Abstract
Splenic marginal zone lymphoma (SMZL) is a rare B-cell malignancy involving the spleen, bone marrow, and frequently the blood. SMZL lymphomagenesis involves antigen and/or superantigen stimulation and molecular deregulation of genes (NOTCH2 and KLF2) involved in the physiological differentiation of spleen marginal zone B cells. Diagnosis requires either spleen histology or, alternatively, the documentation of a typical cell morphology and immunophenotype on blood cells coupled with the detection of intrasinusoidal infiltration by CD20+ cells in the bone marrow. Among B-cell tumors, deletion of 7q and NOTCH2 mutations are almost specific lesions of SMZL, thus representing promising diagnostic biomarkers of this lymphoma. Although the majority of SMZLs show an indolent course with a median survival of approximately 10 years, nearly 30% of patients experience a poor outcome. No randomized trials are reported for SMZL, and few prospective trials are available. A watch-and-wait approach is advisable for asymptomatic patients. Treatment options for symptomatic patients ranges from splenectomy to rituximab alone or combined with chemotherapy. In some geographic areas, a subset of patients with SMZL associates with hepatitis C virus infection, prompting virus eradication as an effective lymphoma treatment. It would be worthwhile to explore deregulated cellular programs of SMZL as therapeutic targets in the future; improved clinical and biological prognostication will be essential for identifying patients who may benefit from novel approaches.
Introduction
Splenic marginal zone lymphoma (SMZL) is a rare indolent B-cell neoplasm involving spleen, bone marrow (BM), and frequently the blood. In some patients, SMZL is occasionaly diagnosed after the detection of peripheral lymphocytosis; in advanced-stage SMZL, symptomatic splenomegaly and cytopenia can be the presenting features. Approximately 20% of patients show an autoimmune manifestation,1 including autoimmune hemolytic anemia, immune thrombocytopenia, cold agglutinin disease, circulating anticoagulants, acquired von Willebrand disease, or angioedema as a result of acquired C1-esterase inhibitor deficiency.2,3
Diagnosing SMZL and distinguishing it from similar indolent B-cell lymphoproliferative disorders can be challenging, especially if it relies on BM morphology and phenotype without the support of spleen histology. Although the majority of patients show an indolent course with a median survival of approximately 8 to 10 years,4,5 the prognosis of SMZL is heterogeneous. Indeed, ∼30% of patients have worse outcome,4 including 5% to 10% of patients undergoing transformation to diffuse large B-cell lymphoma.6-8 Therefore, prognostic scores and biomarkers are needed to sort out the fraction of SMZL patients who will undergo an aggressive clinical course. Within the last few years, molecular genetics has improved our knowledge of SMZL lymphomagenesis, allowed the identification of molecular lesions that are currently translated into diagnostic and prognostic markers, and pointed to deregulated cellular programs worth exploring as therapeutic targets.
The rarity and indolent course of SMZL have limited the development of specific treatment options for this lymphoma. Consequently, no randomized trials are available for SMZL, and few prospective trials are completed or ongoing. Evidence supporting the therapeutic options in SMZL is mostly based on retrospective series or translated from experience in other indolent B-cell lymphomas, and tailoring of treatment is limited by the lack of predictive factors. A dedicated approach that uses antiviral agents should be reserved for SMZL associated with hepatitis C virus (HCV) infection.9
Epidemiology
SMZL generally accounts for <2% of all lymphoid malignancies.10,11 Of the 116 411 cases of non-Hodgkin lymphoma (NHL) in the Surveillance, Epidemiology, and End Results registries, 763 (0.6%) are SMZL. Median age at diagnosis is 69 years. The overall age-adjusted incidence of SMZL is 0.13 per 100 000 persons per year, and the percent change in age-adjusted incidence is 4.81%, with increasing trends among patients who are white, male, or age ≥70 years.12 The International Lymphoma Epidemiology Consortium NHL Subtypes Project, which pooled individual-level data from 20 case-control studies (17 471 NHL cases, 23 096 controls)13 points to an association between SMZL and B-cell activating autoimmune conditions, asthma, and use of hair dye.14
Diagnosis
Cytology
Blood involvement is common, and the typical cell morphology describes lymphocytes with round nuclei, condensed chromatin, and basophilic cytoplasm with polar short villi (so-called “villous lymphocytes”). Heterogeneity in blood morphology is common, ranging from small lymphoid cells without specific features, to various degrees of monocytoid and plasmacytoid differentiation. Large cells, although rare, may suggest disease transformation into a large-cell lymphoma.15
Histology
The first description of SMZL by Schmid et al16 relied on the recognition of a histologic pattern recapitulating the marginal zone (MZ), as observed in the splenic white pulp. The spleen usually has a gross weight of more than 400 g (and may exceed 2000 g), and the cut surface shows a typical multi-micronodular pattern.
SMZL develops in the white pulp with a biphasic picture. Medium-size monocytoid B cells are organized into a pale ring around the follicle with a MZ pattern, whereas small centrocyte-like cells efface the mantle zone and colonize the germinal centers (Figure 1). A variable degree of plasmacytic differentiation may be present. Lymphoma cells may involve the red pulp in patchy or diffuse fashion, with subsequent spread to the sinuses. Infiltration of the walls of the greater vessels is frequent. Large cells, mostly with immunoblastic cytology, are rare and their increase may suggest transformation into a more aggressive lymphoma. Epithelioid histiocytes may be observed, sometimes being enough to obscure the neoplastic infiltrate. A predominant diffuse pattern mandates the exclusion of other lymphoma subtypes within the splenic B-cell lymphoma/leukemia unclassifiable provisional subgroup, including splenic diffuse red pulp small B-cell lymphoma and hairy cell leukemia variant (HCL-v). Hilar lymph nodes are frequently involved, displaying a nodular proliferation with obliteration of the reactive germinal centers and engulfment of the sinuses.
Histopathology of SMZL. In this typical case, (A) a nodular lymphoid proliferation with a biphasic appearance effaces the white pulp, infiltrates the wall of a great vessel (arrow) (hematoxylin and eosin [H&E] stain; magnification ×20) and extends to (B) the red pulp in a patchy distribution (CD79a; magnification ×100). (C) Morphologic pictures show medium sized, monocytoid lymphocytes with only scattered large cells (H&E stain; magnification ×400) and (D) a variable degree of plasmacytic differentiation (Giemsa stain; magnification ×400). (E) Anti-CD23 immunostain (magnification ×100) depicts the CD23+ marginal zone cells as well as the residual dendritic meshwork within the colonized follicles highlighted by (F) Mib/Ki-67 (magnification ×100), which confers a targetoid appearance. (G) A prototypical BM biopsy shows a small to medium size lymphoid population (Giemsa stain; magnification ×400) with (H) a nodular and sinusoidal distribution (CD20; magnification ×400). (G) Note the megaloblastoid features within the erythroblastic lineage, a common finding in cases associated with paraproteinemia and anemia.
Histopathology of SMZL. In this typical case, (A) a nodular lymphoid proliferation with a biphasic appearance effaces the white pulp, infiltrates the wall of a great vessel (arrow) (hematoxylin and eosin [H&E] stain; magnification ×20) and extends to (B) the red pulp in a patchy distribution (CD79a; magnification ×100). (C) Morphologic pictures show medium sized, monocytoid lymphocytes with only scattered large cells (H&E stain; magnification ×400) and (D) a variable degree of plasmacytic differentiation (Giemsa stain; magnification ×400). (E) Anti-CD23 immunostain (magnification ×100) depicts the CD23+ marginal zone cells as well as the residual dendritic meshwork within the colonized follicles highlighted by (F) Mib/Ki-67 (magnification ×100), which confers a targetoid appearance. (G) A prototypical BM biopsy shows a small to medium size lymphoid population (Giemsa stain; magnification ×400) with (H) a nodular and sinusoidal distribution (CD20; magnification ×400). (G) Note the megaloblastoid features within the erythroblastic lineage, a common finding in cases associated with paraproteinemia and anemia.
In BM trephine biopsy, a rather characteristic sinusoidal pattern of infiltration is often detectable, usually combined with an interstitial and nodular component.17 However pathologists must be aware that this pattern may also be observed, although less frequently, in several low-grade B-cell lymphomas. A careful evaluation of cytology and immunophenotype, including a search for the dendritic meshwork more commonly present and disrupted in SMZL,18 is helpful in many instances.
Immunophenotype
SMZL does not harbor a specific immunophenotype; thus, flow cytometry and immunohistochemistry antibody panels should be tailored to exclude other subtypes (Tables 1 and 2).11 The Matutes flow cytometry score is low in SMZL, ranging from 0 to 2, whereas diagnosis of CLL requires a score greater than 3.19,20 By immunohistoichemistry, SMZL consistently expresses CD20, CD79a, BCL2, and surface immunoglobulin M (IgM), variably shows surface IgD and DBA44, and is typically negative for CD5, CD10, BCL6, cyclin D1/BCL1, CD43, annexin A1, LEF1, CD103, and CD123. Monotypic expression of Ig light chains may represent a diagnostic clue. CD5+ cases have been described and should be carefully distinguished from MCL and CLL.21 CD23 and CD21 may be positive in the tumor cells and are useful for delineating the residual follicular dendritic meshwork. Proliferation index (Mib-1/Ki-67) is low (usually <5%) and depicts a distinctive targetoid picture (Figure 1). IRTA1 positivity, reported on the neoplastic cells in cases of extranodal MZL (EMZL), is barely present in SMZL.22
Flow cytometry features of SMZL and other leukemic B-cell lymphoproliferative disorders
| . | SMZL . | CLL . | MCL . | HCL . | HCL-v . |
|---|---|---|---|---|---|
| sIg | Strong | Weak | Strong | Strong | Strong |
| CD5 | + | +++ | +++ | − | − |
| CD23 | + | +++ | − | − | − |
| FMC7 | +++ | + | +++ | +++ | +++ |
| CD11c | ++ | − | − | +++ | +++ |
| CD103 | − | − | − | +++ | ++ |
| CD123 | − | − | − | +++ | − |
| CD25 | + | − | − | +++ | − |
| CD27 | ++ | +++ | +++ | − | ++ |
| CD200 | − | +++ | − | +++ | − |
| . | SMZL . | CLL . | MCL . | HCL . | HCL-v . |
|---|---|---|---|---|---|
| sIg | Strong | Weak | Strong | Strong | Strong |
| CD5 | + | +++ | +++ | − | − |
| CD23 | + | +++ | − | − | − |
| FMC7 | +++ | + | +++ | +++ | +++ |
| CD11c | ++ | − | − | +++ | +++ |
| CD103 | − | − | − | +++ | ++ |
| CD123 | − | − | − | +++ | − |
| CD25 | + | − | − | +++ | − |
| CD27 | ++ | +++ | +++ | − | ++ |
| CD200 | − | +++ | − | +++ | − |
−, <10% of cases positive; +, 11%-35% positive cases; ++, 36%-75% positive cases; +++, >75% positive cases.
CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma; sIg, superficial immunoglobulin expression.
Immunohistochemistry features of SMZL and other small B-cell lymphomas
| . | SMZL . | LPL . | SDRPL . | HCL-v . | HCL . | EMZL/ NMZL . | CLL . | MCL . | FL . |
|---|---|---|---|---|---|---|---|---|---|
| CD20 | + | + | + | + | + | + | −/+ | + | + |
| CD79a | + | + | + | + | + | + | + | + | + |
| CD5 | −/+ | −/+ | −/+ | – | – | −/+ | + | + | – |
| CD21 | −/+ | – | – | – | – | – | – | – | – |
| CD23 | −/+ | −/+ | – | – | – | −/+ | + | – | −/+ |
| BCL1 | – | – | – | −/+ | + | – | – | + | – |
| DBA44 | +/− | – | + | + | + | – | −/+ | – | – |
| Annexin A1 | – | – | – | – | + | – | – | – | – |
| CD103 | – | – | – | +/− | + | – | – | – | – |
| CD123 | – | – | – | – | + | – | – | – | – |
| IRTA1 | – | – | – | – | – | +/− | – | – | – |
| IgM | + | + | + | + | + | + | + | + | + |
| IgD | +/− | – | −/+ | + | + | −/+ | + | + | + |
| CD10 | – | –* | – | – | – | – | – | –* | +/− |
| BCL6 | – | – | – | – | – | −/+ | – | −/+ | + |
| CD43 | −/+ | – | – | – | – | −/+ | + | + | – |
| SOX11 | – | – | – | – | – | – | – | + | – |
| LEF1 | – | – | – | – | – | – | + | −/+ | – |
| . | SMZL . | LPL . | SDRPL . | HCL-v . | HCL . | EMZL/ NMZL . | CLL . | MCL . | FL . |
|---|---|---|---|---|---|---|---|---|---|
| CD20 | + | + | + | + | + | + | −/+ | + | + |
| CD79a | + | + | + | + | + | + | + | + | + |
| CD5 | −/+ | −/+ | −/+ | – | – | −/+ | + | + | – |
| CD21 | −/+ | – | – | – | – | – | – | – | – |
| CD23 | −/+ | −/+ | – | – | – | −/+ | + | – | −/+ |
| BCL1 | – | – | – | −/+ | + | – | – | + | – |
| DBA44 | +/− | – | + | + | + | – | −/+ | – | – |
| Annexin A1 | – | – | – | – | + | – | – | – | – |
| CD103 | – | – | – | +/− | + | – | – | – | – |
| CD123 | – | – | – | – | + | – | – | – | – |
| IRTA1 | – | – | – | – | – | +/− | – | – | – |
| IgM | + | + | + | + | + | + | + | + | + |
| IgD | +/− | – | −/+ | + | + | −/+ | + | + | + |
| CD10 | – | –* | – | – | – | – | – | –* | +/− |
| BCL6 | – | – | – | – | – | −/+ | – | −/+ | + |
| CD43 | −/+ | – | – | – | – | −/+ | + | + | – |
| SOX11 | – | – | – | – | – | – | – | + | – |
| LEF1 | – | – | – | – | – | – | + | −/+ | – |
–, <25% of cases; –/+, 25%-50% of cases; +/–, 50%-75% of cases; +, >75% of cases.
FL, follicular lymphoma; NMZL, nodal marginal zone lymphoma; SDRPL, splenic diffuse red pulp lymphoma.
Sporadic cases reported.
Differential diagnosis
A reactive follicular hyperplasia must always be considered; this pattern is frequent or even the rule in children, adolescents, and young adults. A diagnosis of SMZL should not be proposed if the spleen weighs less than 300 to 400 g or in the absence of a monotypic cell population.
In most cases, architectural and cytologic features along with adequate immunophenotype allow differentiation of SMZL from other small B-cell lymphomas with micronodular patterns, particularly CLL, MCL, and follicular lymphoma, which may occasionally mimic an MZ pattern. In rare CD5+ cases, morphology, cyclin D1/BCL1, and SOX11 negativity and the absence of t(11;14) rule out MCL.11
Among subtypes with privileged splenic involvement, HCL is distinguished for its characteristic morphology and phenotype. Differentiating splenic diffuse red pulp lymphoma 23 and HCL-v24 may be very difficult or even impossible with only blood or BM biopsy,17 because they represent two recognized entities with ill-defined clinicopathologic and immunophenotypic features that partially overlap those of SMZL (Tables 1 and 2). Thus, a definite diagnosis may require detailed clinical information, a comprehensive phenotype, and spleen histology, which usually shows a typical diffuse pattern of infiltration with preserved or atresic white pulp follicles.23 In cases of splenic B-cell lymphomas that do not fulfill the World Health Organization 2008 criteria for better established or provisional entities, a diagnosis of splenic B-cell lymphoma/leukemia unclassifiable should be preferred.11
Differentiating SMZL from lymphoplasmacytic lymphoma (LPL) may be challenging, particularly on BM biopsy, because SMZL may show a monoclonal serum component and plasmacytic morphology, and both entities lack a distinct phenotype. LPL, which develops primarily in the spleen, homogeneously infiltrates the white pulp without MZ pattern and without monocytoid B cells. MYD88 L265P mutation, present in almost all cases of LPL and rare in SMZL, may be a useful diagnostic tool.25 A further diagnostic pitfall may be represented by detection of a BM clonal infiltrate in cases of non-CLL monoclonal B lymphocytosis.26
Finally, secondary splenic localization of EMZL presents a pattern that overlaps with that of SMZL, but clinical dissemination is crucial for differentiation. Splenic involvement virtually excludes a diagnosis of nodal MZL; apart from the differential expression of IRTA1, which is negative in SMZL,11,22 clinical correlation is critical for reaching a correct diagnosis when dealing with a BM biopsy.
Molecular pathogenesis of SMZL
Cell of origin and immunogenetics
The cellular origin of SMZL is still debated, and its identification is essential to correctly classify this lymphoma and to elucidate its pathobiology. According to the World Health Organization classification, the postulated normal counterpart of SMZL is a B cell of unknown differentiation stage.11 According to studies of Ig gene rearrangements, a derivation from antigen-experienced B cells has been postulated in the vast majority of SMZL.27-29 Skewing of the Ig gene repertoire toward the use of the IGHV1-2*04 allele in SMZL suggests that they could derive from a progenitor population adapted in the spleen to particular antigenic challenges, although definitive answers on the issue of the cell of origin of SMZL will admittedly be provided only through multidisciplinary examination of the immune repertoire and transcriptome of normal B-cell populations of the spleen compartments.
The contribution of antigen stimulation to SMZL pathogenesis is suggested by the highly restricted Ig gene repertoire, including stereotyped configuration of the B-cell receptor (BCR) in ∼10% of cases30 and selective usage of the Ig heavy chain variable IGHV1-2*04 allele in ∼30%.31 Although the epitope recognized by IGHV1-2*04-expressing BCR is unknown, the features of IGHV1-2*04 rearrangements, including minimal somatic mutations and the long complementarity-determining region 3 sequence with common motifs, suggest a possible selection of T-cell-independent MZ B cells by superantigens and thus a role of antigenic drive in lymphomagenesis.
Cytogenetic and genetic lesions
SMZL lacks recurrent chromosomal translocations, including translocations that are typical of other lymphoma types such as the t(14;18) translocation affecting BCL2 in follicular lymphoma, the t(11;14) translocation affecting CCND1 in MCL, and the t(11;18), t(14;18), and t(1;14) translocations affecting the BIRC3/MALT1, MALT1, and BCL10 genes, respectively, in EMZL. The lack of these abnormalities may help distinguish SMZL from pathologically mimicking tumors. Approximately 30% of SMZL show hemizygous 7q deletion, which is also frequently seen in splenic B-cell lymphoma/leukemia unclassifiable, but rarely in other lymphoma subtypes.32,33 The gene(s) targeted by the 7q deletion remain obscure despite the combined investigation of genomic and transcriptomic profiles and mutation analysis of a number of candidate genes.33-36
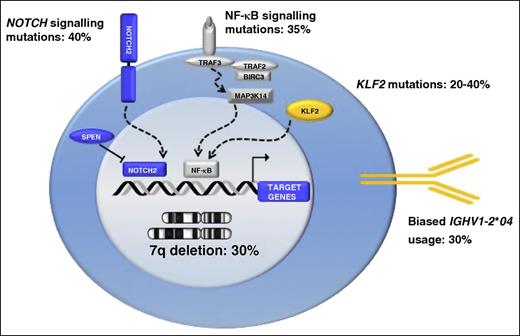
Unbiased genomic studies have unraveled the typical coding genome of SMZL.37-43 However, because of the limited number of SMZL genomes and/or exomes available so far, the full spectrum of lesions that contribute to the malignant transformation of SMZL remains unknown. The notion that the most frequently mutated genes in SMZL (ie, nuclear factor κB [NF-κB] signaling genes, NOTCH2 and other NOTCH pathway genes, and KLF2) are physiologically involved in proliferation and commitment of mature B cells to the MZ points to homing to the spleen compartment and MZ differentiation as the major programs deregulated in this lymphoma. SMZL has an expression signature consistently characterized by the upregulation of genes belonging to the MZ differentiation program, including NF-κB and NOTCH pathway genes.
Active NF-κB signaling is necessary for the generation and/or maintenance of normal MZ B cells. Overall, mutations of positive and negative NF-κB regulators accounted for ∼35% of SMZL cases, which implicates activation of NF-κB as a major contributor to the pathogenesis of this disease. The canonical NF-κB signaling is molecularly deregulated by a variety of mechanisms in 15% of SMZL. IKBKB, the central activating kinase of canonical NF-κB signaling, is constitutively activated by mutations in ∼10% of SMZL, whereas TNFAIP3, the master negative regulator of NF-κB, is inactivated by mutations and/or deletions in ∼5% of cases. The TRAF3/MAP3K14-TRAF2/BIRC3-negative regulatory complex of noncanonical NF-κB signaling is disrupted by mutations in ∼15% of SMZL, allowing the cytoplasmic release, stabilization, and constitutive activation of MAP3K14 (also known as NIK), the central activating kinase of noncanonical NF-κB signaling.44
The NOTCH receptor genes encode a family of heterodimeric transmembrane proteins that function as ligand-activated transcription factors. Upon activation, the cleaved intracellular portion of the NOTCH receptors translocates into the nucleus and recruits the MAML1 and MAML2 transcriptional cofactors to modify the expression of a number of target genes. The most prominent mechanism of NOTCH signal suppression is operated through its PEST domain, which terminates signaling by directing the active intracellular portion of NOTCH toward proteasomal degradation. Other negative regulators of NOTCH signaling include SPEN and DTX1. SPEN represses NOTCH signaling by competing with the active intracellular NOTCH for binding to RBPJ. DTX1 represses NOTCH signaling by binding the NOTCH family proteins and inhibiting their recruitment of transcription coactivators. Genes of the NOTCH pathway are mutated in ∼40% of SMZLs. NOTCH2 shows recurrent mutations in ∼10% to 25% of SMZLs, establishing NOTCH2 as one of the most frequently mutated genes in this lymphoma. NOTCH1, a paralog of NOTCH2, is also mutated in an additional ∼5% of SMZL.37-43 NOTCH2 and NOTCH1 mutations in SMZL are selected to truncate the PEST domain of the protein, thus causing impaired degradation of the NOTCH2 and NOTCH1 proteins and, as a consequence, sustained NOTCH signaling. In addition to NOTCH2 and NOTCH1, other genes involved in NOTCH signaling (SPEN, DTX1, and MAML2) are affected by genomic lesions in SMZL, although at lower frequency.
In additions to the pathogenic implications, NOTCH2 mutations may help inform SMZL diagnosis and prognosis. From a diagnostic standpoint, NOTCH2 mutations are highly specific for SMZL among mature B-cell tumors, including conditions that look like SMZL, thus representing a biomarker with positive predictive value for SMZL specification.37,38 From a prognostic standpoint, SMZL cases with NOTCH2 mutations have an inferior outcome.38
KLF2 is a member of the KLF family of zinc-finger transcription factors that in normal lymphocytes physically bind the promoter and regulate the expression of genes involved in cell cycle/apoptosis, cell trafficking, and NF-κB signaling. KLF2 is somatically mutated in 20% to 40% of SMZL, thus representing one of the most frequently altered genes in this lymphoma along with NOTCH2.40,42,43 Most KLF2 mutations disrupt its nuclear localization signal, which causes the displacement of KLF2 from the nucleus to the cytoplasm and/or affected codons required for the interaction between KLF2 and DNA. Deregulation of the transcriptional program orchestrated by mutant KLF2 consistently leads to NF-κB activation. Indeed, mutations prevent the physiological ability of KLF2 to suppress NF-κB induction by upstream signaling pathways, including the BCR and Toll-like receptor pathways.42 KLF4, a tumor suppressor that is a highly homologous paralog of KLF2, is also frequently inactivated by aberrant promoter methylation in SMZL, suggesting a more general involvement of the Krüppel-like transcription factors in this lymphoma.45 Although KLF2 mutations are not diagnostically useful because they also occur in other indolent B-cell tumors, they are of prognostic relevance in SMZL because they mark cases with an inferior outcome.40,42,43
As is the case for most cancer-associated genetic lesions, NOTCH2 upregulation or inactivation of KLF2 may not be sufficient, as single events, for malignant transformation. In fact, transgenic mice engineered to overexpress NOTCH2 or that lack KLF2 in mature B cells display an expansion of the MZ at the expense of the follicular compartment but do not develop lymphoma.46,47 It is important to note, however, that lymphoma development may require longer times than those observed so far in mice, in line with the indolent course of SMZL and the age of elderly patients affected by this lymphoma. Consistent with a multistep process of lymphomagenesis, IGHV1-2*04 usage, NOTCH2 mutations, KLF2 mutations, KLF4 aberrant methylation, and 7q deletion co-occur in SMZL, thus identifying a disease subset with a distinct genotype characterized by multigenetic/epigenetic changes and suggesting a possible cooperation between genetic/epigenetic abnormalities and BCR configuration in promoting transformation. Key molecular alterations of SMZL are summarized in Figure 2.
Key molecular alterations in SMZL. Schematic representation of genes and pathways that are molecularly deregulated in SMZL. The prevalence of molecular alterations in SMZL is shown as a percentage beside each gene or pathway.
Key molecular alterations in SMZL. Schematic representation of genes and pathways that are molecularly deregulated in SMZL. The prevalence of molecular alterations in SMZL is shown as a percentage beside each gene or pathway.
HCV-associated SMZL
Several epidemiologic studies have investigated the association of HCV with NHL.48 In subtype-specific analyses, HCV is frequently associated with MZL and diffuse large B-cell lymphoma.49 According to other models of lymphomagenesis (eg, Helicobacter pylori in gastric EMZL,50 Borrelia burgdorferi for EMZL of the skin,51 Chlamydophila psittaci for EMZL of the ocular adnexa52 ), chronic stimulation by HCV may also be a factor in the development of a subgroup of SMZL patients. However, the role of HCV in SMZL can reflect geographic difference considering the relatively high seroprevalence in some series4,53 and the rarity of HCV-positive patients in others.1
A clinical triad of SMZL, mixed cryoglobulinemia, and HCV infection has been proposed as a model of infection-driven lymphomagenesis.54 Saadoun et al55 reported SMZL associated with type II cryoglobulinemia and HCV infection: all 18 patients had type II mixed cryoglobulinemia (symptomatic in 13). Accordingly, in a large series from Italy,4 HCV serology was positive in 19% of the patients with more frequent presence of nodal disease, cryoglobulinemia, and serum monoclonal component.
Along with overlapping histologic features between HCV-positive and HCV-negative patients,56 comparative study of genome hybridization array did not find any difference in DNA copy number changes according to HCV status.57 Conversely, supervised analysis of microRNA (miRNA) expression revealed differentially expressed miRNAs and also revealed that miR-26b, an miRNA with tumor suppressive activity, was downregulated in HCV-positive SMZL.58
Prognostic factors
Several prognostic factors have been proposed for SMZL, including leukocytosis,1,59 lymphocytosis,1,60 lymphopenia,59 anemia,32,60 thrombocytopenia,60 use of chemotherapy,59 monoclonal component,1 β2-microglobulin,1 performance status of 2 or greater,61 incomplete response,61 nonhematopoietic site involvement,61 advanced age,7,32,60,62 diffuse pattern of bone marrow infiltration,60 and histologic transformation.7
The first system of scoring for the assessment of SMZL prognosis was proposed by the Italian Lymphoma Intergroup (now Fondazione Italiana Linfomi [FIL]).4 In this series of 309 SMZL patients, the 5-year cause-specific survival rate was 76%. By using 3 laboratory variables (hemoglobin level less than 12 g/dL, elevated serum lactate dehydrogenase level, and albumin level less than 3.5 g/dL), SMZL patients were grouped into 3 prognostic categories: low risk (41%) with no adverse factors, intermediate risk (34%) with 1 adverse factor, and high risk (25%) with 2 or 3 adverse factors. The 5-year cause-specific survival rate was 88% for the low-risk group, 73% for the intermediate-risk group, and 50% for the high-risk group. This latter group accounted for 54% of all lymphoma-related deaths.
Subsequently, an international study of 593 SMZL patients63 identified hemoglobin, platelet count, high lactate dehydrogenase level, and extrahilar lymphadenopathy as parameters independently associated with lymphoma-specific survival (LSS). Three risk groups were identified with significantly different 5-year LSS (94%, 78%, and 69%, respectively). In a subsequent study that aimed at optimizing prognostication,64 clinically acceptable cut points were established: 9.5 g/dL for hemoglobin, and 80 × 109/L for platelet count. The patients were allocated into 3 groups: low risk (36%) with 0 points, intermediate risk (56%) with 1 or 2 factors, and high risk (8%) with 3 or 4 factors. The 3 groups had a 5-year LSS of 95%, 87%, and 68%, respectively. These scores have been validated in an independent series of SMZL patients.65
The clinical scores are neither 100% sensitive nor 100% specific in identifying high-risk patients. Molecular aspects of SMZL (ie, Ig gene mutation status, NOTCH2 and KLF2 mutations, TP53 abnormalities, and aberrant promoter methylation) represent promising prognostic biomarkers associated with inferior outcome,32,38,43,45 and their incorporation into the currently available clinical prognostic models might improve risk stratification of patients.
Treatment
Consensus guidelines recommend treating SMZL only in the presence of symptomatic splenomegaly, cytopenias, systemic symptoms, or progressive nodal disease.15,66,67 Autoimmune cytopenias should be specifically treated.15,66
No randomized trials have been conducted in SMZL and, as consequence, there is no consensus on how to treat newly diagnosed and relapsed patients. The therapeutic options for SMZL have a wide range and include splenectomy,1,7,59,61,68-73 chemotherapy,74-81 and rituximab alone70,82,83 or rituximab with chemotherapy.70,84-87 In addition, antiviral treatment should be considered in patients with SMZL and concurrent chronic infection with HCV-related hepatitis who do not need immediate conventional treatment against the lymphoma.9,55
Staging and response criteria
According to Lugano classification,88 SMZL is not fluorodeoxyglucose-avid disease and must be staged by means of computed tomography. However, positron emission tomography can be considered if a transformation is suspected.66
In addition to Lugano principles,88 specific criteria for response assessment have been proposed15 that consider the particular clinical presentation of SMZL. In particular, a complete response (CR) is achieved when splenomegaly has been resolve, blood cell counts are normalized, flow cytometry on blood is negative, and BM histology is negative by immunohistochemistry.
Splenectomy
As a therapeutic approach, surgical removal of large spleens may eliminate a significant amount of disease by ameliorating abdominal discomfort and resolving cytopenias that result from splenic sequestration.7 After surgery, patients can remain free from treatment for many years.1 Because cytopenias resulting from marrow failure do not resolve after splenectomy, a BM biopsy is advisable during the workup to define the burden of BM infiltration by the disease. One additional advantage of splenectomy is that it allows a definitive diagnosis of SMZL. Drawbacks of splenectomy are short-term (perioperative events) and long-term (immune suppression and infections) complications.
Perioperative complications were registered in one quarter of a recent series of 41 splenectomized patients89 : pulmonary dysfunction in 8 (19.5%), deep vein thrombosis in 1 (2.4%), portal vein thrombosis in 1 (2.4%), and major bleeding in 9 (21.9%).
Infections caused by encapsulated bacteria are the major risk associated with splenectomy,90 and vaccination against capsulated bacteria is mandatory at least 2 weeks before elective splenectomy.91 The risk of infections after splenectomy is low in lymphomas but is still present after many years and is potentially fatal.92 In 2 recent series from France7 and British Columbia,62 about 5% of splenectomized patients died of infectious complications.
Physicians are sometimes reluctant to choose splenectomy in patients with comorbidities and/or advanced age; in these situations, a laparoscopic approach72,93 can extend the indication to splenectomy in case of massive splenomegaly through the hand-assisted approach.94
Splenectomy should be contraindicated in cases with disseminated lymphoma with nodal involvement outside the splenic hilum. Conversely, a strict indication for splenectomy is present in cases with suspected transformation (eg, nodular lesion with augmented fluorodeoxyglucose uptake). Results of SMZL series of splenectomized patients are summarized in Table 3.
Series of SMZL patients treated with splenectomy
| Reference . | Year . | N . | Response . | OS . | Deaths due to surgery . | |
|---|---|---|---|---|---|---|
| ORR (%) . | Duration . | |||||
| Mulligan et al68 | 1991 | 20 | 95 | Median DOR 4 y | NR | 1 |
| Troussard et al59 | 1996 | 28 | 75 | NR | 71% at 5 y | 1 |
| Chacon et al61 | 2002 | 60∗ | 93.3 | Median FFS 40 mo | 65% at 5 y | NR |
| Thieblemont et al1 | 2002 | 48† | 100 | PFS 48% at 5 y | NR | NR |
| Parry-Jones et al60 | 2003 | 33 | NR | NR | LSS 95% at 10 y | NR |
| Iannitto et al69 | 2004 | 21 | 91 | Median DOR 4 y | NR | NR |
| Tsimberidou et al70 | 2006 | 10 | 60 | FFS 80% at 3 y | 89% at 3 y | 0 |
| Olszewski et al71 | 2012 | 652 | NR | NR | 67.8% at 5 y‡ | NR |
| Kalpadakis et al73 | 2013 | 27 | 85 | PFS 58% at 5 y | 77% at 5 y | 1 |
| Lenglet et al7 | 2014 | 100 | 97 | PFS 61% at 5 y | 84% at 5 y | 0 |
| Xing et al62 | 2015 | 52§ | NR | FFS 39% at 10 y | 61% at 10 y | 0 |
| Pata et al89 | 2015 | 41 | 90 | PFS 35% at 5 y | 75% at 5 y | 0 |
| Reference . | Year . | N . | Response . | OS . | Deaths due to surgery . | |
|---|---|---|---|---|---|---|
| ORR (%) . | Duration . | |||||
| Mulligan et al68 | 1991 | 20 | 95 | Median DOR 4 y | NR | 1 |
| Troussard et al59 | 1996 | 28 | 75 | NR | 71% at 5 y | 1 |
| Chacon et al61 | 2002 | 60∗ | 93.3 | Median FFS 40 mo | 65% at 5 y | NR |
| Thieblemont et al1 | 2002 | 48† | 100 | PFS 48% at 5 y | NR | NR |
| Parry-Jones et al60 | 2003 | 33 | NR | NR | LSS 95% at 10 y | NR |
| Iannitto et al69 | 2004 | 21 | 91 | Median DOR 4 y | NR | NR |
| Tsimberidou et al70 | 2006 | 10 | 60 | FFS 80% at 3 y | 89% at 3 y | 0 |
| Olszewski et al71 | 2012 | 652 | NR | NR | 67.8% at 5 y‡ | NR |
| Kalpadakis et al73 | 2013 | 27 | 85 | PFS 58% at 5 y | 77% at 5 y | 1 |
| Lenglet et al7 | 2014 | 100 | 97 | PFS 61% at 5 y | 84% at 5 y | 0 |
| Xing et al62 | 2015 | 52§ | NR | FFS 39% at 10 y | 61% at 10 y | 0 |
| Pata et al89 | 2015 | 41 | 90 | PFS 35% at 5 y | 75% at 5 y | 0 |
DOR, duration of response; FFS, failure-free survival; LSS, lymphoma-specific survival; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
Splenectomy alone in 29 patients.
Splenectomy alone in 25 patients.
Survival of entire series of 1251 patients with no impact of splenectomy on OS.
Splenectomy alone in 42 patients.
Rituximab-based treatment
The clinical scenario of systemic therapy in SMZL was changed with the introduction of the anti-CD20 monoclonal antibody rituximab, and rituximab-based treatment has become a valid alternative to splenectomy.95,96
Rituximab monotherapy yield results similar to those of splenectomy, avoid the toxicity of chemotherapy, and potentially eradicate the disease at the molecular level. Kalpadakis et al73 reported 58 patients treated with rituximab once per week for 6 weeks, followed by a maintenance phase once every 2 months for 1 to 2 years. At the end of the induction phase, the CR rate was 45%, unconfirmed CR 26%, and partial response 24%; the 5-year overall survival and progression-free survival (PFS) were 92% and 73%, respectively.
According to the European Society for Medical Oncology guidelines,66 rituximab monotherapy is a reasonable first-line therapy and a less traumatic alternative to splenectomy. According to the Italian Society of Hematology guidelines,67 rituximab monotherapy is an option for patients without disseminated disease who need treatment and for patients with contraindications to surgery.
The combination of rituximab with chemotherapy is considered standard therapy for symptomatic, indolent B-cell NHL.66 In SMZL, this approach is indicated for eligible patients with disseminated disease,66,67 constitutional symptoms, and/or signs of high-grade transformation.66
In the FIL trial,87 51 patients with SMZL were treated with rituximab with cyclophosphamide, vincristine, non-pegylated liposomal doxorubicin, and prednisone (R-COMP). The overall response rate was 84%, 6-year PFS was 54%, and overall survival was 72%. Toxicity was not negligible (grade >3 neutropenia, 26%; grade >3 infections, 8%; 2 deaths as a result of infection).
The combination of rituximab with bendamustine (BR) is effective in indolent NHL, although it has never been tested in a dedicated trial of SMZL.97,98 In the Bright study,97 the overall response rate to BR was 92% in 25 patients with MZL. In the StiL trial,98 the PFS for BR therapy was not longer than that for rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) among patients with MZL, but the study was not powered to find differences in the MZL subset. The BRISMA (International Extranodal Lymphoma Study Group 36; EudraCT Number 2011-000880-28) phase II trial using BR may soon provide specific information on the safety and activity of this regimen in SMZL. The results of rituximab-based treatment in patients with SMZL are summarized in Table 4.99,100
Series of SMZL patients treated with rituximab-based approach
| . | . | . | . | . | . | Response . | . | |
|---|---|---|---|---|---|---|---|---|
| Reference . | Year . | Study type . | Scheme . | Patient status . | N . | ORR . | Duration . | OS . |
| Rituximab monotherapy | ||||||||
| Bennett et al82,102 | 2005 | Retrospective | R monotherapy | RR | 11 | 91% | PFS 60% at 5 y | 70% at 5 y |
| Tsimberidou et al70 | 2006 | Retrospective | R monotherapy | First line | 25 | 88% | FFS 86% at 3 y | 95% at 3 y |
| Kalpadakis et al83,96 | 2007 | Retrospective | R monotherapy | First line | 16 | 100% | PFS 92% at 2.4 y | 100% at 2.1 y |
| Else et al103 | 2012 | Retrospective | R monotherapy | First line and RR | 10 | 100% | DFS 89% at 3 y | NR |
| Kalpadakis et al73 | 2013 | Retrospective | R monotherapy | First line | 58 | 95% | PFS 73% at 5 y | 92% at 5 y |
| Rituximab + chemotherapy | ||||||||
| Tsimberidou et al70 | 2006 | Retrospective | R-chemo | First line | 6 | 83% | FFS 100% at 3 y | 100% at 3 y |
| Cervetti et al85,86 | 2010 | Retrospective | R-2CDA | First line and RR | 47* | 87% | PFS 80% at 5 y | 86% at 5 y |
| Else et al103 | 2012 | Retrospective | R-chemo | First line and RR | 33 | 100% | DFS 71% at 3 y | NR |
| Iannitto et al87 | 2015 | Prospective | R-COMP | First line | 51 | 84% | PFS 54% at 6 y | 72% at 6 y |
| . | . | . | . | . | . | Response . | . | |
|---|---|---|---|---|---|---|---|---|
| Reference . | Year . | Study type . | Scheme . | Patient status . | N . | ORR . | Duration . | OS . |
| Rituximab monotherapy | ||||||||
| Bennett et al82,102 | 2005 | Retrospective | R monotherapy | RR | 11 | 91% | PFS 60% at 5 y | 70% at 5 y |
| Tsimberidou et al70 | 2006 | Retrospective | R monotherapy | First line | 25 | 88% | FFS 86% at 3 y | 95% at 3 y |
| Kalpadakis et al83,96 | 2007 | Retrospective | R monotherapy | First line | 16 | 100% | PFS 92% at 2.4 y | 100% at 2.1 y |
| Else et al103 | 2012 | Retrospective | R monotherapy | First line and RR | 10 | 100% | DFS 89% at 3 y | NR |
| Kalpadakis et al73 | 2013 | Retrospective | R monotherapy | First line | 58 | 95% | PFS 73% at 5 y | 92% at 5 y |
| Rituximab + chemotherapy | ||||||||
| Tsimberidou et al70 | 2006 | Retrospective | R-chemo | First line | 6 | 83% | FFS 100% at 3 y | 100% at 3 y |
| Cervetti et al85,86 | 2010 | Retrospective | R-2CDA | First line and RR | 47* | 87% | PFS 80% at 5 y | 86% at 5 y |
| Else et al103 | 2012 | Retrospective | R-chemo | First line and RR | 33 | 100% | DFS 71% at 3 y | NR |
| Iannitto et al87 | 2015 | Prospective | R-COMP | First line | 51 | 84% | PFS 54% at 6 y | 72% at 6 y |
2CDA, 2-chlorodeoxyadenosine; chemo, chemotherapy; DFS, disease-free survival; R, rituximab; RR, relapsed/refractory.
Rituximab in 32 patients.
Novel agents
No trial with novel agents was specifically dedicated to SMZL, although some data about activity in MZL of different histology can be derived from published studies conducted in indolent NHL (Table 5).101-104
Novel agents in MZLs
| Agent . | Year . | Phase . | Patient status . | No. of patients . | MZL . | Response . | |
|---|---|---|---|---|---|---|---|
| ORR . | Duration . | ||||||
| Vorinostat104 | 2011 | 1 | RR | 35 | 9 | 22% | Median PFS 18.8 mo |
| Ibrutinib105 | 2013 | 1 | RR | 56 | 4 | 25% | NR |
| Idelalisib106 | 2014* | 2 | RR | 125 | 15 | 47% | Median PFS 6.6 mo Median DOR 18.4 mo |
| Lenalidomide + Rituximab107 | 2014 | 2 | First line | 103 | 27 | 89% | Median PFS 53.8 mo |
| Agent . | Year . | Phase . | Patient status . | No. of patients . | MZL . | Response . | |
|---|---|---|---|---|---|---|---|
| ORR . | Duration . | ||||||
| Vorinostat104 | 2011 | 1 | RR | 35 | 9 | 22% | Median PFS 18.8 mo |
| Ibrutinib105 | 2013 | 1 | RR | 56 | 4 | 25% | NR |
| Idelalisib106 | 2014* | 2 | RR | 125 | 15 | 47% | Median PFS 6.6 mo Median DOR 18.4 mo |
| Lenalidomide + Rituximab107 | 2014 | 2 | First line | 103 | 27 | 89% | Median PFS 53.8 mo |
Updated at the 56th American Society of Hematology Annual Meeting and Exposition, San Francisco, CA, December 6-9, 2014.
Ongoing clinical trials specifically dedicated to SMZL or including SMZL among other indolent NHLs are evaluating new anti-CD20 monoclonal antibodies alone or in combination (EudraCT Number 2013-004916-23 and NCT01332968), ibrutinib (NCT01980628 and NCT01974440), and PI3K inhibitors (NCT01282424, NCT01732926, NCT02369016, NCT02367040, and NCT01732913).
HCV infection and antiviral treatment
The causal role of HCV in lymphomagenesis is strongly supported by the regression of lymphoma after eradicating the HCV infection.105 The first experience was reported by Hermine et al9 in 9 SMZL patients with HCV infection treated with interferon (IFN): 7 patients obtained a complete hematologic remission and HCV-RNA negativity. In a cohort of 704 consecutive HCV-positive patients with indolent NHL (137 patients had SMZL) reported by FIL, 36 SMZL patients were treated with IFN-based antiviral treatment as a first-line approach, and 65% showed a response.105
Although there is a clear association across the studies between lymphoma regression and the clearance of HCV, the direct antilymphoma activity of IFN cannot be ruled out. Data on new IFN-free regimens with direct-acting antivirals in HCV-associated lymphoproliferative disorders are based on clinical reports and they describe rapid response.106 Because antiviral treatment has a favorable impact on the outcome of HCV-infected NHL patients,105,107 it should be considered the first option for HCV-associated SMZL if cytoreductive treatment is not immediately necessary.66,67
Conclusions
Despite the relative rarity of SMZL, there has been a major effort in the last decade to define diagnostic criteria and the prognosis and molecular landscape of this neoplasm and of disorders that mimic SMZL. Although understanding of the genetics of this disease has improved significantly over the last 5 years, the pathogenetic implications of newly discovered genetic lesions still remain to be formally documented. In addition, although NOTCH2 and KLF2 mutations represent promising biomarkers, their broad application in clinical practice requires studies of diagnostic accuracy and their incorporation into the currently available clinical prognostic models for SMZL.
Prospective studies dedicated to SMZL will clarify clinical benefit and the toxicity profile of the immunochemotherapy approach. Specific molecular targets seem to be reasonably actionable in SMZL but in the era of precision medicine, splenectomy and rituximab monotherapy are still effective options for the majority of the patients with SMZL.
Acknowledgment
The authors thank Giorgio Croci for help in preparing the histology portion of the manuscript.
Authorship
Contribution: All authors contributed to the writing of the manuscript and approved the final version.
Conflict-of-interest disclosure: L.A. received honoraria or consulted for Roche, Celgene, Gilead, and Bayer. The remaining authors declare no competing financial interests.
Correspondence: Luca Arcaini, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: luca.arcaini@unipv.it.

![Figure 1. Histopathology of SMZL. In this typical case, (A) a nodular lymphoid proliferation with a biphasic appearance effaces the white pulp, infiltrates the wall of a great vessel (arrow) (hematoxylin and eosin [H&E] stain; magnification ×20) and extends to (B) the red pulp in a patchy distribution (CD79a; magnification ×100). (C) Morphologic pictures show medium sized, monocytoid lymphocytes with only scattered large cells (H&E stain; magnification ×400) and (D) a variable degree of plasmacytic differentiation (Giemsa stain; magnification ×400). (E) Anti-CD23 immunostain (magnification ×100) depicts the CD23+ marginal zone cells as well as the residual dendritic meshwork within the colonized follicles highlighted by (F) Mib/Ki-67 (magnification ×100), which confers a targetoid appearance. (G) A prototypical BM biopsy shows a small to medium size lymphoid population (Giemsa stain; magnification ×400) with (H) a nodular and sinusoidal distribution (CD20; magnification ×400). (G) Note the megaloblastoid features within the erythroblastic lineage, a common finding in cases associated with paraproteinemia and anemia.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/17/10.1182_blood-2015-11-624312/4/m_2072f1.jpeg?Expires=1770977279&Signature=hP803zqR5l3rURHUEB-JZXPQdKhNK1JSZyvjm0386bQ3FVaYhGlDkW0D8O7h87lY8WajKpkNUCBq0PtXPPuXPRNiDK2DSfaXUWAp7hbpBENUBf80aKqrAO1twWhMaaR3x4OCi8EJICucQqQKznydibzIa9xmwyuZtbUZRbztZWYVNY6CrQCZYMW7SrFZPq9i3ucL4b7B9K8lCJQ--CoBvIz2Lm3hcmvJb~dvmn7e7zuKKClKIs~KZjHlFw07vePtYeCfuDEyfSErNH5WTau-4IkHQd7ScLYxOq1DUTrAk~AJeQ37zL2IuHRvIJljPVFTqisX98bS2epwk5PKc0hgVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)