwvFF08EG4MxNHZQhoCCO~KSFEzUCNita1CZat7zIquFO3DY3pOalmPFpa4vwBz4ebc6tp3eJ6pGrEDQrTW~2uA8IUlY3-UUz0oJbxmKUmUJMQ5nzJ9LTupB8w682R-Oc2fkgt-POZ6l2DsCAab6jxqE~Wy4d10eMW1siuuxulxX8MTUlHR2GWfMxeMZETqkfL~E5SbTOA1e1xKHKcPq-Yadyu8GJDAo4Nc2J0d0sTdnr3KDxliRGWlHC9le-BuwJL8jl7YM~fx9~qamAEjh1VhTKAWg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Histology and somatic mutations of histiocytoses of group L, C, R, M, and H. (A) L group: Histology of LCH (skin [i-ii] and bone [iii]) and of ECD (perirenal [iv-v]). Pie chart of relative frequencies of activating kinase mutations in LCH (vi) and ECD (vii). (B) C group: Histology of JXG (i-ii). (C) R group: Histology of RDD (meningeal with high IgG4+ plasma cell infiltration [i-ii]). (D) M group: Histology of MH (i-ii). (E) H group: Histology of inherited HLH (liver [i-ii]). Staining with CD1a (Lii in red), IgG4 (Rii in brown), CD163 (Hii in brown), or hematoxylin and eosin (all others). NOS, not otherwise specified.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/22/10.1182_blood-2016-01-690636/4/m_2672f1.jpeg?Expires=1768035274&Signature=5O0TeG-CUQhxANfeqiy-KXA17o2bIaX-TcNZXk0OnCnrXUllVySYuaOHgiSyYtnHOEEWZfNE88GtF4Zxgb7UD4CaQrAIOmPF5Snm06dqtN1l4fviEx4aWGZPkAM~Dz1qRW8O0s4245KONZmc~0pPY-JYKymAGDI5poDolerEtfndNNJoPEDmb9F9Jlw5XA~hteURoeCNv7GfM~91wVmPnlig1lOTqvlUe1W1amE1aGa~UsaCOapMsh8H5PdfYd4i75OAthVBv~15lFKKl6qivziso-OCdUeuvrJpWak~9x2BtUPcEb0~ayGLZN04xaYwG0mS8JSpMS~BT0EcGfQr2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)