Abstract
Postremission therapy in patients with acute myeloid leukemia (AML) may consist of continuing chemotherapy or transplantation using either autologous or allogeneic stem cells. Patients with favorable subtypes of AML generally receive chemotherapeutic consolidation, although recent studies have also suggested favorable outcome after hematopoietic stem cell transplantation (HSCT). Although allogeneic HSCT (alloHSCT) is considered the preferred type of postremission therapy in poor- and very-poor-risk AML, the place of alloHSCT in intermediate-risk AML is being debated, and autologous HSCT is considered a valuable alternative that may be preferred in patients without minimal residual disease after induction chemotherapy. Here, we review postremission transplantation strategies using either autologous or allogeneic stem cells. Recent developments in the field of alternative donors, including cord blood and haploidentical donors, are highlighted, and we discuss reduced-intensity alloHSCT in older AML recipients who represent the predominant category of patients with AML who have a high risk of relapse in first remission.
Introduction
Although the majority of patients with acute myeloid leukemia (AML) enter remission upon induction chemotherapy, the risk of relapse is considerable. That risk varies greatly according to age and genetic subtype as major denominators.1-3 Taking an increasingly detailed risk evaluation into account, the intensity and type of postremission therapy is generally tailored according to risk profile, whereby chemotherapeutic consolidation is favored in good-risk AML and allogeneic hematopoietic stem cell transplantation (alloHSCT) is favored in poor-risk AML.1,4,5 These approaches are currently weighed and debated in intermediate-risk AML, and interest in autologous HSCT (autoHSCT) has recently been revived.6
The last decade has witnessed an enormous increase in knowledge of the pathogenesis of AML. Genetic characterization has yielded important prognostic information, which has necessitated taking the most important mutational aberrations into account for therapeutic decision making.1,7 Furthermore, translational research has now established AML as a multiclonal disease that develops stepwise according a Darwinian model of leukemogenesis.8 The latter insight has important consequences for therapy, because it became unlikely that targeted monotherapies aimed at a single AML oncogene could match the efficacy of the tyrosine kinase inhibitors in monoclonal malignancies (eg, chronic myeloid leukemia). Indeed, current therapeutic developments aim to incorporate targeted therapy into established intensive chemotherapeutic schedules.9,10 In addition, the advent of new treatment modalities such as chimeric antigen receptor T cells and various monoclonal antibodies has renewed interest in immunotherapeutic approaches for treatment of multiclonal leukemias such as AML and acute lymphoblastic leukemia.11,12 A particularly potent immunotherapeutic approach is the allogeneic graft-versus-leukemia (GVL) effect, which has been shown to exert its influence across all genomic subsets with similar relative potency.13 However, its inherent toxicity is increasingly being debated, which makes using transplantation protocols associated with an acceptable limited toxicity profile a greater necessity. Conversely, the urge to preserve and exploit the GVL effect is an important issue, especially in the poor-risk genetic subtypes of AML. Here, we address 3 important issues in the field of postremission therapy by alloHSCT or autoHSCT in first complete response (CR1) in patients with AML: What is the current place of reduced-intensity conditioning (RIC) and nonmyeloablative (NMA) alloHSCT in both older and younger patients with AML in CR1? Which type of alternative donor is currently preferred and what is the current status of haploidentical donor transplantation in AML? Is autoHSCT a valuable alternative for alloHSCT in intermediate-risk AML?
RIC alloHSCT in AML CR1 recipients
Following the observation that both organ toxicity and severe graft-versus-host disease (GVHD) are related to the intensity of the conditioning regimen,14 it was hypothesized that reducing the intensity may limit these complications, while still allowing a sufficient GVL effect.15,16 Nearly 20 years later, it is clear that the hypothesis has been proved by showing reduced non-relapse mortality (NRM) and persistent GVL effects.17 GVL effects after RIC appear to depend on several factors, including a stronger reduction of relapse in patients experiencing chronic GVHD (cGVHD) and higher relapse risk after more stringent T-cell depletion or T-cell inhibition.18-20 As the first and major consequence, the development of RIC alloHSCT has enabled the use of alloHSCT in older and medically infirm patients. It represents a major change because this population is at greater risk for complications and has the poorest prognosis.3,21 That poor prognosis is mainly related to the underlying mutational profile22 and ineligibility to qualify for and benefit from intensive chemotherapy.23 Early attempts have confirmed the feasibility of RIC alloHSCT in older recipients with AML,24,25 which has resulted in a worldwide increase in alloHSCT activity.26 Although recent reviews27-29 have highlighted the development of RIC alloHSCTs and weighed their efficacy, several questions remain.
Is there an optimal conditioning dose intensity that allows for a well-balanced antileukemic effect and limited toxicity?
By definition, myeloablative conditioning (MAC) regimens do not allow for autologous recovery and require stem cell support whereas NMA regimens do not require stem cell support. Regimens that do not fit these criteria can then be classified as RIC, whereby the dose of total body irradiation or the alkylating agent is usually reduced by at least 30% compared with an ablative regimen.30 The NMA conditioning regimen developed by Storb et al consists of low-dose total body irradiation and fludarabine, which effectively allows for engraftment and appears to be associated with limited early mortality.16 However, antileukemic activity may be compromised, notably in advanced or high-risk diseases in which higher rates of relapse have been observed.31 In a prospective trial not limited to AML, it was reported that such an NMA regimen is indeed associated with low NRM but at the expense of a somewhat higher relapse rate, eventually resulting in the same outcome as that in patients treated with more intensive conditioning.32 Other centers developed RIC regimens based on the combination of alkylating agents with a purine analog28,33-35 followed by attempts to increase antileukemic activity by increasing the dose of the alkylating drug.36 Overall, such RIC regimens were suggested to be associated with lower relapse rates compared with NMA regimens, but prospective randomized studies are lacking. Most retrospective comparisons are hampered by patient selection and also by incomplete genetic characterization of the underlying AML as the most important prognostic parameter. To address the question of whether an increased dose of busulfan as part of RIC may be associated with better leukemia-free survival, a French cooperative group has set out to prospectively compare 3 different dosages of busulfan when combined with fludarabine prior to HLA-identical sibling alloHSCT (NCT01985061). Meanwhile, the experience of the local team and the number of transplantations being performed annually also have an impact on results.37 Thus, it is very difficult to favor one regimen over many others at this time, although an NMA regimen may not be preferred in more advanced AML.
Do anti-T-cell antibodies in the conditioning regimen allow for more effective GVHD prophylaxis without compromising GVL?
To effectively prevent GVHD and especially cGVHD in the elderly, some teams have proposed adding additional immunosuppression that incorporates antithymocyte immunoglobulins (ATGs) or alemtuzumab into the conditioning regimen. Finke et al38 reported a lower incidence of acute GVHD (aGVHD) and cGVHD using ATG before myeloablative unrelated donor (UD) alloHSCT. However, a survival benefit could not be demonstrated in that study or in a meta-analysis of several prospective studies.39 No prospective comparative study evaluated these agents in RIC alloHSCT, and registry data (Center for International Blood and Marrow Transplant Research [CIBMTR]; European Society for Blood and Marrow Transplantation [EBMT]) have not precisely defined the place for ATG.40,41 However, in the context of RIC using an alkylating agent (busulfan) and fludarabine, an intermediate ATG dose (5 mg/kg total dose) was suggested to be associated with effective prevention of cGVHD while preserving GVL effects.42,43 In contrast, it was suggested that a higher dose of ATG blunted the GVL effect and resulted in a high rate of opportunistic infections as a result of more stringent T-cell depletion.44 Whether such a strategy applies to both matched unrelated donors and sibling donors is currently unknown. In a recent phase 2 study that included patients older than age 55 years conditioned with fludarabine, intravenous busulfan, and 2 days of ATG before sibling alloHSCT, an acceptable NRM of 9% and a favorable quality of life was reported, whereas relapse appeared to be limited.45 More stringent in vivo T-cell depletion may be achieved by using alemtuzumab, which has been used extensively in the United Kingdom. A recent study of AML patients age 35 to 60 years suggested a low NRM and very effective prevention of GVHD in those who received matched sibling transplants, but results in recipients of UD grafts were less favorable, which might be explained by a higher incidence of opportunistic infections.46 Altogether these studies suggested that GVHD may be controlled in a more effective way by incorporating ATG or alemtuzumab, but the comparative value of these approaches with conventional prevention of GVHD is still unclear because randomized studies are lacking. Finally, the strategy of posttransplant immunosuppression may be profoundly changed by the introduction of posttransplant high-dose cyclophosphamide (PT-HDCy), which was developed in haploidentical alloHSCT but is currently being explored after matched sibling alloHSCT.47
Age and comorbidities
Considering the significant impact of comorbidities, one may wonder whether age itself or whether age-associated comorbidities have a significant impact on risk for NRM. Several retrospective studies addressed that question, but an independent impact of age was often absent.45,48-51 Those studies suggested no definite age limit, but they noted that the assessment of comorbidities was an essential part of the workup for every patient for whom alloHSCT was considered.52 In addition, the use of a geriatric evaluation in patients older than age 60 to 65 years is also important. Recently, Muffly et al53 have shown that the combined evaluation of geriatric and comorbidity status has a discriminative impact on outcome. Younger patients (younger than age 60 years) would benefit from conditioning regimen with a more acceptable toxicity profile. Several meta-analyses of prospective trials that used a donor vs no-donor comparison have established that donor availability may be associated with a strong reduction of relapse, which is generally compromised by NRM in patients older than age 40 years.54,55 All patients who have received transplants in the latter studies received standard MAC, combining either full-dose total body irradiation and an alkylating agent or 2 alkylating agents at a myeloablative dosage. Because of increased NRM in patients older than age 40 years and the favorable experience in older patients with RIC alloHSCT, several cooperative groups introduced RIC alloHSCT for patients in the 40 to 60 years age category. Two recent retrospective studies suggested better outcome following transplantation in patients older than age 40 years compared with chemotherapy because of the reduced NRM and preserved GVL effects.46,56 These studies suggest that in the context of intensified and optimized induction chemotherapy, a RIC regimen prior to alloHSCT might offer an attractive approach in AML patients in CR1.
Recommendations for RIC alloHSCT in AML patients in CR1
Given the paucity of prospective randomized studies, one needs to be cautious about making firm recommendations. But it seems reasonable to state that RIC alloHSCT is an established type of postremission therapy in elderly patients with AML and may offer a strong antileukemic effect.1,2,57-59 However, the use of alloHSCT would need to be tailored according to risk for NRM and morbidity,51,60 whereby patients at higher risk for NRM might qualify for NMA conditioning or, alternatively, an autograft61 or chemotherapy.2 Weighing risk factors might be done as earlier suggested by the European Leukemia Net5 and as presented in Table 1. Risk factors and their individual or composite characteristics will evolve, but the need to discuss their impact on relapse and NRM continues. Although studies that evaluate alloHSCT by donor availability or by using transplantation as a time-dependent covariate provide the main basis for decision making,59,62 the opposite effects of reduced relapse and increased NRM limit the precise applicability of those studies for individual patients. Therefore, we recommend weighing these effects by taking into account their projected, personalized risks for an individual patient, according to the European Leukemia Net model presented in Table 1. The place of RIC alloHSCT in patients younger than age 60 years is less clear. A recent meta-analysis comparing RIC and MAC regimens did not reveal any significant differences in terms of overall survival (OS) or progression-free survival, although RIC recipients were older and had more advanced disease.63 The prospective randomized study by Bornhäuser et al64 yielded a similar picture, although the RIC regimen came close to myeloablation, and the study was prematurely closed. Meanwhile, results of the prospective randomized study comparing RIC and MAC that was performed in the United States (BMT CTN 0901) are eagerly awaited.
It seems fair to state that patients with poor-risk AML primarily qualify for alloHSCT, whereas patients at higher risk for NRM might be offered a RIC regimen before transplantation. The option of alloHSCT in patients with intermediate-risk AML would need to be weighed, with autoHSCT as an alternative option (discussed in “Autologous transplantation”). The subset of patients with very-poor-risk AML needs special consideration. Although the immunologic GVL effect is operational in patients with very-poor-risk AMLs,13 outcomes with alloHSCT are poor, and experimental approaches that explore ways to exploit the GVL effect in those patients are urgently needed. Such approaches include timing of transplantation, new combinations of conditioning after preceding chemotherapy, and development of new immunotherapeutic and chemotherapeutic approaches after transplantation.65,66 In that respect, it is especially encouraging to note that new immunotherapeutic approaches are being developed to be used either pre- or posttransplant.11,12,67 Overall, recommendations apply to transplants performed with matched sibling and well-matched UDs, because these donor types have been demonstrated to result in fairly similar outcomes.68 However, the last decade has witnessed an enormous development in alternative stem cell donors or stem cell sources, which are also currently used in RIC and NMA alloHSCT and therefore need to be discussed separately.
Alternative donor alloHSCT
Adult UDs and umbilical cord blood
The lack of potential stem cell donors has long been a major limitation for using alloHSCT. Fortunately, recent advances have greatly expanded the pools of potential adult volunteer donors and stem cell donors. First, international registries of adult stem cell donors have expanded to approximately 25 million donors. In addition, the use of high-resolution typing has allowed selection of UDs who are matched for HLA-A, -B, -C, -DRB1, and -DQB1 at the molecular level for an increasing percentage of patients.69 Results with respect to OS and disease-free survival with well-matched donors currently compare well with those obtained with sibling donors.70-74 Few clinical trials have prospectively addressed this question, but nearly 30 000 patients have been compared in different studies that overall show similar results.73 Second, after initial experience in pediatric patients, umbilical cord blood (UCB) transplantation was extended to adult patients. Although both MAC and RIC regimens are used,75 the RIC regimen developed by the transplantation team of the University of Minnesota combines strong immunosuppression, antileukemic activity, and an acceptable NRM.76,77 The incidence of graft failure, however, may still be higher in pediatric patients, and cell dose was identified as a pivotal parameter.78 These results initiated attempts to increase the cell dose, including double UCB transplantation79 and ex vivo expansion.80 Overall, when a minimal cell dose is used, survival appears to be similar to that in HSCT using adult UDs. Indeed, in a large retrospective study from the CIBMTR and EBMT with more than 1500 patients, UCB transplantation was associated with higher NRM but lower relapse and GVHD rates, resulting in similar leukemia-free survival.81 The results of that study and others established UCB transplantation as an alternative stem cell source for patients lacking an HLA-matched sibling donor. Results with double UCB transplantation suggested that the use of two cord blood (CB) donors may be associated with less graft failure and also a somewhat stronger GVL effect.82 With that background, a randomized trial was performed that compared one-unit vs two-unit UCB transplantation in young patients, including those with myeloid malignancies.83 All patients were to receive a minimum of 2.5 nucleated cells per kilogram. Survival rates were similar after one-unit and two-unit UCB transplantations. However, a one-unit CB transplantation appeared to be associated with a lower risk of GVHD, suggesting that a single unit may be preferred if the unit contains sufficient nucleated cells. Mature studies addressing the value of expanded UCB in AML patients are currently lacking.
Haploidentical family donors
More recently, T-cell repleted haploidentical transplantation has become a third player in the field. Interest in haploidentical HSCT started in the mid 1990s with the transplantation team of the University of Perugia.84 However, their sophisticated approach using a highly myeloablative regimen and extensive in vitro and ex vivo T-cell depletion appeared to be associated with impaired immune reconstitution, compromising both the GVL effect and anti-infectious immunity.85 More recently, T-cell repleted haploidentical HSCT was developed based on different immunosuppressive approaches varying from pretransplant ATG to PT-HDCy. Initial reports of T-cell repleted haploidentical HSCT showed high rates of engraftment, acceptable rates of severe aGVHD and NRM, and low incidences of severe cGVHD.86-88 Subsequently, many reports have confirmed these initial findings, although development varied worldwide.89-94 Collectively, the experience successfully challenged the earlier notion that haploidentical HSCT would generally be associated with a high NRM. Some cooperative groups or registries76,95-97 have already addressed the question of which alternative donor is to be preferred.98-100 Overall, these reports suggest similar results between alternative donor or stem cell sources (Table 2), but prospective studies are scarce. The US clinical trial network is conducting a prospective study comparing PT-HDCy haploidentical HSCT and CB transplantation. Because the preferred alternative donor is currently unknown, it is of utmost importance to include as many patients as possible in prospective studies. Finally, it should be noted that the number of studies specifically reporting on patients with AML is very limited (Table 2). However, Wang et al101 recently reported results from a prospective multicenter trial that compared matched sibling alloHSCT (n = 219) with haploidentical HSCT (n = 231) (Table 2). The results suggested that outcome after haploidentical alloHSCT may be comparable to survival after alloHSCT using a matched sibling donor for patients with AML in CR. However, more studies with long-term follow-up and molecular characterization of the underlying AML are needed to better define the place of each type of alternative donor and to develop more detailed guidelines. An increase in transplantation activity may be expected in the years to come, because alternative donors have now become available for nearly every individual patient in need of alloHSCT. However, a number of questions remain, precluding the precise formulation of guidelines, donor hierarchy, and preferred conditioning regimen.
Autologous transplantation
Although alloHSCT is the preferred type of postremission therapy in poor- and very-poor-risk AML, the place of alloHSCT in intermediate-risk AML is being debated, and chemotherapy or autoHSCT may also offer long-term survival, although the risk of relapse may be higher than that after alloHSCT. Chemotherapeutic postremission therapy seems to be favored in the United States, but several European collaborative groups continue to use autoHSCT, especially since the introduction of peripheral blood stem cells (PBSCs), which have been shown to improve hematologic recovery and thereby treatment-related morbidity and mortality.
Results with PBSCs
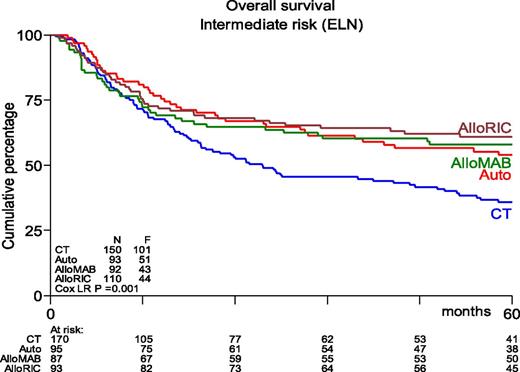
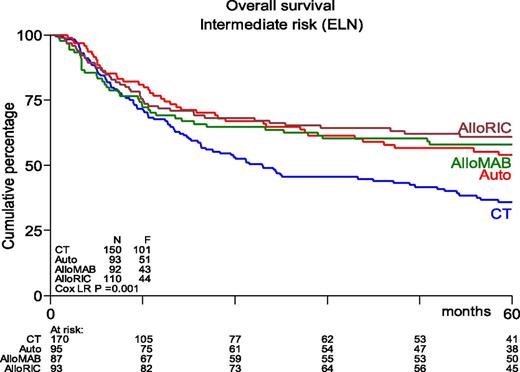
Many phase 2 and retrospective studies have been reported, but few prospective randomized studies are available. The study performed by the Dutch-Belgian Hemato-Oncology Cooperative Group/Swiss Group for Clinical Cancer Research (HOVON-SAKK) Cooperative Consortium included 517 patients who were randomly assigned between 1995 and 2006.102 With a mature follow-up of more than 5 years, the actual rates of relapse after chemotherapy vs after autoHSCT were 70% vs 58%, respectively (P = .02). The study showed a nonsignificant difference in relapse-free survival (RFS) of 29% vs 38% (P = .065). OS did not differ between the 2 groups and was estimated to be 41% vs 44%, respectively, at 5 years from randomization. Salvage treatment by alloHSCT was more frequently performed in relapsing chemotherapy recipients compared with autograft recipients (25% vs 17%). Of note, those percentages seem rather low given the current availability of alternative donors and the increased use of RIC. Therefore, the HOVON-SAKK Cooperative Consortium more recently re-analyzed the use of alloHSCT in relapsing autograft patients and showed that the rate of salvage alloHSCT had increased to approximately 30%.56 As a result, a retrospective comparison of patients age 40 to 60 years suggested better OS after autoHSCT in intermediate-risk patients as a result of improved salvage possibilities.56 Furthermore, outcome after alloHSCT or autoHSCT no longer differed in terms of OS, whereas alloHSCT after either MAC or RIC still appeared to be associated with better RFS (Figure 1). These results compare well to results from a retrospective study by the CIBMTR that suggested similar outcome for younger AML patients in CR1 receiving either alloHSCT from an HLA-identical sibling or an autograft using PBSCs.103 Although recipients of alloHSCT exhibited more high-risk features, had longer follow-up, and experienced a lower risk of treatment failure, no significant difference in OS was noted. The EBMT noted similar observations in older AML patients. Herr et al61 reported that RIC alloHSCT was similar to postremission therapy with autologous PBSC transplantation in terms of OS and RFS if it was used in CR1. Of note, a lower relapse rate of 36% vs 50% was counterbalanced by increased NRM in alloHSCT recipients. Moreover, autoHSCT may be used in patients up to age 70 years with an acceptable NRM of approximately 8%, which compares favorably to 17% as was observed after RIC alloHSCT.61 The conditioning regimen for autoHSCT was based on busulfan in 85% of patients. Although both oral and intravenous busulfan were used in that study,61 it has become clear that the intravenous administration of busulfan should be preferred because of fewer complications and higher reproducibility of levels that were aimed for.35 Given the higher NRM and nonrelapse toxicity (primarily GVHD and its consequences) associated with RIC alloHSCT, these results compel us to redefine the place of RIC alloHSCT and autoHSCT, especially in intermediate-risk AML patients in CR1 who harbor a risk of relapse that may vary between 40% and 50%.
Kaplan-Meier estimates of overall survival of AML intermediate-risk patients in CR1, age 40 to 60 years, by type of postremission therapy (updated results from Cornelissen et al53). HSCT recipients showed significantly better OS than patients receiving chemotherapeutic postremission therapy (P = .001). AlloMAB, myeloablative alloHSCT; AlloRIC, reduced intensity conditioning alloHSCT; Auto, autologous HSCT; CT, chemotherapy; ELN, European Leukemia Net. F, female; LR, logistic regression.
Kaplan-Meier estimates of overall survival of AML intermediate-risk patients in CR1, age 40 to 60 years, by type of postremission therapy (updated results from Cornelissen et al53). HSCT recipients showed significantly better OS than patients receiving chemotherapeutic postremission therapy (P = .001). AlloMAB, myeloablative alloHSCT; AlloRIC, reduced intensity conditioning alloHSCT; Auto, autologous HSCT; CT, chemotherapy; ELN, European Leukemia Net. F, female; LR, logistic regression.
Prognostic parameters
Several studies have suggested using dedicated risk scores for the application of autoHSCT. For example Pfirrmann et al104 developed a composite risk score based on age, CD34+ blast count, Flt3 mutant:wild-type ratio, cytogenetic risk, and secondary origin of AML. Three groups could be discriminated. AutoHSCT appeared to be associated with better survival compared with alloHSCT in their newly defined intermediate risk group. Although the principle of using a risk score in intermediate-risk patients is attractive, disadvantages include the poor reproducibility of parameters such as CD34 blast count and Flt3 mutant:wild-type ratio. In addition, the recent study by the HOVON-SAKK Cooperative Consortium could not reproduce the predictive power of the Pffirmann score in AML patients age 40 to 60 years in CR1 for whom all parameters were available.56 Alternatively, decision-making might benefit from taking minimal residual disease (MRD) into account. Following a 3- to 4-decade-long development, it is now well established that the assessment of MRD after induction and/or consolidation therapy in AML by either multiparameter flow cytometry or by quantitative polymerase chain reaction for specific molecular markers significantly predicts subsequent outcome.105-109 Moreover, MRD has been shown to predict outcome after different modes of postremission therapy such as continued chemotherapy, autoHSCT, or alloHSCT. As a consequence, several collaborative groups such as the Italian Gruppo Italiano Malattie Ematologiche dell’ Adulto (GIMEMA) and the HOVON-SAKK Cooperative Consortium have now incorporated MRD as a decisive parameter for choice of postremission therapy type in intermediate-risk AML patients upon achieving CR1 (Table 1; www.HOVON.nl), whereby MRD-negative patients may be consolidated by autoHSCT and MRD-positive patients may proceed to alloHSCT. The preferred type of postremission therapy in intermediate-risk AML is not definitely settled and continues to evolve. Currently, the choice may depend on multiple parameters, including the risk score of the underlying leukemia, the response to chemotherapy as assessed by morphology and MRD markers, and the projected risks associated with type of transplantation. However, alloHSCT is increasingly used selectively in poor- and very-poor-risk patients, whereas autoHSCT is generating new interest, especially in intermediate-risk patients who became MRD negative upon induction chemotherapy. Good-risk AML patients qualify for chemotherapeutic consolidation, but a recent report by Schlenk et al110 suggested favorable outcome for good-risk patients with autoHSCT, which provides a possible option in that category of patients. Importantly, new developments in the field of immunotherapy11,12,111 that will be evaluated in the context of either autoHSCT or alloHSCT are currently being pursued. Hopefully, they will yield better prospects for older patients with AML and help us to redefine the preferred type of postremission therapy and possibly also type of immunotherapeutic maintenance after transplantation.
Authorship
Contribution: J.J.C. and D.B. contributed equally to outlining this review, formulating questions, searching for and selecting literature, and writing and final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan J. Cornelissen, Erasmus MC Cancer Institute/Erasmus University Medical Center, Groene Hilledijk 301, Rotterdam, 3075 EA, The Netherlands; e-mail: j.cornelissen@erasmusmc.nl.