Abstract
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma in the Western hemisphere. After decades of stagnation, the natural history of FL appears to have been favorably impacted by the introduction of rituximab. Randomized clinical trials have demonstrated that the addition of rituximab to standard chemotherapy induction has improved the overall survival. Maintenance rituximab strategies can improve progression-free survival. Even chemotherapy platforms have changed in the past 5 years, as bendamustine combined with rituximab has rapidly become a standard frontline strategy in North America and parts of Europe. Recent discoveries have identified patients at high risk for poor outcomes to first-line therapy (m7–Follicular Lymphoma International Prognostic Index [m7-FLIPI]) and for poor outcomes after frontline therapy (National LymphoCare Study). However, several unmet needs remain, including a better ability to identify high-risk patients at diagnosis, the development of predictive biomarkers for targeted agents, and strategies to reduce the risk of transformation. The development of targeted agents, exploiting our current understanding of FL biology, is a high research priority. A multitude of novel therapies are under investigation in both the frontline and relapsed/refractory settings. It will be critical to identify the most appropriate populations for new agents and to develop validated surrogate end points, so that novel agents can be tested (and adopted, if appropriate) efficiently.
Introduction
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (NHL) in the Western hemisphere. Although FL is considered incurable with standard chemotherapy, advances in treatment and our understanding of its biology have improved disease management and clinical outcomes. Here, we briefly review the biology of FL and discuss evolving therapeutic strategies.
The biology of follicular lymphoma
Pathology
FL is derived from germinal center (GC) B cells. Its pathogenesis is closely linked to the normal GC reaction where naive B cells from the bone marrow undergo somatic hypermutation and class switching of the B-cell receptor (BCR) in a process that generates immunoglobulin diversity and selects B cells producing high-affinity antibodies.1,2 The hallmark t(14;18)(q32;q21) translocation of FL occurs early in B-cell development, from an error in V(D)J recombination. Like normal naive B cells, those carrying t(14;18) home to follicles and are selected for entry and proliferation in GCs by follicular helper T cells (Figure 1).3 Here, t(14;18)+ B cells likely have a survival advantage due to ectopic expression of BCL2. Although normal B cells exit the GC as mature memory B cells or plasma cells, t(14;18)+ FL-like B cells that exit the GC can traffic and acquire the additional genetic changes necessary for developing their full malignant phenotype.3
Model of FL pathogenesis. Naive B cells in the bone marrow (BM) acquire the t(14;18) translocation due an error in V(D)J recombination and subsequently home to B-cell follicles where they undergo the GC reaction. In the dark zone of the GC, the B cells proliferate as centroblasts and undergo somatic hypermutation (SHM) and class switching of their BCRs. Centroblasts then become smaller centrocytes and migrate to the light zone of the GC where they interact with follicular dendritic cells (FDCs) and are selected to either undergo apoptosis or rescue by follicular helper T cells (TFH) based on antigen (Ag) affinity of their BCRs. Ectopic expression of BCL2 provides mutant B cells with t(14;18) an avenue to escape apoptosis, independent of BCR affinity. These FL-like B cells then exit the GC and enter the circulation where they might be prone to traffic between follicles and/or the BM and have the opportunity to acquire additional genetic changes necessary for transformation to FL. Reprinted from Roulland et al3 with permission. CSR, class switching recombination; IgM, immunoglobulin M; sIgM, surface immunoglobulin M.
Model of FL pathogenesis. Naive B cells in the bone marrow (BM) acquire the t(14;18) translocation due an error in V(D)J recombination and subsequently home to B-cell follicles where they undergo the GC reaction. In the dark zone of the GC, the B cells proliferate as centroblasts and undergo somatic hypermutation (SHM) and class switching of their BCRs. Centroblasts then become smaller centrocytes and migrate to the light zone of the GC where they interact with follicular dendritic cells (FDCs) and are selected to either undergo apoptosis or rescue by follicular helper T cells (TFH) based on antigen (Ag) affinity of their BCRs. Ectopic expression of BCL2 provides mutant B cells with t(14;18) an avenue to escape apoptosis, independent of BCR affinity. These FL-like B cells then exit the GC and enter the circulation where they might be prone to traffic between follicles and/or the BM and have the opportunity to acquire additional genetic changes necessary for transformation to FL. Reprinted from Roulland et al3 with permission. CSR, class switching recombination; IgM, immunoglobulin M; sIgM, surface immunoglobulin M.
FL is characterized by a proliferation of neoplastic GC B cells, both centrocytes and centroblasts, with at least a partial follicular pattern.4 The current grading system for FL evaluates the proportion of centrocytes to centroblasts; cases with more centroblasts behave more aggressively and have a higher likelihood of transformation to diffuse large-cell lymphoma.5 Grade 1-2 FL is defined as ≤15 centroblasts per high-powered field, whereas grade 3 FL has >15 centroblasts per high-powered field. Grade 3 FL is further classified as 3A or 3B, with the latter characterized by an absence of centrocytes. Accumulating evidence suggests that FL3B is a biologically distinct entity, with frequent absence of t(14;18) and CD10 expression and increased p53 and MUM1/IRF4 expression.6 Accordingly, a large retrospective analysis of >500 FL cases confirmed that the clinical course of FL3A is similar to FL1-2, whereas FL3B had a clinical course more similar to diffuse large B-cell lymphoma, with no relapses beyond 5 years.7
Mutational landscape
The mutational landscape of FL is dominated by 2 recurrent alterations: (1) the t(14;18) translocation and (2) inactivating mutations of the MLL2 gene. The t(14;18) translocation is found in 85% of FL and places the BCL2 gene under the IGH regulatory elements. Dysregulation of BCL2 expression alone is not sufficient to induce lymphomagenesis, but it provides a survival advantage through activation of antiapoptotic programs that are typically repressed by BCL6 in GC B cells.8 Inactivating mutations of MLL2 are found in >80% of FL and interfere with the ability of MLL2 to activate gene transcription through H3K4 methylation.9 Like t(14;18), MLL2 inactivation appears to be an early event in FL, suggesting that epigenetic dysregulation combined with dysregulated BCL2 may the drive malignant transformation of GC B cells.10 Mutations of other histone modifiers (CREBBP, EZH2, MEF2B, and EP300) are found in ∼33%, 27%, 15%, and 9% of FL, respectively.9,11,12 Finally, analysis of candidate genes within recurrent nonrandom losses of chromosome 1p36 and 6q in FL revealed that inactivating mutations of TNFRSF1413,14 and loss of tumor suppressors TNFAIP3/A20 and EPHA7 contribute to the pathogenesis of FL.15
Follicular lymphoma microenvironment
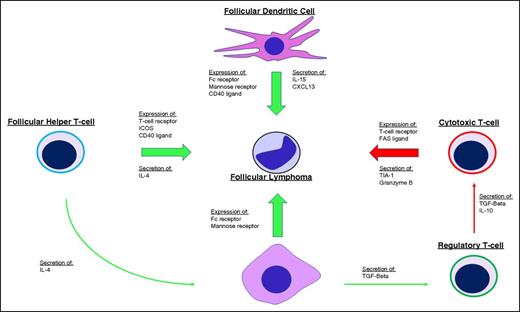
Dave et al demonstrated the significance of the tumor microenvironment when gene expression signatures of the nonmalignant stromal cells were found to be prognostically more important than the neoplastic B cells.16 The gene expression signature associated with favorable outcomes was enriched for genes expressed by T cells whereas the expression signature associated with less favorable outcomes was enriched for genes expressed by macrophages and follicular dendritic cells, suggesting the balance between immune surveillance and a permissive tumor microenvironment plays a role in dictating the disease course (Figure 2). Many subsequent immunohistochemical studies have attempted to translate these findings to the clinical laboratory by enumerating T-cell subsets and macrophages on biopsy specimens, but results have been inconsistent and have yet to engender any practice changes.17-22 Understanding and translating the complex relationship between the neoplastic cells and their tumor microenvironment to actionable prognostic assays or companion diagnostics remains an active area of investigation.
Influence of the tumor microenvironment on FL. Tumor growth and survival are supported (green arrows) by follicular dendritic cells, follicular helper T cells (TFH), and M2-type tumor-associated macrophages (TAMs) through expression of various ligands and receptors on the cell surface, including the FC receptor and mannose receptors that bind the FL BCR and stimulate BCR signaling. Secretion of various cytokines and chemokines also supports tumor growth by directly stimulating FL cells and by shaping the microenvironment into one that is more accommodating for FL, including secretion of interleukin-4 (IL-4) by TFH cells to skew TAM activation toward a tumor-supportive M2 phenotype and secretion of transforming growth factor β (TGF-β) by TAMs to promote T-helper cell differentiation toward regulatory T cells that inhibit (red arrows) antitumor cytotoxic T cells. ICOS, inducible T-cell costimulator.
Influence of the tumor microenvironment on FL. Tumor growth and survival are supported (green arrows) by follicular dendritic cells, follicular helper T cells (TFH), and M2-type tumor-associated macrophages (TAMs) through expression of various ligands and receptors on the cell surface, including the FC receptor and mannose receptors that bind the FL BCR and stimulate BCR signaling. Secretion of various cytokines and chemokines also supports tumor growth by directly stimulating FL cells and by shaping the microenvironment into one that is more accommodating for FL, including secretion of interleukin-4 (IL-4) by TFH cells to skew TAM activation toward a tumor-supportive M2 phenotype and secretion of transforming growth factor β (TGF-β) by TAMs to promote T-helper cell differentiation toward regulatory T cells that inhibit (red arrows) antitumor cytotoxic T cells. ICOS, inducible T-cell costimulator.
Emerging evidence suggests that tonic signaling through the BCR and its downstream pathways may provide a key survival signal to FL cells. Somatic hypermutation of the BCR is capable of introducing N-glycosylation sites to the FL BCR variable regions that ultimately bear mannose-terminated glycans.23 Introduction of these mannose-terminated glycans facilitates BCR interaction with mannose-binding lectins found on dendritic cells, macrophages, and commonly occurring bacteria, thereby allowing the GC microenvironment to support malignant B-cell survival in the absence of cognate antigen.24,25 Specifically, dendritic cell–specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) is a mannose-binding lectin, overexpressed in FL dendritic cells and tumor-associated macrophages (TAMs), that binds FL BCR and triggers BCR signaling. DC-SIGN–mediated FL BCR signaling can be attenuated by Bruton tyrosine kinase (BTK) or SYC inhibitors and reduces the viability of FL cells in vitro, illuminating the therapeutic potential of targeting the FL microenvironment.26,27
Clinical course and estimating prognosis
FL is a biologically heterogeneous disease, and the prognosis varies widely among individuals. The prognosis for an individual patient can be estimated based on clinical and laboratory findings. The Follicular Lymphoma International Prognostic Index (FLIPI) was derived from a database of over 4000 FL patients treated largely in the prerituximab era.28 This index is often remembered by the acronym “No-LASH” as the 5 strongest prognostic factors in multivariate analysis were: (1) number of nodal sites of disease (>4); (2) elevated lactate dehydrogenase (LDH); (3) age >60 years; (4) stage III or IV disease; and (5) hemoglobin <12 g/dL. The FLIPI provides a roughly equal distribution of patients across low-risk (0-1 factor), intermediate-risk (2 factors), or high-risk (>3 factors) categories. The 10-year overall survival (OS) rates were 71% (low risk), 51% (intermediate risk), and 36% (high risk). An index developed in the rituximab-chemotherapy (R-chemo) era, called FLIPI-2, identified age >60 years, elevated β2-microglobulin (B2M), hemoglobin <12 g/dL, bone marrow involvement, and lymph node diameter >6 cm as independent risk factors for progression-free survival (PFS) (Table 1).29
Identifying biologic prognostic factors, which can be measured in practice, has been challenging. A recent international, multigroup effort produced a clinicogenetic risk model that integrates the mutational status of 7 genes (EZH2, ARID1A, MEF2B, EP300, FOX01, CREBBP, and CARD11) with the FLIPI (termed m7-FLIPI).30 In the validation set, the m7-FLIPI identified a high-risk group of patients (22% of the cohort) with a 5-year failure-free survival of 25%. In contrast, the 5-year failure-free survival of the high-risk cohort identified by FLIPI alone was 46%, indicating the superiority of the m7-FLIPI for identifying a high-risk population. The m7-FLIPI provides an example of an integrated risk model and will need to be validated prospectively.
Perhaps the strongest predictor of long-term outcomes is the length of first remission after a standard immunochemotherapy induction. An analysis from the National LymphoCare Study (NLCS) examined outcomes in 588 patients receiving rituximab, cyclophosphamide, vincristine, prednisone (R-CHOP) as initial FL therapy.31 Approximately 20% of patients experienced progressive disease (PD) within 2 years of diagnosis. The 5-year OS was 50% in the early PD group compared with 90% in patients without early PD. Neither the FLIPI, nor the FLIPI-2, nor the m7-FLIPI is able to identify a group of young (age <60 years) patients at such a high risk for early death. The optimal management strategy for these patients is unclear, and clinical trials are needed specifically for this patient population.
Transformation
The FL disease course, which can spontaneously remit, even in the absence of treatment, may be best modeled by the idea of a dominant clone that fluctuates under the selective pressure of inherent mutations and the associated microenvironment.32 The tracking of multiple clones in patients shows that disease progression occurs either by direct clonal evolution or by divergent evolution from a common progenitor cell. Similarly, transformation to an aggressive B-cell lymphoma can also occur by either of these routes.33 Up to 45% of patients will experience transformation during the course of their disease.34-36 Although no cytogenetic or molecular biomarkers are in routine use to assess a patient’s risk for transformation, mutations in the neoplastic cells including upregulation of MYC expression37,38 and TP53 mutations,39 as well as changes in the tumor microenvironment,21,40 have been associated with transformation.
A recent report from the NLCS evaluated outcomes in 2652 patients, and found the risk of transformation remains 2% to 3% per year in the R-chemo era.41 The risk was similar in R-CHOP– and rituximab, cyclophosphamide, prednisone (R-CVP)–treated patients, suggesting no risk reduction with the upfront inclusion of anthracyclines. However, the risk was reduced in patients receiving maintenance rituximab (hazard ratio, 0.67; 95% confidence interval, 0.46-0.97). Of particular note, the median OS after transformation was 5 years, which was markedly better than historical reports.
Evolving management strategies for follicular lymphoma
Management of localized follicular lymphoma
Limited-stage (Ann Arbor I or II) FL is relatively uncommon, and as a result, there are no randomized studies indicating the optimal management strategy. Rather, most of the data are observational, derived from single-institution databases. Older studies suggested 40% to 50% of patients might be cured with external beam radiation therapy (XRT).42,43 More recent data indicate that radiation fields can be reduced without adversely impacting disease control.44 Studies evaluating chemotherapy plus radiation (combined modality therapy) have demonstrated improved PFS without an obvious effect on OS.45 An initial strategy of observation can also be considered. A Stanford report of stage I and II patients who received no initial therapy showed that more than half of the 43 patients did not require therapy at a median of 6 years, and 85% of patients were alive at 10 years.46 Regarding contemporary practice patterns in the United States, a report from the NLCS database found the following treatment approaches were used for 471 stage I FL patients: R-chemo, 28%; XRT, 27%; observation, 17%; combined modality therapy, 13%; rituximab, 12%; and other, 3%.47 Approaches utilizing systemic therapy produced better PFS outcomes than XRT alone. There were no OS differences between any of the approaches. Given the lack of OS differences, any of the mentioned strategies can be defended, and the approach must be individualized based upon patient-specific factors such as age, comorbidities, and goals. Certainly for patients with a life expectancy of 15 years or less, a watch-and-wait strategy (or strictly palliative approach) is often most appropriate, as aggressive therapy is unlikely to alter the life expectancy and could have detrimental effects on quality of life. For patients with longer life expectancies, treatment including involved field or involved site radiation, delivered with curative intent, seems most appropriate.
Approach to patients with advanced-stage follicular lymphoma
Advanced stage grade 1-2 and grade 3a FL is generally considered “incurable” yet the disease is highly responsive to most therapies, and the median OS likely exceeds 12 years. The approach to a newly diagnosed patient should factor the presence or absence of symptoms, the tumor burden, the patient age and comorbidities, and the goals of therapy. An algorithmic approach distinguishing patients by symptoms and by tumor burden is depicted in Figure 3. Four patient categories are generated by the algorithm: (1) asymptomatic, low tumor burden; (2) asymptomatic, high tumor burden; (3) symptomatic, low tumor burden; and (4) symptomatic, high tumor burden, with suggested management strategies for each. The 2 most common scenarios are discussed in the next 2 sections.
An algorithmic approach to FL. (A) Approach to the patient with newly diagnosed FL. Patients with newly diagnosed, advanced-stage, grade 1-2 or grade 3a FL can be managed using the proposed algorithm. Patients with FL grade 3b are generally managed according to the principles for diffuse large B-cell lymphoma. (B) Approach to the patient with recurrent FL. Management decisions must be individualized and are dependent upon a number of factors including patient age, comorbidities, goals of therapy, number and types of prior therapies, and efficacy of prior therapies. AlloSCT, allogeneic stem cell transplantation; AutoSCT, autologous stem cell transplantation.
An algorithmic approach to FL. (A) Approach to the patient with newly diagnosed FL. Patients with newly diagnosed, advanced-stage, grade 1-2 or grade 3a FL can be managed using the proposed algorithm. Patients with FL grade 3b are generally managed according to the principles for diffuse large B-cell lymphoma. (B) Approach to the patient with recurrent FL. Management decisions must be individualized and are dependent upon a number of factors including patient age, comorbidities, goals of therapy, number and types of prior therapies, and efficacy of prior therapies. AlloSCT, allogeneic stem cell transplantation; AutoSCT, autologous stem cell transplantation.
Management of asymptomatic, low-tumor-burden follicular lymphoma
Asymptomatic, low-tumor-burden patients may be candidates for a strategy of watch and wait. The Groupe d’Etude des Lymphomes Folliculaires (GELF) criteria are commonly used criteria to assess tumor burden. For high-tumor-burden FL, GELF criteria include at least 1 of the following: 3 distinct nodal sites, each ≥3 cm; single nodal site ≥7 cm; symptomatic splenomegaly; organ compression or compromise; pleural effusions, ascites; B symptoms or any systemic symptoms; LDH or B2M above the upper limit of normal. The watch-and-wait strategy was first advocated at Stanford University when 2 retrospective studies suggested no detriment in patient outcome.48,49 Three randomized clinical trials later confirmed the Stanford observations.50-52 Low-tumor-burden FL patients assigned to watch and wait had the same OS compared with patients assigned immediately to treatment. All of these studies, however, were conducted in the prerituximab era. To date, there are no studies comparing rituximab plus chemotherapy to watch and wait. One randomized clinical trial has compared single-agent rituximab to watch and wait in patients with previously untreated, asymptomatic, low-tumor-burden FL.53 Patients were assigned to watch and wait (arm A), rituximab at 4 weekly doses (arm B), or rituximab at 4 weekly doses plus a single dose every 2 months for 2 years (arm C). A significant prolongation in PFS as well as time to first chemotherapy was observed for the patients randomized to rituximab. With a median follow-up of 32 months, the proportion of patients without progression at 3 years was 33%, 60%, and 81% in arms A, B, and C, respectively. The proportion of patients free of chemotherapy or radiation at 3 years was 48%, 80%, and 91% in arms A, B, and C, respectively. There was no difference, however, in the OS at 3 years (95% in all arms). The study found that anxiety and depression were more common in patients with low-tumor-burden FL than in the general population but that patients in all treatment arms adapted to their illness over time. The patients identified as “anxious” adapted more readily when assigned to rituximab treatments. From these data, it is reasonable to conclude that: (1) given no OS difference observed to date, watch and wait remains a reasonable standard for the asymptomatic, low-tumor-burden FL population; (2) some benefits are associated with immediate rituximab therapy, such as improved PFS and a longer time to first chemotherapy (these benefits should be discussed with patients); and (3) a subset of patients (perhaps 15%) with particular difficulty adjusting to their diagnosis may experience a quality-of-life benefit from single-agent rituximab.
The question of whether to use a maintenance rituximab dosing strategy in this population was addressed in the Rituximab Extended Schedule or Re-Treatment Trial (RESORT) study.54 After induction therapy with single-agent rituximab, patients with low-tumor-burden FL were randomized to receive maintenance rituximab until treatment failure or to be periodically re-treated with rituximab (re-treated with 4 weekly doses at each progression) until treatment failure. The trial revealed no difference in the time to treatment failure between the 2 dosing strategies. Patients on the maintenance arm, however, received 4 times as much rituximab. There was no difference in quality-of-life, depression, or anxiety between the 2 strategies. On the basis of these results, a re-treatment strategy is preferred if opting for single-agent rituximab in this patient population.
Therapy of symptomatic, high-tumor-burden follicular lymphoma
The addition of rituximab to conventional chemotherapy has improved outcomes in FL, including response rates, PFS, event-free survival, and OS. The results were remarkably consistent across 4 randomized clinical trials.55-58 Clearly, rituximab added to chemotherapy is a therapeutic advance in FL. The optimal chemotherapy backbone to partner with rituximab remains unsettled. Before the introduction of bendamustine, the most commonly used regimens in the United States were R-CHOP (60%), R-CVP (27%), and R-fludarabine based (13%).59 A randomized comparison of these regimens indicated R-CHOP had the best risk-benefit profile of the 3, as it was more active than R-CVP and less toxic than rituximab-fludarabine-mitoxantrone.60
The alkylating agent bendamustine has gained widespread adoption as the chemotherapy platform of choice in FL. A phase 3 trial from the Study group indolent Lymphoma (StiL) comparing bendamustine-rituximab (BR) to R-CHOP demonstrated better efficacy and reduced toxicity with BR.61 In this multicenter phase 3 study, 549 patients with high-tumor-burden indolent NHL and mantle cell lymphoma (median age, 64 years) were randomized to receive bendamustine 90 mg/m2 on days 1 and 2, with rituximab 375 mg/m2 on day 1, every 28 days (the BR group) or to receive standard R-CHOP chemotherapy every 21 days. The overall response rates (ORRs) were similar in the BR vs R-CHOP groups (92.7% vs 91.3%, respectively), but the complete response (CR) rate was significantly higher in the BR group (39.8%) compared with the R-CHOP group (30.0%) (P = .03). When evaluating just the FL patients, with a median follow-up of 45 months, the median PFS was significantly longer after BR compared with R-CHOP (median PFS, not reached vs 40.9 months, P = .007). OS did not differ between both groups. There was less hematologic toxicity, alopecia, infections, peripheral neuropathy, and stomatitis with BR. Drug-associated erythematous skin reactions were seen more frequently in the BR group. These data suggest that BR is a better option for untreated high-tumor-burden FL. A confirmatory randomized phase 3 trial (BRIGHT study) was conducted in North America.62 Previously untreated indolent NHL patients with high tumor burden were randomized to BR or R-CHOP/R-CVP. Control arm patients were identified as an R-CHOP or R-CVP candidate prior to randomization. The primary end point was to show noninferiority of BR in the CR rate. Seventy percent of the 447 enrolled patients had FL, and in these patients, BR therapy was found to be noninferior to the R-CHOP/R-CVP control arm for CR rate (30% vs 25%) and the ORR (99% vs 94%). Time-to-event data were not reported. Side-effect profiles were distinct, with more gastrointestinal toxicity and rash with BR, and more neuropathy and alopecia with R-CHOP/R-CVP. Although, the BRIGHT data do not exactly replicate the StIL data for BR, they do suggest that BR remains an attractive alternative to R-CHOP or R-CVP in FL.
The question of whether to administer maintenance rituximab after frontline R-chemotherapy was addressed in the phase 3 PRIMA trial.63 The study evaluated the efficacy and safety profile of maintenance rituximab in newly diagnosed FL patients who responded to initial treatment with rituximab plus chemotherapy. Induction treatment was selected by center; R-CHOP (75%), R-CVP (22%), or rituximab-fludarabine-cyclophosphamide-mitoxantrone (3%). Patients were randomized to either observation or a single dose of rituximab every 2 months for 2 years. At a median follow-up of 36 months from randomization, the 2-year PFS in the maintenance rituximab arm was 75% vs 58% in the observation arm (P < .0001). The beneficial effect of maintenance rituximab was seen irrespective of the induction chemotherapy backbone and in both CR and partial remission patients. Grade 3-4 adverse events were slightly higher in the maintenance rituximab arm (24% vs 17%). No difference in OS was observed. Given the lack of OS benefit, the decision regarding the use of maintenance rituximab can be individualized. Regarding the duration of maintenance rituximab, available data suggest that 2 years is safe, but data beyond 2 years is lacking.
A novel strategy, combining the immunomodulatory agent lenalidomide with rituximab, for the initial management of FL was reported by investigators from the MD Anderson Cancer Center.64 Lenalidomide was administered orally at 20 mg per day on days 1 to 21 of each 28-day cycle. Rituximab was given at 375 mg/m2 IV on day 1 of each cycle. Patients responding after cycle 6 could continue on therapy for up to 12 cycles. Of 46 evaluable patients with FL, 87% had a complete response and 11% had a partial response (ORR 98%). The 3-year PFS was 78.5%. The main grade 3-4 toxicities were neutropenia (35%), muscle pain (9%), and rash (7%). Based upon these promising results, an international phase 3 study entitled RELEVANCE (clinicaltrials.gov identifier NCT01476787) comparing this regimen to R-chemo in untreated FL was initiated. This trial is fully enrolled and outcomes are pending.
Therapy for relapsed and refractory follicular lymphoma
Multiple options exist for the treatment of patients who have failed first-line therapy, and the decision of which therapy to use depends on a number of factors, including the prior treatment used, duration of prior response, patient age, comorbid illnesses, and goals of therapy (Figure 3B).
Bendamustine is approved in the United States for use in patients with rituximab-refractory indolent B-cell lymphoma. A pivotal trial in 100 patients reported an ORR of 75% with a median PFS of 9.3 months.65 The US Food and Drug Administration (FDA)–approved dose of single-agent bendamustine is 120 mg/m2 given IV on days 1 and 2 of 21-day cycles. Approximately two-thirds of patients required dose modifications or delays, mainly due to cumulative myelosuppression. In addition, most practitioners prefer to administer bendamustine with rituximab. An expert panel has published guidelines on bendamustine dosing when combined with rituximab, and recommends 90 mg/m2 on days 1 and 2 repeated every 28 days.66 Lower doses (such at 70 mg/m2) may be more appropriate in the elderly.
Fludarabine-based regimens are another option for patients who relapse after an alkylator-based therapy. They should be used with caution in heavily pretreated or elderly patients, however, due to immunosuppression. Radioimmunotherapy is also an option for patients with nonbulky, indolent B-cell NHL if the bone marrow is minimally involved. With 90Y ibritumomab tiuxetan, response rates are ∼70% and response duration is, on average, 11-15 months. Single-agent rituximab can be used in relapsed FL, but more patients are becoming rituximab refractory after receiving the drug with primary therapy and as maintenance. For patients who are still rituximab sensitive, it is an attractive option for elderly patients who will not tolerate cytotoxic agents as well. For patients who are rituximab refractory, the combination of bendamustine and obinutuzumab (type II anti-CD20 monoclonal antibody) was shown to be superior to bendamustine alone, approximately doubling the PFS in a randomized clinical trial.67 Obinutuzumab is under investigation in the frontline setting. The GALLIUM study (clinicaltrials.gov identifier NCT01332968) is an international randomized phase 3 study comparing obinutuzumab plus chemotherapy (with obinutuzumab maintenance) to rituximab plus chemotherapy (with rituximab maintenance). GALLIUM is fully enrolled and results are pending.
A new option for relapsed FL is the phosphatidylinositol 3-kinase δ (PI3Kδ) inhibitor idelalisib. Idelalisib targets the δ isoform of PI3K, an enzyme downstream from the BCR, which eventually signals through AKT and mammalian target of rapamycin. In a phase 2 study of 125 patients with indolent NHL who were refractory to both rituximab and an alkylating agent, idelalisib was administered at a dose of 150 mg twice daily until PD or patient withdrawal.68 The response rate was 57% with a median duration of 12.5 months. Response rates and duration in the FL subset were similar to the overall population. Grade 3 or higher toxicities included neutropenia (27%), transaminase elevations (13%), diarrhea (13%), and pneumonia (7%). Based upon this data, idelalisib received accelerated approval by the FDA in 2014.
A variety of novel agents are in development in FL, including other PI3K inhibitors, immunomodulatory agents, inhibitors of BTK, antibody-drug conjugates, novel anti-CD20 monoclonal antibodies, chimeric antigen receptor T-cell therapy, immune checkpoint inhibitors, inhibitors of nuclear export proteins, and others. An exhaustive review of agents in development is beyond the scope of this review, but examples of novel agents with outcome data in FL are summarized in Table 2.69-74
Stem cell transplantation
High-dose chemotherapy with autoSCT and alloSCT are both useful strategies in the management of FL, particularly for younger patients with high-risk features, such as a brief remission to previous therapy. A review of 904 patients in the International Bone Marrow Transplant Registry who underwent autologous or allogeneic transplantation for FL revealed that durable remissions could be induced with either technique.75 A lower 5-year recurrence rate with allogeneic transplantations was offset by a higher treatment-related mortality compared with autologous transplantation, leading to similar 5-year survival rates of 51% to 62%. To reduce the treatment-related mortality of alloSCT, most centers now favor a nonmyeloablative strategy in FL. Results utilizing a nonmyeloablative alloSCT strategy vary widely in the literature. For example, a series of 62 patients treated at the Fred Hutchinson Cancer Center demonstrated a 3-year OS and PFS of 67% and 54%, respectively.76 Alternatively, a highly selected group (n = 47) treated at the MD Anderson Cancer Center achieved an 11-year OS and PFS of 78% and 72%, respectively.77
There is 1 small, randomized clinical trial (the Chemotherapy vs Unpurged stem cell transplant vs Purged stem cell transplant [CUP] trial) examining autoSCT vs standard therapy in patients with relapsed FL.78 The study, conducted in the prerituximab era, found improved PFS and a trend toward improved OS with autoSCT. An interesting long-term analysis of patients receiving myeloablative chemotherapy followed by autoSCT comes from investigators at St. Bartholomew’s Hospital (London, United Kingdom) and the Dana-Farber Cancer Institute (Boston, MA).79 A cohort of 121 patients, with a median follow-up of 13.5 years, was noted to have a plateau in the remission duration curve beginning around year 8. Nearly half of the patients were still in remission at 10 to 15 years, suggesting some patients may be cured. Results were substantially better for patients treated in second remission as opposed to later in the disease course, suggesting the optimal window to consider autoSCT in FL is second or third remission. Later application appears to be associated with diminishing returns, and patients with multiply-relapsed FL are more appropriately considered for alloSCT or novel agents. Trials evaluating autoSCT in first remission do not support its use in that setting.80,81
Summary
Despite “incurability,” outcomes are good in FL with median OS exceeding 12 years. As a result, it is sometimes deprioritized as a cancer in need of therapeutic advances. Certainly there are FL patients who never require therapy or who only require 1 line of therapy and can be considered functional cures. On the other hand, subsets of FL patients are at high risk of death from their disease. These include patients with high-risk m7-FLIPI scores, patients who experience recurrence within 2 years of R-chemo, patients who experience histologic transformation, and FL patients under the age of 60 years at diagnosis. Future research should: (1) seek to identify prognostic biomarkers capable of identifying high-risk patients at diagnosis; (2) continue to develop targeted therapies (with predictive biomarkers); and (3) test interventions designed to reduce the risk for histologic transformation. Achievement of these goals would facilitate a more personalized approach to the management of FL.
Acknowledgments
The authors thank Eunhye Oak for outstanding editorial assistance.
Authorship
Contribution: B.S.K. and D.T.Y. wrote the manuscript.
Conflict-of-interest disclosure: B.K. provided consulting services to Roche, Teva, Gilead, Celgene, and Pharmacyclics. D.T.Y. declares no competing financial interests.
Correspondence: Brad S. Kahl, Division of Oncology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8056, St. Louis, MO 63110; e-mail: bkahl@dom.wustl.edu.