Abstract
Multiple new drugs are being developed to treat acute myeloid leukemia (AML), including novel formulations of traditional chemotherapy-antibody drug conjugates and agents that target specific mutant enzymes. Next-generation sequencing has allowed us to discover the genetic mutations that lead to the development and clinical progression of AML. Studies of clonal hierarchy suggest which mutations occur early and dominate. This has led to targeted therapy against mutant driver proteins as well as the development of drugs such as CPX-351 and SGN-CD33A whose mechanisms of action and efficacy may not be dependent on mutational complexity. In this brief review, we discuss drugs that may emerge as important for the treatment of AML in the next 10 years.
Introduction
Promising novel agents evaluated over the past 40 years for the treatment of acute myeloid leukemia (AML), with rare exceptions, have been relegated to the dustbin of history. Nevertheless, the revolution in understanding the genetics of AML facilitated by next-generation sequencing has led to many new investigational drugs against potential driver mutations such as mutant isocitrate dehydrogenase (IDH) and Fms-related typrosine kinase 3 (FLT3) (Table 1). To date, a targeted agent is not available against DNMT3A mutations, which are thought to arise in the preleukemic hematopoietic stem cell population.1
The current outcomes for patients with AML are well known. Multiple international retrospective studies show that only 40% of patients younger than age 60 years survive more than 5 years.2 Indeed, even favorable-risk core-binding factor leukemias have an unacceptably high mortality rate of 56% at 10 years.3-5 Few patients who relapse after achieving a complete remission (CR) survive more than 5 years. Treatment approaches for patients unfit for traditional induction chemotherapy—hypomethylating agents or low-dose cytarabine (LDC)—are modestly effective but inadequate.6,7 It is estimated that 20 830 people will be diagnosed with AML in the United States in 2015, making AML a relatively rare malignancy. Because of this, AML is considered an orphan disease by the US Food and Drug Administration. Challenges in the development of drugs for AML are formidable and have recently been addressed.8
We present a mechanism-based overview of novel agents currently in clinical trials that have published data or have been presented at a major international meeting. In our opinion, these drugs may be used to treat AML, either alone or in combination with the current standard of care during the next 5 years. This review is not meant to be exhaustive. The landscape of targets is well known, and new drugs are introduced at a rapid pace. Rather, we focus on those agents that will likely pave the road to success.
Novel formulations of cytotoxic chemotherapy
Despite the excitement over new therapeutic agents against genetic and epigenetic targets, in the last 10 years improvements in overall survival (OS) have been driven by refinements in the use of conventional chemotherapy.9,10 With history as a guide, continued modifications and reformulations of traditional chemotherapy may incrementally improve OS.
CPX-351
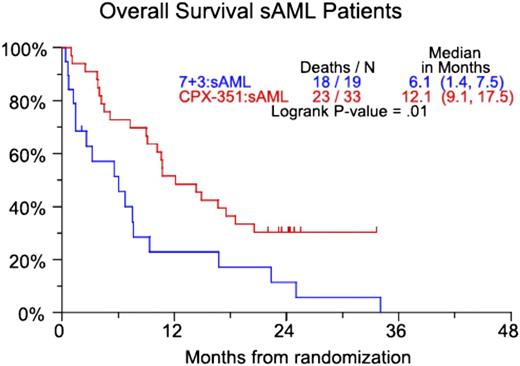
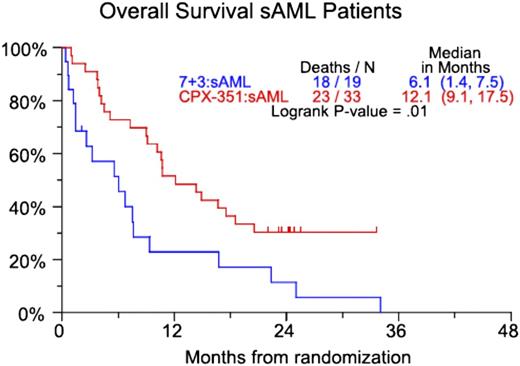
CP-351 is a liposomal formulation of cytarabine and daunorubicin in a 5:1 molar ratio. In vitro studies demonstrate that this ratio has the highest level of synergy and the lowest level of antagonism.11 In a randomized, open-label phase 2 trial, 127 patients age 60 to 75 years with or without secondary AML (sAML; defined as AML with a history of an antecedent hematologic disorder or therapy-related AML) were randomly assigned in a 2:1 fashion to induction chemotherapy with CPX-351 or daunorubicin (60 mg/m2) and cytarabine (100 mg/m2/d for 7 days). Patients who received CPX-351 for induction could receive CPX-351 for consolidation. Those patients who received daunorubicin and cytarabine during induction could receive cytarabine-based consolidation.12 Primary end points were the rate of CR and CR with incomplete blood count recovery (CRi). Secondary end points were event-free survival (EFS) and OS. The composite CR (CRc) rate (CR + CRi) was 66.7% for all patients treated with CPX-351 and 51.2% in the group receiving daunorubicin and cytarabine. The improved response rate in the CPX-351 group did not reach statistical significance (P = .07). There was neither an EFS nor OS benefit when all patients were considered. However, the higher rate of CRc in the CPX-351 group did lead to a statistically significant 6-month survival benefit in patients with sAML (Figure 1).
CPX-351 vs 7+3 (7 days of cytarabine and 3 days of daunorubicin). OS for patients with secondary AML. Numbers in parentheses represent confidence intervals. Reprinted from Ravandi et al12 with permission.
CPX-351 vs 7+3 (7 days of cytarabine and 3 days of daunorubicin). OS for patients with secondary AML. Numbers in parentheses represent confidence intervals. Reprinted from Ravandi et al12 with permission.
In a separate study, patients between the ages of 18 and 65 years with AML in first relapse were randomly assigned 2:1 to CPX-351 or investigator’s choice of salvage regimens. In the entire cohort, there was a trend toward increased CRc in the CPX-351 group but no difference in EFS or OS.13 In a subset analysis of poor-risk patients as defined by the European Prognostic Index (a prognostic index for adult patients with AML in first relapse), median OS was improved by 2.4 months in patients treated with CPX-351 although EFS was statistically similar.14
On the basis of these results, an open-label, randomized phase 3 study of CPX-351 vs daunorubicin (60 mg/m2)-cytarabine was initiated for sAML in patients between the ages of 60 and 75 years (NCT01696084). The trial is fully accrued and results are expected in late 2016.
If CPX-351 results in an OS benefit in the randomized phase 3 trial, it may become the preferred treatment for patients older than age 60 years with sAML. However, subgroup analysis of a phase 3 study demonstrated that daunorubicin 90 mg/m2 leads to an increased OS in AML patients age 60 to 65 years.15 It may be that CPX-351 is equivalent to daunorubicin 90 mg/m2 while being superior to daunorubicin 60 mg/m2. Furthermore, the phase 2 study that demonstrated an OS benefit with CPX-351 strictly defined sAML; only patients with a known history of an antecedent hematologic disorder or therapy-related AML were included. The current phase 3 study broadens the sAML definition to include patients with AML with myelodysplasia-related changes and AML with cytogenetic abnormalities characteristic of myelodysplastic syndrome. Dysplasia also occurs in patients with de novo AML, and myelodysplasia-related changes are in the eye of the pathologist. The group enrolled in the phase 3 study may be different from the group with an OS benefit in the randomized phase 2 trial. Despite these caveats, that phase 3 study offers a significant possibility of improving on the current standard of care for older adults who are eligible for induction chemotherapy.
Vosaroxin
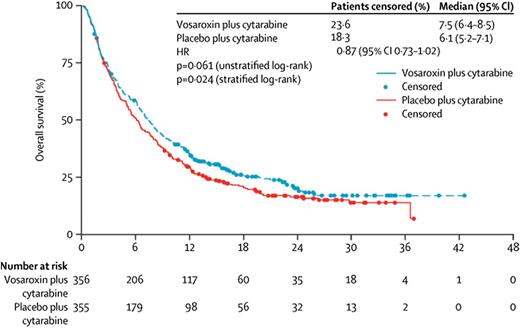
Daunorubicin is a critical component of standard induction chemotherapy, but it can cause cardiac toxicity, particularly in patients with preexisting heart failure. Vosaroxin is a quinolone derivative that inhibits topoisomerase II without the production of oxygen free radicals that lead to the cardiac toxicity observed with other topoisomerase II inhibitors. Based on encouraging early clinical data, a randomized placebo-controlled phase 3 study of cytarabine (1 g/m2 days 1-5) with or without vosaroxin (90 mg/m2 days 1-4) was initiated for patients with primary refractory AML or AML in first relapse.16 Of the 711 patients enrolled on the study, the CR rate in the vosaroxin arm was 30.1%, nearly double the 16.3% rate seen in the placebo arm. Despite this, OS trended toward but did not meet statistical significance, with median OS of 7.5 months for cytarabine-vosaroxin compared with 6.1 months for cytarabine-placebo (P = .06) (Figure 2). After censoring at the time of allogeneic stem cell transplantation, patients who received vosaroxin had a statistically significant (P = .02) median OS benefit of 1.4 months (median OS, 6.7 months vs 5.3 months favoring vosaroxin). In a preplanned intention-to-treat analysis of patients older than age 60 years, median OS was 7.1 months vs 5.0 months favoring vosaroxin. Fifteen percent of patients on the vosaroxin arm developed grade 3 or 4 stomatitis that either interfered with oral intake or had life-threatening consequences.
Two separate open-label randomized phase 2 trials, one of vosaroxin vs low-dose LDC and the other of vosaroxin with LDC vs LDC alone, led to no differences in CR rate or OS between the arms treated with vosaroxin and the arms treated with LDC alone.17 In addition, toxicity in patients treated with vosaroxin mirrored that seen in the phase 3 study, with gastrointestinal toxicity significantly more common in the patients treated with vosaroxin. However, the 30-day and 60-day mortality were similar in the 2 arms of the study.
The future of vosaroxin at the dose and schedule used in the phase 3 study is in doubt. Despite the OS benefit seen when patients were censored at the time of allogeneic transplantation, the clinical significance of a 6-week OS benefit is unclear. Nevertheless, vosaroxin has proven clinical activity, and future studies may clarify the best dose and schedule for optimizing that activity.18
Guadecitabine
Along with LDC, hypomethylating agents have emerged as the de facto treatment for patients with AML who are ineligible for conventional cytotoxic induction chemotherapy. The CR rate in randomized clinical trials of hypomethylating agents is no better than 30%, and median OS is similar to that of conventional care regimens.6,7 Guadecitabine (SGI-110) is a dinucleotide of decitabine and deoxyguanosine that increases the in vivo exposure of decitabine by protecting it from deamination. In a randomized study comparing guadecitabine at 60 mg/m2 to 90 mg/m2 in elderly patients with newly diagnosed AML, there were no significant differences between groups in CR rate. In a combined analysis of 51 patients who received either dose of guadecitabine, the CRc was 57%, with 37% of patients achieving a true CR. This translated into a median OS of 10.5 months in the entire patient population and 18.2 months in those patients who achieved a CR.19 On the basis of these data, a large, randomized phase 3 study of guadecitabine vs 5-azacitidine, decitabine, or cytarabine has been initiated (NCT02348489).
If the randomized phase 3 study shows an OS benefit, guadecitabine may supplant hypomethylating agents as the agent of choice for patients with AML unfit for traditional induction chemotherapy. However, both decitabine and 5-azacitidine are now available as generic formulations, and there may be a substantial difference in cost between guadecitabine, decitabine, and 5-azacitidine. If guadecitabine shows a marginal OS benefit, its use may be restricted by payors.
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) against a variety of cell surface receptors on leukemic myeloblasts are in clinical development. CD33 is a transmembrane cell surface receptor expressed on cells with a myeloid lineage. Because of its presence on myeloblasts, targeting CD33 on the surface of leukemic blasts either with naked antibodies or ADCs is variably successful.20-22 The only example of a successful ADC to date is gemtuzumab ozagomicin, which received accelerated approval but was subsequently withdrawn from the market.23 Despite this, efforts to improve upon its dosing and schedule have led to clinical trials that suggest a survival benefit in certain subsets of patients treated with gemtuzumab ozagomicin.24,25
SGN-CD33A is a novel ADC with highly stable dipeptide linkers that enable uniform drug loading of a pyrrolobenzodiazapine dimer that crosslinks DNA leading to cell death. An interim analysis of a phase 1 dose-escalation study of SGN-CD33A in patients with relapsed CD33+ AML and those patients who declined intensive therapy, the CRc rate was 29%. Seventy-seven percent of patients who received doses of 40 μg/kg or higher had at least a 50% reduction in bone marrow blasts.26 There is an ongoing study of SGN-CD33A alone and in combination with hypomethylating agents.
All anti-CD33 ADCs induce myelosuppression; the term CR with incomplete platelet count recovery was coined as a direct result of the bone marrow blast clearance but ongoing thrombocytopenia seen in patients treated with gemtuzumab ozagomicin. SGN-CD33A appears to have the antileukemic activity of gemtuzumab ozagomicin without liver toxicity. In this way, it represents the pinnacle of an anti-CD33 ADC.
Molecularly targeted agents
Volasertib inhibits polo-like kinase 1 (PLK10), PLK2, and PLK3. PLKs are a family of 5 conserved serine/threonine kinases that are important for checkpoint regulation and cell cycle progression. Inhibition of PLK1, which is overexpressed in human AML cells, leads to a disorganized spindle assembly and cellular apoptosis.
In a phase 2 study, 89 patients were randomly assigned 1:1 to volasertib with LDAC or LDAC alone.27 The dose of volasertib was 350 mg on days 1 and 15 of a 28-day cycle. CRc was 31% in the combination arm compared with 13.3% with LDAC monotherapy with a strong trend toward statistical significance (P = .052). Both EFS and OS were significantly longer in the combination arm; median OS was 8.0 months with volasertib with LDAC and 5.2 months with volasertib alone (Figure 3). Clinical trials of volasertib in combination with induction chemotherapy (NCT02198482) and decitabine (NCT02003573) are ongoing.
OS estimates according to randomization and European Leukemia Net genetic group. (A) Favorable and intermediate I/II groups. Median OS times for patients who received LDC (n = 28) and LDC + volasertib (n = 22) were 7.8 and 9.8 months, respectively (HR, 0.54; 95% confidence interval [CI], 0.29-1.02). (B) Adverse group. Median OS times for patients who received LDC (n = 14) and LDC + volasertib (n = 14) were 3.3 and 5.9 months, respectively (HR, 0.63; 95% CI, 0.29-1.40). Database snapshot November 7, 2013.
OS estimates according to randomization and European Leukemia Net genetic group. (A) Favorable and intermediate I/II groups. Median OS times for patients who received LDC (n = 28) and LDC + volasertib (n = 22) were 7.8 and 9.8 months, respectively (HR, 0.54; 95% confidence interval [CI], 0.29-1.02). (B) Adverse group. Median OS times for patients who received LDC (n = 14) and LDC + volasertib (n = 14) were 3.3 and 5.9 months, respectively (HR, 0.63; 95% CI, 0.29-1.40). Database snapshot November 7, 2013.
FLT3 inhibitors
Fms-related tyrosine kinase 3 (FLT3) is a type 3 receptor tyrosine kinase expressed on hematopoietic progenitors and on most leukemic myeloblasts. FLT3 internal tandem duplications (FLT3-ITDs) are seen in approximately 30% of patients with de novo AML. Patients with FLT3-ITD have a poor prognosis.28,29 The development of FLT3 inhibitors is a priority. Despite a litany of first-generation FLT3 inhibitors—those that are approved for treatment of other malignancies (sorafenib and sunitinib) and unapproved drugs that have been explored in late-stage clinical trials (lestaurtinib and midostaurin)—the search continues for a novel durable inhibitor of FLT3 that leads to a durable OS benefit. However, there is considerable interest regarding 4 agents currently in clinical trials: midostaurin, quizartinib, crenolanib, and gilteritinib (ASP-2215).
Midostaurin
Midostaurin is an inhibitor of FLT3, c-KIT, PDGFRB, VEGFR-2, and protein kinase C. A phase 1B study explored different dose levels and schedules of midostaurin in combination with induction chemotherapy. The trial enrolled patients between the ages of 18 and 60 years, both FLT3 wild-type (FLT3-WT) and FLT3 mutant. In the cohort that received 50 mg twice per day (either sequentially or concomitant with chemotherapy), the CR rate was 92% in patients with FLT3-ITD or FLT3 tyrosine kinase domain (FLT3-TKD) and 74% in patients who were FLT3-WT.30 These encouraging results led to the development of the RATIFY study, an international, randomized, placebo-controlled trial of midostaurin (PKC412) in combination with daunorubicin (60 mg/m2 for 3 days) and cytarabine (200 mg/m2 for 7 days). For patients who achieve a CR, consolidation chemotherapy is continued with either midostaurin or placebo. In addition, maintenance therapy after consolidation is given with midostaurin or placebo. There is no crossover in treatment arms after patients achieve CR. The primary study outcome is OS, not censored at the time of allogeneic bone marrow transplant.31 The trial has been open since 2008, and results are eagerly awaited.
Quizartinib
Quizartinib (formerly known as AC220) was developed as a highly selective second-generation FLT3 inhibitor.32 In a phase 1 study of quizartinib in patients with relapsed or refractory AML, regardless of FLT3 mutation status, the maximum tolerated dose was 200 mg/d.33 A variety of phase 2 studies demonstrated the effectiveness of quizartinib in the relapsed and refractory settings (see Levis et al,34 Cortes et al,35 and Schiller et al36 ). What is notable about all of these studies (whether patients were in first relapse or later, young or old) is the relatively remarkable CRc rate, partial remission (PR) rate, and median duration of response. The CRc ranged between 44% and 54% whereas the overall response rate (CRc + PR) was between 61% and 72%. Of crucial importance, the PR definition did not require normalization of platelet and neutrophil counts as defined in the International Working Group Criteria. Median duration of response ranged between 11.3 and 12.7 weeks.
Despite the promising single-agent activity of quizartinib, 50% of patients relapsed within 3 months. Further studies suggest that the mechanism of resistance to quizartinib is acquired mutations in the tyrosine kinase domain of the FLT3 gene, including mutations in D835 and F691.37,38 Because of this, agents that overcome this resistance and lead to a longer duration of response are seen as crucial to the development of targeted inhibitors of mutant FLT3. Crenolanib is such an agent.
Crenolanib
The tyrosine kinase inhibitor crenolanib has activity against the D835 mutation found in the activation loop of FLT3. Crenolanib, as a pan selective FLT3 inhibitor overcomes quizartinib resistance. A phase 2 study of crenolanib in patients with relapsed or refractory AML and an FLT3-ITD or FLT3-TKD was presented at the American Society of Hematology annual meeting in 2014.39 All patients received crenolanib at a dose of 200 mg 3 times per day, and patients were stratified on the basis of whether they had received prior FLT3-directed therapy (eg, quizartinib, midostaurin, sorafenib, or PLX3397).
Crenolanib induced a CRi in 23% of patients who were FLT3 inhibitor naïve but in only 5% of patients who had received prior FLT3 therapy. Both treatment groups had similar degrees of hematologic improvement (31% and 33%, respectively), although it is unclear whether this modest improvement translated into clinical benefit. Crenolanib is now being investigated in combination with induction chemotherapy in patients with newly diagnosed AML with an FLT3-ITD or FLT3-TKD mutation (NCT02283177).
Gilteritinib
Gilteritinib (ASP-2215) is a potent inhibitor of both FLT3-ITD and FLT3-TKD mutations. A phase 1/2 trial was initiated in 2013 in FLT3-WT and FLT3-mutant patients, and interim results were reported in 2015.40 A total of 166 patients were enrolled in a combination of dose-escalation and in-parallel dose-expansion cohorts. The maximum tolerated dose was 300 mg once per day. Of note, both FLT3-WT and FLT3-mutant patients were enrolled on the study. FLT3-WT patients derived minimal benefit from gilteritinib, with a CRc of 8% and a PR rate of 3%. In the FLT3-mutant patient population, the overall response rate was 57% with a CRc rate of 43% and a PR rate of 15%. A phase 1 study of gilteritinib in combination with induction consolidation chemotherapy is ongoing (NCT02236013), and a randomized phase 3 study of gilteritinib vs salvage chemotherapy is planned (NCT02421939).
The new generation of FLT3 inhibitors are remarkably active in patients with relapsed/refractory FLT3-positive AML. These inhibitors are able to clear blasts from the bone marrow, but they are less effective at restoring normal hematopoiesis. The rate of CRi and isolated blast decrease in the marrow is much higher than the rate of true CR. For example, in the initial results from gilteritinib, the true CR rate was only 5%. This may be because suppressing the FLT3 clone does not fully eradicate other clonal drivers of disease that contribute to leukemogenesis and abnormal hematopoiesis. In addition, the relatively short duration of response in patients receiving quizartinib and gilteritinib (mean duration of response, 126 days) makes both of these agents potentially ideal candidates to bridge patients to an allogeneic stem cell transplantation but are less likely to benefit older patients for whom allogeneic transplantation is not an option. For this reason, combining FLT3 inhibitors with other novel agents, induction chemotherapy, or hypomethylating agents may lead to improved CR and OS. The RATIFY study, when reported, should provide robust data about whether combining an FLT3 inhibitor with chemotherapy improves survival.
IDH1 and IDH2 inhibitors
IDH2 (found in the mitochondria) and IDH1 (found in the cytoplasm) function in their WT state to catalyze the conversion of isocitrate to α-ketoglutarate. IDH2 is mutated in 10% to 15% and IDH1 is mutated in 5% to 10% of adult AML.41-43 The prevalence of both IDH mutations appears to increase as patients age; IDH mutations are relatively rare in patients with pediatric AML.42,44 Mutations in IDH2 are enriched in patients with normal karyotypes, but up to 30% of patients with IDH2 mutations have abnormal cytogenetics at the time of diagnosis. These generally fall into the intermediate or unfavorable cytogenetic risk groups as defined by the European Leukemia Net classification. Mutant IDH enzymes acquire neomorphic activity and catalyze the conversion of α-ketoglutarate into β-hydroxyglutarate, increase levels of β-hydroxyglutarate, and cause hypermethylation of target genes leading to a block in myeloid differentiation (Figure 2).45-47 Inhibitors of mutant IDH1 and IDH2 are currently in phase 1 clinical trials (NCT02381886, NCT01915498, NCT02074839), and early results have demonstrated that these agents have encouraging efficacy in patients with relapsed disease. In phase 1 clinical trials, inhibitors of mutant IDH1 and IDH2 are remarkably active with minimal toxicity.
Interim results of a phase 1/2 study of AG-221 (Agios/Celgene), the first IDH2 inhibitor currently in clinical trials, were presented at the annual meeting of the European Hematology Association in 2015 and demonstrated an overall response rate of 41% in patients with relapsed/refractory AML.48 Twenty-seven percent of patients cleared their bone marrow of blasts with various levels of count recovery (true CR, CRi, and morphologic leukemia-free states), and 18% of patients achieved a true CR. An additional 14% of the patients had a true PR with normalization of platelet count and absolute neutrophil count. Seventy-six percent of the responding patients have been on treatment for at least 6 months. Interestingly, an additional 44% of patients had clinical stable disease defined as a stable or decreased blast percentage in the bone marrow that does not meet the criteria for PR. Some of these patients with stable disease are red cell transfusion independent, have normal platelet counts, and a normal absolute neutrophil count, despite persistence of blasts in the bone marrow and peripheral blood. The number of patients with clinically meaningful stable disease and those with smoldering AML is an area of active investigation because it has implications for the OS benefit that may be observed in these patients.
Inhibitors of mutant IDH1 in clinical development include AG-120 (Agios) and IDH305 (Novartis). Early results for AG-120 in patients with relapsed AML have shown evidence of efficacy similar to that of the IDH2 inhibitor, with an overall response rate of 31% and a true CR rate of 15%. An additional 27 patients had stable disease. Dose escalation continues, and expansion cohorts in patients with relapsed/refractory AML have been initiated.
DOT1L, BCL-2, BET bromodomain inhibitors, and histone deacetylase inhibitors
Other agents against molecular targets are in the midst of ongoing clinical trials. These include the DOT1L inhibitor EPZ-5676, the BCL2 inhibitor ABT-199, and inhibitors of bromodomain (BET) proteins.
The prognosis of patients with acute leukemia and a translocation or partial tandem duplication of the mixed lineage leukemia (MLL) gene is poor. In a study of 1897 patients with AML treated within German AML Cooperative Group trials between 1992 and 1999, 2.8% of patients were found to have a rearrangement involving 11q23. OS at 3 years was 12.5%.49 Elegant in vitro and in vivo experiments demonstrated that a key mediator of MLL-rearranged leukemia is the histone methyltransferase DOT1L.50-54 Similarly, preclinical studies of DOT1L inhibition in acute leukemia associated with translocations involving the MLL gene have shown remarkable effectiveness.55,56 Translating these results to patients has been more difficult. Inhibition of DOT1L with the small molecule inhibitor EPZ-5676 produced a CR in 2 of 34 patients with an MLL rearrangement or MLL-partial tandem duplication. In 1 patient, not only did morphologic evidence of myeloid disease disappear, but leukemia cutis resolved and the patient achieved a cytogenetic remission.57
The BCL2 inhibitor ABT-199 showed a CR/CRi in 5 of 32 patients, the majority of whom had relapsed or refractory disease. Interestingly, 3 of 11 patients with IDH mutation in the study were among the 5 patients achieving CR/CRi.58 Although there is preclinical data suggesting that IDH1 and IDH2 mutations induce BCL2 dependence in AML, the small numbers of responders in this early study makes any statement about the clinical effectiveness of BCL2 inhibition in IDH-mutant AML premature.59
Similar to the interest in DOT1L inhibition, small molecular inhibition of BET proteins has a robust preclinical rationale that is currently being tested in multiple clinical trials of AML (NCT02158858, NCT02308761, NCT01943851).60-62 To date, the only report from a clinical trial has been of the BET bromodomain inhibitor OTX-015.63 In that study, 36 patients with relapsed and refractory leukemia (33 with AML, 2 with acute lymphoblastic leukemia, and 1 with myelodysplastic syndrome) were enrolled on this phase 1 dose-escalation study, and 28 were evaluable for dose-limiting toxicity. Of these, 1 patient had incomplete platelet count recovery and one patient had a true CR. Three other patients had evidence of clinical activity (decrease in blast percentage and resolution of gingival hypertrophy).
Finally, pracinostat is an orally available competitive inhibitor of histone deacetylase (HDAC). In vitro studies show that it has >1000-fold selectivity for class I, II, and IV HDACs compared with class III HDACs with no effects on other zinc-binding enzymes. A phase 2 study of pracinostat in combination with 5-azacitidine in patients with previously untreated AML who are unfit for induction chemotherapy was completed in December 2014. The overall response rate was 54% (27 of 50 patients), and the CR rate was 42% (21 of 50 patients). Follow-up continues in an effort to calculate a median OS for the patients treated on study.
The results above speak to the difficulty of translating preclinical studies into therapeutically effective treatments in the clinic. The path forward for each of these drugs is either to identify a biomarker of response or to consider combining these agents with other drugs that are synergistic.
Incorporating emerging therapies into the treatment of AML
The new chemotherapies, hypomethylating agents, ADCs, and molecularly targeted agents all have clinical activity as single agents and may increase OS in patients with AML. However, it is likely that the best responses will be seen when these agents are combined with conventional induction chemotherapy or with each other. For example, one can envision a future in which it will be routine to combine FLT3 inhibitors and IDH inhibitors and perhaps even SGN-CD33A with induction chemotherapy and use molecularly targeted agents and ADCs in induction, consolidation, and as maintenance therapy after consolidation. We may use CPX-351 in patients with sAML, but clinical trials with CPX-351 in combination with molecularly targeted agents should also be explored. For patients with AML who are unfit for induction chemotherapy, a winner may emerge among volasertib, guadecitbine, and SGN-CD33A, but given their nonoverlapping mechanisms of action, we would encourage clinical trials that combine 1 or more of these agents and also combine them with molecularly targeted therapies. A decade from now, we believe the outcomes of patients with AML will improve because of these novel agents and other future drugs that build on their successes. Although drug development for AML may be a boulevard of broken dreams,64 as the comedienne Lily Tomlin noted, “The road to success is always under construction.”
Authorship
Contribution: E.S. and M.T. contributed equally to researching and writing this review article.
Conflict-of-interest disclosure: M.T. is on the Advisory Board for Agios Pharmaceuticals and has received research funding from Boehringer Ingleheim, Bioline, and Epizyme. E.S. is on the Advisory Boards for Agios Pharmaceuticals and Seattle Genetics and has received research funding from Agios Pharmaceuticals and Seattle Genetics.
Correspondence: Eytan M. Stein, Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, 1275 York Ave, New York, NY 10065; e-mail: steine@mskcc.org.


![Figure 3. OS estimates according to randomization and European Leukemia Net genetic group. (A) Favorable and intermediate I/II groups. Median OS times for patients who received LDC (n = 28) and LDC + volasertib (n = 22) were 7.8 and 9.8 months, respectively (HR, 0.54; 95% confidence interval [CI], 0.29-1.02). (B) Adverse group. Median OS times for patients who received LDC (n = 14) and LDC + volasertib (n = 14) were 3.3 and 5.9 months, respectively (HR, 0.63; 95% CI, 0.29-1.40). Database snapshot November 7, 2013.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/1/10.1182_blood-2015-07-604538/5/m_71f3.jpeg?Expires=1771045186&Signature=ML9IjaBdc-P1-OyYC~RoMcEKJKBfBPMZJsV6LBrlZSNswrlbRI2u7OSNrj4CVzwdzexF1NWT35RRVYsM9RocjM92Hv9uu2SXODXlKjYjdAU1NB0QcwTVnRPDaDvjQuQi~Aokscm~9Lic4HFk-Yg3ge3aUaG7pmoliFrw5Yosvs8MGSremEcxv8xQQSGFEKddHFWYngF8lJ~oscBjMx5NOpZ20~0Yn8hRrQH46JUAvSKl~AP2nmtRJ6TWu9Q-l-VwVOfRe3gPEnmxkuTSZKBKEhBKUzOKi1XIlv5kzMse460-7-IoNsfNqTVNH6-oS8mjf0A78DRoqaRtOEhTpsEWTQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. OS estimates according to randomization and European Leukemia Net genetic group. (A) Favorable and intermediate I/II groups. Median OS times for patients who received LDC (n = 28) and LDC + volasertib (n = 22) were 7.8 and 9.8 months, respectively (HR, 0.54; 95% confidence interval [CI], 0.29-1.02). (B) Adverse group. Median OS times for patients who received LDC (n = 14) and LDC + volasertib (n = 14) were 3.3 and 5.9 months, respectively (HR, 0.63; 95% CI, 0.29-1.40). Database snapshot November 7, 2013.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/1/10.1182_blood-2015-07-604538/5/m_71f3.jpeg?Expires=1771080961&Signature=DjL5TPdUoe7UpSQJ5WyWAaw~jRDv5i53f2Q-tIOvEptGKjoYxXncyvH0aP-SVdJedeGRzYXS2v-jMGR0J~fkm0WBG8Qu2~jvN4vQs1nX~c55o0X4c6mudx~TrF-byG-O4yMCafkXKxpbBZ-a6UQhQ6zk9F-kkRnylsJxfO5s7S4MEWFTyvOXSJlklYg3X~OJFqk~Pa5VLtqfMNqOqDIaDV6RyqGFo~B~LzfsU1MKtCfSKbZoDYInDY-0-Y5WpIYofJlQza6JA7EKLUPr4sesvPUuqpnH2T4yFJ2vlVY6cTIp8W62gzJPE0NHDWTD8sbsNb3y68Qey6T4LJJ0jtaENA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)