Key Points
Obinutuzumab monotherapy demonstrates an increased ORR with 2000 mg over 1000 mg, but no difference in progression-free survival.
No meaningful difference was observed in the overall safety profile across the 2 treatment arms.
Abstract
Obinutuzumab is a glycoengineered, type 2 anti-CD20 humanized antibody with single-agent activity in relapsed chronic lymphocytic leukemia (CLL). With other CD20 antibodies, a dose-response relationship has been shown. We therefore performed a randomized phase 2 study in symptomatic, untreated CLL patients to evaluate if an obinutuzumab dose response exists. Obinutuzumab was given at a dose of 1000 mg (100 mg IV day 1, 900 mg day 2, 1000 mg day 8 and day 15 of cycle 1; 1000 mg day 1 of cycles 2-8) or 2000 mg (100 mg IV day 1, 900 mg day 2, 1000 mg day 3, 2000 mg day 8 and day 15 of cycle 1; 2000 mg day 1 of cycles 2-8). The primary end point was overall response rate (ORR). Eighty patients were enrolled with similar demographics: median age 67 years, 41% high-risk Rai disease, and 10% del(17p)(13.1). ORR (67% vs 49%, P = .08) and complete response (CR) or CR with incomplete cytopenia response (20% vs 5%) favored 2000 mg obinutuzumab. Overall, therapy was well tolerated, and infusion events were manageable. This study demonstrates significant efficacy of obinutuzumab monotherapy, for 1000 mg as well as for 2000 mg, in untreated CLL patients with acceptable toxicity. Although exploratory, a dose-response relationship may exist, but its relevance to improving progression-free survival is uncertain and will require further follow-up. This trial was registered at www.clinicaltrials.gov as #NCT01414205.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent type of adult leukemia and is defined by a typical immunophenotype of mature B-cell markers (CD19, CD20[dim], CD23, sIg [dim]) with coexpression of the T-cell marker CD5. At the present time, CLL is incurable with available therapies. Early therapeutic intervention has not demonstrated improvements in outcome, so therapy is generally deferred until disease-related symptoms or cytopenias emerge. Treatment of CLL has evolved significantly over the past 2 decades, first with the demonstration that fludarabine was more effective than chlorambucil as measured by overall response rate (ORR), complete response (CR), and progression-free survival (PFS).1,2 Combination of fludarabine with alkylator-based cyclophosphamide (FC) improved further the CR rate and PFS as compared with fludarabine monotherapy but did not lead to improved overall survival (OS).3-5 Thus, the impact of chemotherapy for CLL has generally been palliative with little evidence of impact on natural disease history.
Contrasting with this, targeted therapy has improved outcomes for patients with CLL. When given in combination with fludarabine or FC, rituximab demonstrated increased ORR, CR, PFS, and OS over historical control chemotherapy-only treatments.6-8 The CLL8 study then randomized FC vs FC with rituximab (FCR) and demonstrated definitive improvement in ORR, CR, PFS, and OS among all genetic groups except those patients with normal interphase cytogenetics and del(17)(p13.1).9 National Comprehensive Cancer Network guidelines reference FCR as the preferred frontline regimen for fit younger (age <70) patients with CLL lacking del(17)(p13.1). Despite the success of anti-CD20 therapy as part of chemoimmunotherapy, early studies of monotherapy demonstrated low response rates in CLL and small lymphocytic lymphoma.10 This low response rate was addressed in part through subsequent studies evaluating dose intensification, which showed improved outcomes when given as a single agent, although this did not yield additional benefit when given in combination with chemoimmunotherapy.11-13
The success of rituximab as part of chemoimmunotherapy in CLL has fostered development of several other therapeutic anti-CD20 antibodies in this disease. The first to be approved by the Food and Drug Administration for use in relapsed CLL was ofatumumab.14,15 This type 1 humanized antibody binds to a different epitope than rituximab that mediates improved complement, natural killer cell antibody–dependent cellular cytotoxicity, and direct killing (with cross-linking) as compared with rituximab against CLL cells.16 Although monotherapy dosing of ofatumumab was established empirically for its relapsed indication, a subsequent report in previously untreated CLL demonstrated improved response with the higher 2000-mg/wk dose as compared with a 1000-mg/wk dosing when given alone.17 This provides further support that despite even improved biological properties, a dose of anti-CD20 antibody may contribute to the expected response and PFS observed with these treatments.16,17
Obinutuzumab is a third-generation, humanized, afucosylated, type 2 anti-CD20 antibody that also binds to a separate epitope that facilitates enhanced direct killing and the ability of effector cells to bind to the Fc region of the antibody.18 Given the documented advantage of obinutuzumab in preclinical studies against CLL cells by several groups,18-21 this agent entered phase 1 clinical trials in CLL where obinutuzumab was administered once every 21 days for a total of 8 cycles with an additional dose on day 8 of the first cycle (maximum of 9 infusions).22 A total of 13 patients with CLL received obinutuzumab at doses with a range of 400 to 2000 mg across 4 cohorts. The ORR was 62% (all partial responses [PRs]), and a maximally tolerated dose was not reached. The dose and schedule selected for a phase 2 study was chosen based on a population pharmacokinetics (PK) analysis of all phase 1 data indicating that saturation of the CD20 target could be attained at a 1000-mg dose once every 21 days for up to 8 cycles with 2 additional doses administered on day 8 and day 15 of cycle 1 comprising a loading dose (maximum of 10 infusions). The 20 relapsed and refractory CLL patients from the phase 2 study achieved a best ORR of 30%.22 The CLL11 study randomly assigned a comorbid (often older) population of previously untreated patients with CLL to chlorambucil, chlorambucil + rituximab, or chlorambucil + obinutuzumab. This study was recently published demonstrating that obinutuzumab + chlorambucil was superior to both of these alternative treatments as measured by ORR, CR rate, and PFS.23 Additionally, the addition of obinutuzumab to chlorambucil significantly prolonged OS over chlorambucil alone, making this the first treatment to effectively improve survival in the population of patients not appropriate for FCR. Less obinutuzumab was used in this combination trial because of diminished target tumor cells as a consequence of the addition of chlorambucil.
Despite the positive results of CLL11, there remains considerable interest in optimizing the single-agent activity of obinutuzumab and exploring whether a dose response to this treatment exists that might guide future investigation of this agent. To address each of these questions, we performed a randomized phase 2 study of obinutuzumab in previously untreated, symptomatic CLL patients who were either inappropriate for or declined FCR-based therapy exploring 2 separate doses (1000 mg and 2000 mg). Herein, we present the results of this study.
Methods
Patients
Patients were randomized into this multiple institution company-sponsored clinical study (#NCT01414205, GAGE) following approval by each participating site’s institutional review board. All patients provided written informed consent. Patients had diagnosis of CLL that required therapy according to International Working Group for CLL (IWCLL) criteria. Patients were required to have CD20-positive CLL, no previous treatment of CLL or autoimmune cytopenias, Eastern Cooperative Oncology Group performance status of 2 or less, life expectancy of >6 months, measurable disease by computed tomography (CT) scan, adequate organ function (creatinine ≤1.5 the upper limit of normal, aspartate aminotransferase or alanine aminotransferase ≤2.5× the upper limit of normal, and total bilirubin <3.0 mg/dL). Patients were not eligible if they had prior anaphylaxis to monoclonal antibody therapy, uncontrolled infection, evidence of chronic hepatitis B or C infection, or concomitant illness that could compromise ability to interpret the study results.
Treatment
Following confirmation of eligibility, patients were centrally randomized 1:1 to each treatment arm stratifying for advanced vs intermediate Rai staging and presence or absence of bulky (>5 cm) lymph nodes. Premedication with oral acetaminophen (eg, 650-1000 mg) and an antihistamine such as diphenhydramine (50-100 mg) was administered 30 to 60 minutes prior to starting each infusion of obinutuzumab. For the first 3 infusions of obinutuzumab, 100 mg of IV prednisolone (or equivalent) was administered as premedication. Following this, corticosteroid use was at the discretion of the treating physician. Obinutuzumab was administered by IV infusion as an absolute (flat) dose of 1000 mg or 2000 mg, depending on the study arm. Three doses of obinutuzumab were given during cycle 1, 1 each on days 1, 8, and 15. Splitting the first dose of obinutuzumab in cycle 1 was mandatory. For the 1000-mg arm, the first dose was split over 2 consecutive days (100 mg on day 1 and 900 mg on day 2). For the 2000-mg arm, the first dose could be split over 3 consecutive days (100 mg on day 1, 900 mg on day 2, and 1000 mg on day 3). Alternatively, for the 2000-mg arm, if the patient tolerated 100 mg on day 1, then the rest of the dose (1900 mg) could be administered on day 2. The day 8 and day 15 doses could each be administered over 1 day (ie, not split). Thereafter, obinutuzumab was given on day 1 of each subsequent 21-day cycle, for up to 8 cycles.
Assessment of toxicity and response
The National Cancer Institute (NCI) Common Terminology Criteria (CTC) for Adverse Events (version 4.0) were used to define and grade toxicity associated with therapy. Infusion-related reactions (IRRs) were defined as any study treatment–related adverse event that occurred during or within 24 hours from the end of infusion. Patients were assessed initially for clinical response after each cycle with laboratory studies and physical exam. CT was performed at baseline and 2 months after the end of treatment. Response assessment was evaluated using the 2008 IWCLL response criteria at the 2-month time period posttherapy.24 Thereafter, all patients were asked to return to the clinic every 3 months until patient discontinues or study ends.
PK studies
PK of obinutuzumab was assessed utilizing a validated enzyme-linked immunosorbent assay–based assay with a sensitivity of 0.004 μg/mL (PPD, Richmond, VA). Samples were obtained before and after the first dose of obinutuzumab (predose on day 1 and postdose on day 2 or day 3); 72 (± 48) hours after first dose; before and after the day-8 infusion; and before the day-15 infusion during cycle 1. Additional samples were obtained before and after the infusion on day 1 of cycles 2, 4, and 8; days 5 (±1), 8 (±1), and 12 (±1) of cycle 8; and then every 3 months for 1 year following the completion of therapy. PK data collected were compared with population PK model predictions, using simulation methods with the software NONMEM. The population PK model of obinutuzumab, using nonlinear mixed-effects modeling (with software NONMEM), was developed from a large Roche database composed of 590 patients (254 patients with CLL and 336 patients with non-Hodgkin lymphoma [NHL]) from 4 clinical studies.25
Statistical considerations
Sample size considerations were based on ORR at the end of treatment. Assuming an ORR of 50% in all patients, the 95% confidence interval (CI) was projected to be 0.39 to 0.61. The sample size was calculated in an exploratory manner based on a difference of 20% in response rates between the treatment dose arms, and assuming that the true observed ORR for the low-dose arm is ∼41%, a sample size of 40 patients per treatment would ensure ∼83% power to detect a 20% difference, using a 1-sided test with a significance level of .2. The end of treatment response assessment took place ∼2 months and not earlier than 51 days (56 days ± 5-day window) after the last study treatment. The primary analysis was based on the intent-to-treat population. ORR was summarized for each of the 2 treatment dose arms with corresponding 95% CI using the Pearson-Clopper method. In addition, as supportive analyses, ORRs between the treatment dose arms were evaluated using the Cochran-Mantel-Haenszel (CMH) test adjusted for stratification factors applied at randomization: (1) tumor burden at baseline (high or low) and (2) Rai stage at baseline (study entry; I/II or III/IV). The results of the CMH analysis were presented as odds ratios and corresponding 95% CI. Further, the effect of the stratification/prognostic factors was to be assessed in an exploratory analysis using logistic regression. Patients with missing, early (before 51 days), or no response assessments were classified as nonresponders. Duration of response (DOR) was assessed from the time of the first CR, CR with incomplete marrow recovery (CRi), or PR to the time of disease progression, relapse, or death from any cause. PFS was measured from the time of randomization to disease progression, relapse, or death from any cause. If a patient had not experienced progressive disease (PD) or death, DOR and PFS were censored on the day of the last tumor assessment. OS was assessed from the time from randomization to death from any cause. If a patient was still being followed or had been lost to follow-up at the time of the analysis, OS was censored at this patient’s last known alive date. The Kaplan-Meier approach was used to estimate the time patients live event free for the following time-to-event end points: DOR, PFS, and OS. Median PFS (if available) rates, together with 95% CIs, were estimated using the Kaplan-Meier survival methodology. PFS rates at 6 months and at 1 year following randomization were also provided, with 95% CIs. Cytogenetic abnormalities were displayed by chromosomal abnormality and the hierarchical model of Dohner.26
Results
Patient characteristics
A total of 80 patients from 19 separate sites were randomized in a 1:1 ratio to receive either obinutuzumab 1000 mg or 2000 mg, with 41 patients in the 1000-mg arm and 39 patients in the 2000-mg arm. One patient in each treatment arm did not receive therapy with obinutuzumab. The demographics of patients enrolled in each arm are summarized in Table 1. The overall median age of patients enrolled on this study was 67 (34-91) years with 41% having advanced (Rai III/IV) stage disease.
Pretreatment demographics
| . | Obinutuzumab 1000 mg (n = 41) . | Obinutuzumab 2000 mg (n = 39) . | All patients (n = 80) . |
|---|---|---|---|
| Median age, y (range) | 67 (37-87) | 65 (38-91) | 67 (34-91) |
| Female, n (%) | 16 (39) | 13 (33) | 29 (36) |
| Rai stage III/IV, n (%) | 16 (39) | 17 (43) | 33 (41) |
| Binet C, n (%) | 14 (34) | 14 (36) | 28 (35) |
| ECOG performance status, n (%) | |||
| 0 | 18 (45) | 22 (58) | 40 (51) |
| 1 | 21 (53) | 14 (37) | 35 (45) |
| 2 | 1 (3) | 2 (5) | 3 (4) |
| Median white blood cell count, 109/L (range) | 58.90 (8.0-350.2) | 77.85 (7.4-319.7) | 68.75 (7.4-350.2) |
| Median platelet count, 109/L (range) | 130.5 (14-300) | 144.5 (59-294) | 137.5 (14-300) |
| Median hemoglobin, g/dL (range) | 12 (8.2-15.1) | 12.2 (7.8-15.7) | 12.1 (7.8-15.7) |
| Splenomegaly, n (%) | 5 (18) | 4 (15) | 9 (17) |
| Cytogenetics, n (%) | n = 41 | n = 37 | n = 78 |
| 13q− | 12 (29) | 16 (43) | 28 (36) |
| Trisomy 12 | 7 (17) | 7 (19) | 14 (18) |
| 11q− | 7 (17) | 2 (5) | 9 (12) |
| 17p− | 4 (10) | 4 (11) | 8 (10) |
| No abnormality | 11 (27) | 8 (22) | 19 (24) |
| IGHV unmutated, n (%) | n = 38 | n = 38 | n = 76 |
| Mutated, n (%) | 11 (29) | 15 (40) | 26 (34) |
| Unmutated, n (%) | 22 (58) | 19 (50) | 41 (54) |
| Not done, n (%) | 5 (13) | 4 (10) | 9 (12) |
| CD38 expression ≥30%, n (%) | 25/35 (71) | 20/36 (56) | 45/71 (68) |
| β2-Microglobulin ≥3.5 mg/L, n (%) | 26/35 (74) | 15/25 (60) | 41/60 (68) |
| Serum thymidine kinase ≥140 DU/L, n (%) | 15/21 (71) | 13/21 (62) | 28/42 (67) |
| . | Obinutuzumab 1000 mg (n = 41) . | Obinutuzumab 2000 mg (n = 39) . | All patients (n = 80) . |
|---|---|---|---|
| Median age, y (range) | 67 (37-87) | 65 (38-91) | 67 (34-91) |
| Female, n (%) | 16 (39) | 13 (33) | 29 (36) |
| Rai stage III/IV, n (%) | 16 (39) | 17 (43) | 33 (41) |
| Binet C, n (%) | 14 (34) | 14 (36) | 28 (35) |
| ECOG performance status, n (%) | |||
| 0 | 18 (45) | 22 (58) | 40 (51) |
| 1 | 21 (53) | 14 (37) | 35 (45) |
| 2 | 1 (3) | 2 (5) | 3 (4) |
| Median white blood cell count, 109/L (range) | 58.90 (8.0-350.2) | 77.85 (7.4-319.7) | 68.75 (7.4-350.2) |
| Median platelet count, 109/L (range) | 130.5 (14-300) | 144.5 (59-294) | 137.5 (14-300) |
| Median hemoglobin, g/dL (range) | 12 (8.2-15.1) | 12.2 (7.8-15.7) | 12.1 (7.8-15.7) |
| Splenomegaly, n (%) | 5 (18) | 4 (15) | 9 (17) |
| Cytogenetics, n (%) | n = 41 | n = 37 | n = 78 |
| 13q− | 12 (29) | 16 (43) | 28 (36) |
| Trisomy 12 | 7 (17) | 7 (19) | 14 (18) |
| 11q− | 7 (17) | 2 (5) | 9 (12) |
| 17p− | 4 (10) | 4 (11) | 8 (10) |
| No abnormality | 11 (27) | 8 (22) | 19 (24) |
| IGHV unmutated, n (%) | n = 38 | n = 38 | n = 76 |
| Mutated, n (%) | 11 (29) | 15 (40) | 26 (34) |
| Unmutated, n (%) | 22 (58) | 19 (50) | 41 (54) |
| Not done, n (%) | 5 (13) | 4 (10) | 9 (12) |
| CD38 expression ≥30%, n (%) | 25/35 (71) | 20/36 (56) | 45/71 (68) |
| β2-Microglobulin ≥3.5 mg/L, n (%) | 26/35 (74) | 15/25 (60) | 41/60 (68) |
| Serum thymidine kinase ≥140 DU/L, n (%) | 15/21 (71) | 13/21 (62) | 28/42 (67) |
ECOG, Eastern Cooperative Oncology Group.
Treatment administration and adverse events
A total of 97.4% of patients received 90% or more of the planned study treatment day-1 dose in cycle 1. Of the patients who reached cycle 2 and beyond, 100% received ≥90% of the planned study treatment. The median total cumulative dose of obinutuzumab in each treatment arm was 10 000 mg in the 1000-mg arm (range: 40-10 292 mg) and 20 000 mg in the 2000-mg arm (range: 100-20 045 mg). The median treatment duration was the same in the 2 treatment arms together with the mean dose intensity received.
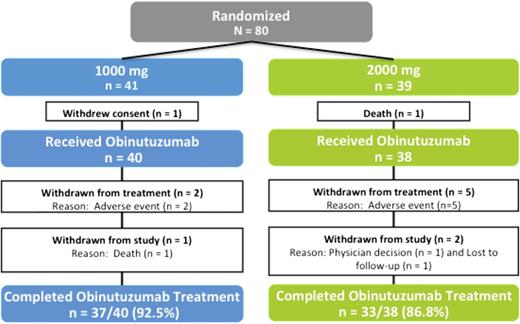
Safety analyses were based on the safety evaluable population, which comprised all patients who were randomized and received any amount of obinutuzumab: 40 patients in the 1000-mg arm and 38 patients in 2000-mg arm, respectively (Figure 1). Two patients did not receive any study drug; 1 patient in the 1000-mg arm withdrew consent because of fever, which began 3 days after randomization, and another patient in the 2000-mg arm experienced fluid overload as a result of preinfusion prophylactic hydration and died as a result of cardiac failure 7 days after randomization without receiving obinutuzumab.
Four deaths occurred on study. In the 1000-mg arm, 1 death occurred on study because of myocardial infarction (1000-mg treatment arm) in a patient who received 5 treatment cycles (1 of 40 patients, 2.5%), which the investigator considered unrelated to study treatment, and 2 deaths in del(17)(p.13.1) patients were because of disease progression after completion of treatment. In the 2000-mg arm, a patient with a history of chronic obstructive pulmonary disease died on study day 712 of emphysema.
The incidence of NCI CTC grade 3 to 5 adverse events was similar between arms (55.0% in the 1000-mg arm vs 65% in the 2000-mg arm). Adverse events led to withdrawal of study medication in 5 patients randomized to the 2000-mg arm (13%) for 1 case of serious grade 4 acute coronary syndrome following treatment on day 1, cycle 1; 2 cases of neutropenia (grade 3 on day 3 and grade 4 on day 100); and 2 cases of thrombocytopenia (grade 3 on day 20 and grade 4 on day 78). In contrast, withdrawal of study medication occurred only twice in the 1000-mg arm (5%) for 1 case of grade 3 IRR, hypotension, hypoxia, and decreased consciousness (believed secondary to sedation medications) on day 1 of therapy and a case of grade 3 thrombocytopenia on day 139.
Table 2 summarizes the toxicity observed in this treatment trial. Selected toxicity including infusion events and cytopenias (early and late) are summarized subsequently.
Reported adverse events with frequency of 20% or greater
| Patients, n (%) . | Obinutuzumab 1000 mg (n = 40) . | Obinutuzumab 2000 mg (n = 38) . | ||
|---|---|---|---|---|
| All grades, n (%) . | Grade 3-4, n (%) . | All grades, n (%) . | Grade 3-4, n (%) . | |
| IRR | 28 (70) | 1 (2.5) | 24 (63.2) | 0 (0) |
| Pyrexia | 16 (40) | 0 | 18 (47.4) | 1 (2.6) |
| Fatigue | 15 (37.5) | 1 (2.5) | 12 (31.6) | 2 (5.3) |
| Chills | 9 (22.5) | 1 (2.5) | 7 (18.4) | 0 |
| Nausea | 15 (37.5) | 0 | 10 (26.3) | 0 |
| Vomiting | 11 (27.5) | 1 (2.5) | 5 (13.5) | 0 |
| Dizziness | 10 (25) | 0 | 7 (18.4) | 0 |
| Headache | 11 (27.5) | 0 | 4 (10.5) | 0 |
| Neutropenia | 15 (37.5) | 12 (30) | 12 (31.6) | 12 (31.6) |
| Thrombocytopenia | 10 (25) | 6 (15) | 6 (15.8) | 4 (10.5) |
| Dyspnea | 6 (15) | 0 | 10 (26.3) | 1 (2.6) |
| Cough | 5 (12.5) | 0 | 8 (21.1) | 0 |
| Flushing | 8 (20) | 0 | 9 (23.7) | 0 |
| Patients, n (%) . | Obinutuzumab 1000 mg (n = 40) . | Obinutuzumab 2000 mg (n = 38) . | ||
|---|---|---|---|---|
| All grades, n (%) . | Grade 3-4, n (%) . | All grades, n (%) . | Grade 3-4, n (%) . | |
| IRR | 28 (70) | 1 (2.5) | 24 (63.2) | 0 (0) |
| Pyrexia | 16 (40) | 0 | 18 (47.4) | 1 (2.6) |
| Fatigue | 15 (37.5) | 1 (2.5) | 12 (31.6) | 2 (5.3) |
| Chills | 9 (22.5) | 1 (2.5) | 7 (18.4) | 0 |
| Nausea | 15 (37.5) | 0 | 10 (26.3) | 0 |
| Vomiting | 11 (27.5) | 1 (2.5) | 5 (13.5) | 0 |
| Dizziness | 10 (25) | 0 | 7 (18.4) | 0 |
| Headache | 11 (27.5) | 0 | 4 (10.5) | 0 |
| Neutropenia | 15 (37.5) | 12 (30) | 12 (31.6) | 12 (31.6) |
| Thrombocytopenia | 10 (25) | 6 (15) | 6 (15.8) | 4 (10.5) |
| Dyspnea | 6 (15) | 0 | 10 (26.3) | 1 (2.6) |
| Cough | 5 (12.5) | 0 | 8 (21.1) | 0 |
| Flushing | 8 (20) | 0 | 9 (23.7) | 0 |
Reported adverse events are by MedDRA (Medical Dictionary for Regulatory Activities) preferred terms.
Infusion-related events with obinutuzumab generally started early during infusion and were similar between the 1000-mg and 2000-mg treatment arms (95.0% and 89.5%), of which the majority (75.6%) were of NCI CTC grade 1/2. No grade 5 events were observed, and 8 serious infusion events were reported in 5 patients with 2 patients being withdrawn from the study treatment. These infusion events typically occurred very early with the initial treatment and only infrequently occurred with subsequent administrations. Only 2 patients had elevated liver function tests during the first month of therapy, which was reversible and did not necessitate discontinuation of therapy.
Cytopenias occurred but in general were manageable. The incidence of neutropenia (all grades) during therapy was not statistically higher in the 1000-mg treatment arm (42.5% vs 34.2% in the 2000-mg arm), although the study was not designed to test this. A total of 3 patients (10.0%) were withdrawn from study drug because of neutropenia, including 1 patient who experienced febrile neutropenia. Fourteen of 40 (35%) patients in the 1000-mg arm and 13 of 38 (34.2%) patients received colony-stimulating factor as a concomitant medication. Late-onset neutropenia, defined as occurring more than 24 days posttherapy in a patient with pretreatment normal neutrophil count was observed in 1/37 (2.7%) patients in the 1000-mg arm and 3/33 (9.1%) patients in the 2000-mg arm. Grade 3/4 infection occurred in 1 patient in the 1000-mg arm and 2 patients in the 2000-mg arm. No grade 5 thrombocytopenia events were observed; 1 serious thrombocytopenia event was reported, and 3 were withdrawn from the study drug because of thrombocytopenia. Three patients experienced tumor lysis syndrome in the first cycle; all cases resolved. There were no obvious dose-IRR/cytopenia/infection relationships observed.
Efficacy data
The primary analysis (ORR at end of treatment) was conducted in April 2013 (median follow-up time was 10.7 months) when all patients had reached the end of treatment assessment. Safety and PFS data were updated in February 2014 (median follow-up time was 20.3 months).
The response data for both treatment arms are summarized in Table 3. A total of 20/41 (49%) patients in the 1000-mg treatment arm responded with a PR, Cri, or CR, whereas 26/39 (67%) patients in the 2000-mg treatment arm responded (2-sided P = .08). Furthermore, the CR or CRi rate was 5% on the 1000-mg treatment arm, whereas it was 20% on the 2000-mg treatment arm (P < .05). At the primary analysis, PD occurred for 3 (7%) patients on the 1000-mg treatment arm. Two of these 3 patients on the 1000-mg arm were assessed to have SD by the investigator at 50 and 49 days after last dose of obinutuzumab and had a subsequent response assessment with evidence of disease progression at 91 and 98 days after last dose of obinutuzumab, respectively. Two patients had del(17)(p13.1). No patient progressed while on the 2000-mg treatment arm prior to completion of therapy.
ORR at the end of treatment
| Patients, n (%) . | Obinutuzumab 1000 mg (n = 41) . | Obinutuzumab 2000 mg (n = 39) . |
|---|---|---|
| ORR*(CR + CRi + PR) | 20 (49) | 26 (67) |
| P | 0.08† | |
| CR | 2 (5) | 6 (15) |
| CRi | 0 | 2 (5) |
| PR | 18 (44) | 18 (46) |
| SD | 11 (27) | 9 (23) |
| PD | 3‡ (7) | 0 |
| Not evaluable/missing§|| | 7 (17) | 4 (10) |
| Patients, n (%) . | Obinutuzumab 1000 mg (n = 41) . | Obinutuzumab 2000 mg (n = 39) . |
|---|---|---|
| ORR*(CR + CRi + PR) | 20 (49) | 26 (67) |
| P | 0.08† | |
| CR | 2 (5) | 6 (15) |
| CRi | 0 | 2 (5) |
| PR | 18 (44) | 18 (46) |
| SD | 11 (27) | 9 (23) |
| PD | 3‡ (7) | 0 |
| Not evaluable/missing§|| | 7 (17) | 4 (10) |
SD, stable disease.
ORR based on investigator-reported end-of-therapy response, which includes CT scan performed 51+ days from last obinutuzumab dose.
P value is 2-sided, based on stratified CMH test on ORR.
The 1000-mg arm: 2 of 3 patients were assessed as SD by the investigator at 50 and 49 days after last dose of obinutuzumab and had a subsequent response assessment with evidence of disease progression at 91 and 98 days after last dose of obinutuzumab, respectively.
Reasons for unevaluable patients in 1000-mg arm: no study treatment received (n = 1), death (n = 1), adverse event leading to discontinuation (n = 1), and concurrent metastatic squamous cell carcinoma (n = 1). In the 2000-mg arm: no study treatment received (n = 1) and adverse event leading to discontinuation (n = 1).
Patients classified as missing if no postbaseline response assessments were available or all postbaseline response assessments were performed <51 days from last obinutuzumab dose (1000-mg arm, n = 3; 2000-mg arm, n = 2).
For the majority of patients with missing data in both treatment arms, reasons were because of either a response assessment date outside the prescribed 56-day ± 5-day time window (1000-mg arm, 3/7 patients; 2000-mg arm, 2/4 patients) or no end of treatment response because of patient withdrawal before end of treatment (1000-mg arm, 4/7 patients; 2000-mg arm, 2/4 patients). Prognostic factors of age, sex, Rai stage, tumor burden, and β2-microglobulin (reference: <3.5 mg/L) had no influence on response. There were 4 of 41 (9.8%) patients in the 1000-mg arm and 4/37 (10.8%) patients in the 2000-mg arm who were considered high-risk CLL patients because they had a del(17)(p13.1). Among these patients, none in the 1000-mg arm (0/4 patients) and all in the 2000-mg arm (4/4 patients) achieved an objective PR. Also, 3/8 patients with del(17p) deletion had additional abnormalities, including 1 patient each who had 11q deletion, 13q deletion, and trisomy 12. Three of 8 patients had progressed.
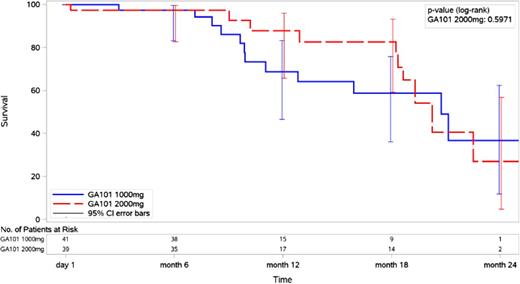
Results of the secondary end point PFS by treatment arm are shown in Figure 2. At a median follow-up of 20.3 months, a total of 23 patients experienced a PFS event: 13 patients in the 1000-mg arm (1 death by 6 months and 12 disease progressions by 22 months) and 10 patients in the 2000-mg arm (1 death at study start and 9 disease progression by 23 months). Although the 18-month PFS was 59% (95% CI, 39% to 79%) in the 1000-mg arm and 83% (95% CI, 67% to 99%) in the 2000-mg arm, the data show no difference in PFS rates because the curves merge after month 18 and the CIs overlap (Figure 2). Further follow-up will be required to determine if dose-response translates into improved PFS. The median OS for both treatment arms was not reached at the time of these analyses.
PFS following treatment with obinutuzumab on 1000-mg or 2000-mg treatment arm.
PK
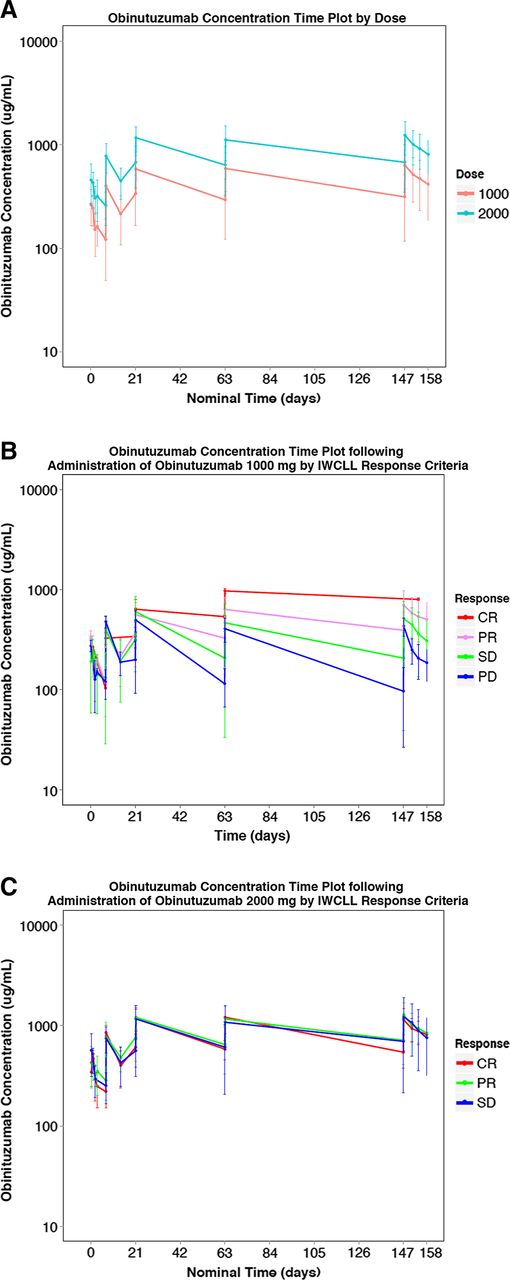
Of 78 patients who received obinutuzumab, 77 (40 in the 1000-mg arm and 37 in the 2000-mg arm) were included in the PK analysis. A total of 1028 PK samples were collected. Noncompartmental analysis was also performed at cycle 8 on all patients, and the observed PK parameters are summarized by dose cohort in Table 4 (with software Phoenix WinNonlin 6.2). A population PK model of obinutuzumab using nonlinear mixed-effect modeling (with software NONMEM) was developed previously based on a large database composed of 590 patients (254 with CLL and 336 with NHL) from a total of 4 phase 1, 2, and 3 studies with obinutuzumab. This model comprises a 2-compartment model with 2 pathways of obinutuzumab clearance, a linear clearance pathway that corresponds to immunoglobulin G catabolism, and a nonlinear time-varying clearance pathway, which is high at the start of treatment but reduces over time as treatment continues. This pathway is consistent with target-mediated drug disposition such that at the start of treatment, when CD20 levels are highest, the clearance of obinutuzumab is highest; however, as repeated administrations are given, the CD20 target becomes saturated, and the impact of the clearance pathway diminishes. The patients in this phase 2 study (GAGE, GAO4768g) were similar to the patients used in the population PK analysis, and consequently, no difference in their PK characteristics was expected. The population PK model was used to simulate the PK profile of obinutuzumab in the current study. Results of this showed that the observed PK data were in full agreement with the previously established PK characteristics of obinutuzumab in patients with CLL. Plasma concentration profiles demonstrate a distinct difference between the 1000- and 2000-mg doses (Figure 3A). Exploration of these data with respect to response reveals an exposure response relationship at 1000 mg: patients with the lowest concentrations had PD, whereas patients with the highest obinutuzumab concentrations had CR (Figure 3B). This relationship disappeared at the 2000-mg dose level, and all patients had similar serum concentrations irrespective of their response (Figure 3C).
PK parameters summarized by dose cohort
| Dose (mg) . | Serum obinutuzumab on cycle 8 (geometric mean, % CV) . | ||||
|---|---|---|---|---|---|
| Cmax (μg/mL) . | AUC (d × μg/mL) . | CLss (mL/d) . | Vss (L) . | t1/2 (d) . | |
| 1000 mg (n = 37) | 600 (45.6) | 8230 (58.3) | 121 (58.3) | 7.08 (73.2) | 30.6 (87.1) |
| 2000 mg (n = 33) | 1190 (34.9) | 16 500 (50.3) | 122 (50.3) | 6.68 (74.7) | 26.3 (80.1) |
| Dose (mg) . | Serum obinutuzumab on cycle 8 (geometric mean, % CV) . | ||||
|---|---|---|---|---|---|
| Cmax (μg/mL) . | AUC (d × μg/mL) . | CLss (mL/d) . | Vss (L) . | t1/2 (d) . | |
| 1000 mg (n = 37) | 600 (45.6) | 8230 (58.3) | 121 (58.3) | 7.08 (73.2) | 30.6 (87.1) |
| 2000 mg (n = 33) | 1190 (34.9) | 16 500 (50.3) | 122 (50.3) | 6.68 (74.7) | 26.3 (80.1) |
Serum obinutuzumab samples were analyzed using a validated enzyme-linked immunosorbent assay. At cycle 7, Ctrough (geometric mean, % CV) was 241 μg/mL (62.8) in the 1000-mg arm and 566 μg/mL (48.3) in the 2000-mg arm. A population PK model of obinutuzumab using nonlinear mixed-effect modeling (with software NONMEM) was developed based on a large database composed of 590 patients (254 with CLL and 36 with NHL). In addition to the noncompartmental analysis, the PK analysis made use of the population PK model to simulate patient PK profiles for comparison with the observed study data. The population PK model accurately predicted the patient PK profiles for those who received both 1000 mg and 2000 mg of obinutuzumab.
AUC, area under the curve; CV, coefficient of variation.
PK plots of obinutuzumab. (A) Obinutuzumab concentration vs time plot by dose (1000 mg or 2000 mg). (B) Obinutuzumab concentration time plot following administration of obinutuzumab 1000 mg by IWCLL response. (C) Obinutuzumab concentration time plot following administration of obinutuzumab 2000 mg by IWCLL response.
PK plots of obinutuzumab. (A) Obinutuzumab concentration vs time plot by dose (1000 mg or 2000 mg). (B) Obinutuzumab concentration time plot following administration of obinutuzumab 1000 mg by IWCLL response. (C) Obinutuzumab concentration time plot following administration of obinutuzumab 2000 mg by IWCLL response.
Discussion
We describe an 80-patient, randomized phase 2 study examining the efficacy of obinutuzumab monotherapy at 2 different doses (1000 mg vs 2000 mg) in symptomatic, previously untreated CLL patients. The study was set up to explore whether dose response to therapy exists with obinutuzumab as has been previously demonstrated with nonengineered anti-CD20 antibodies rituximab and ofatumumab. Additionally, although the 30% best ORR in relapsed CLL was improved,22 the ORR, PFS, and OS seen in CLL11 suggest that significant activity might exist when obinutuzumab is administered earlier in treatment.22,23 Our data demonstrate that obinutuzumab produces a higher response rate in symptomatic, previously untreated patients than previously observed in relapsed patients. More importantly, the study results suggest some benefit of 2000-mg over 1000-mg monotherapy in end-of-treatment responses (ORR). These data establish that obinutuzumab in both dose strengths, 1000-mg and 2000-mg monotherapy, is safe and has significant clinical activity in symptomatic, previously untreated CLL.
Currently, obinutuzumab is approved for use in previously untreated CLL patients inappropriate for aggressive chemoimmunotherapy in combination with chlorambucil. The ORR, CR rate, and 18-month PFS in the 2000-mg obinutuzumab monotherapy arm very closely approximates that observed with the combination of obinutuzumab + chlorambucil.23 In contrast, the 1000-mg dose cohort of obinutuzumab monotherapy had a lower response and shorter 18-month PFS than observed with the previously reported combination of obinutuzumab + chlorambucil.23 Thus, these results suggest some benefit to the addition of chlorambucil for cytoreduction using the 1000-mg dose of obinutuzumab. This is also consistent with the population PK analysis of obinutuzumab, which indicates a prominent target-mediated component that must be overcome to attain high serum concentrations. The addition of chlorambucil is likely to assist in this, and in the absence of other cytoreductive therapeutic agents, a higher dose of obinutuzumab may be required. Examination of obinutuzumab efficacy in high-risk genomic subtypes such as del(17)(p13.1) in this study was not possible because of the low frequency of patients included with this aberration. Importantly, this study looks at obinutuzumab monotherapy and does not address dose escalation with more effective cytoreductive chemotherapy or newer targeted therapies such as ibrutinib27 or idelalisib.28 Although dose escalation of rituximab in combination with FC did not offer a therapeutic advantage from historical controls in 1 single-center study,11 obintuzumab mediates cell death through both antibody-dependent cellular cytotoxicity and direct killing, the later mechanism which could be influenced by dose. Thus, further exploration of higher doses of obinutuzumab as part of combination therapy with chemotherapy or new targeted therapies should be considered. Additionally, given that PK of obinutuzumab change with tumor cytoreduction, consideration of earlier use of higher doses of obinutuzumab followed by reduced dosing when significant tumor elimination has occurred might also be pursued. These data support a dose effect of obinutuzumab with increasing trend toward higher response at the 2000-mg dose; however, conducting a larger study to definitively address this will be required because the relevance to improving PFS is uncertain. Such analysis would require a cost assessment analysis and also consideration of the impact of new therapies such as the B-cell receptor signaling agents that have been approved for this disease. These data from our study justify such a study in the future once additional long-term follow-up emerges from both this study and also others with the B-cell receptor signaling inhibitor agents.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this study and their families who supported them. Also, we thank the GAGE study management team (Roche/Genentech) and Mijanur Rahman for statistical programming support.
J.C.B. was supported by a grant from the National Institutes of Health National Cancer Institute (P01 CA95426), the D. Warren Brown Foundation, and the Four Winds Foundation.
Authorship
Contribution: J.C.B. was the national principal investigator of the study, wrote the first draft of the manuscript, handled the modifications required during peer review, and as corresponding author had full access to all study data and takes responsibility for the accuracy of data in the manuscript; J.M.F. was the lead investigator at The Ohio State University, reviewed drafts of the manuscript, and approved the final version; T.J.K., M.B., K.S.K., and J.P.S. enrolled patients in the study, reviewed drafts, and approved the final version of the paper; D.J.C. performed the pharmacoanalysis of obinutuzumab; G.F.-R. was the Roche Global Development Team Leader who reviewed drafts of the manuscript and approved the final version of the manuscript; N.T. was the statistician who designed and analyzed the data in this study; and J.H. was the Genentech study medical monitor and participated in overseeing the study nationally, working with J.C.B. to assess response, analyze the results, review versions of the manuscript, and approve the final version of the manuscript.
Conflict-of-interest disclosure: T.J.K. has served as a consultant for, and received honoraria and research funding from, F. Hoffmann-La Roche. M.B. has received honoraria from Celgene, Janssen, and Incyte. K.S.K. and J.P.S. have served as consultants for Gilead and received research funding from Genentech Inc., Gilead, TG Therapeutics, and Pharmacyclics. D.J.C., G.F.-R., and N.T. are employees of F. Hoffmann-La Roche. J.H. is an employee of Genentech Inc. D.J.C., G.F.-R., N.T., and J.H. own stock in F. Hoffmann-La Roche. J.P.S. has served as a consultant for Gilead, Pharmacyclics, and Celgene; has received research funding from Gilead, Celgene, Seattle Genetics, Foundation Medicine, Novartis, Pharmacyclics, and Janssen; and has received honoraria from Gilead and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: John C. Byrd, Ohio State University Comprehensive Cancer Center Building, Room 455B, 410 West 12th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu.
References
Author notes
J.C.B. and J.M.F. contributed equally to this study.