Abstract
Background- Introduction of tyrosine kinase inhibitors in the treatment of patients with Philadelphia (Ph) chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) has led to a significant improvement in the long term outcome of patients, in particular those who are unsuitable to undergo allogeneic stem cell transplant (alloSCT) in first complete remission (CR1).
Design- This multicenter, phase II, clinical trial was conducted in 37 centers across the US to determine whether the addition of dasatinib to chemotherapy followed by an alloSCT in the eligible patients would lead to a significant improvement in relapse-free survival. The study also examined the duration of CR in patients unable to receive an alloSCT and the potential value of detection of minimal residual disease (MRD) using quantitative PCR for BCR-ABL fusion transcripts at defined intervals. Patients ≥ 18 and ≤ 60 years of age with newly diagnosed Ph+ ALL with an ECOG performance status of 0-2 and adequate organ function were enrolled to receive 8 cycles of alternating hyperCVAD and high dose cytarabine and methotrexate chemotherapy in addition to dasatinib 100 mg daily for the first 14 days of each cycle. Later the protocol was amended and patients received dasatinib 70 mg daily continuously from cycle 2 onwards. Central nervous system prophylaxis was in the form of alternating doses of cytarabine and methotrexate administered intrathecally twice per cycle for 8 doses. Patients who had received one cycle of induction therapy before the Ph abnormality was identified were allowed to participate in the study. All patients with an available matched sibling or unrelated donor were encouraged to undergo an alloSCT in CR1. Others received maintenance therapy with continuous dasatinib 100 mg daily as well as monthly vincristine and prednisone for 2 years and followed by dasatinib indefinitely. Patients receiving alloSCT would receive dasatinib 100 mg daily starting from day 100 for up to 5 years, with dose adjustments as necessary.
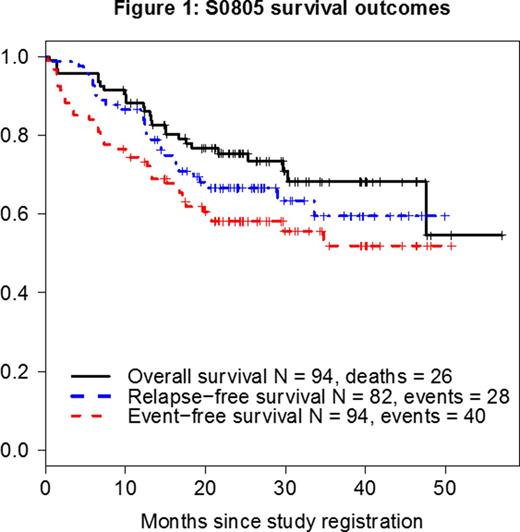
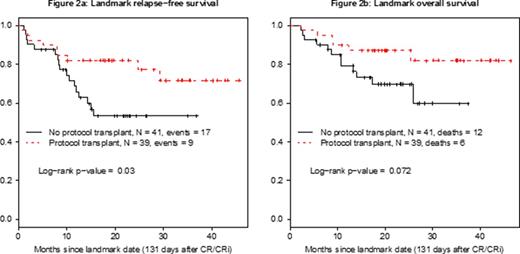
Results- The study has accrued 97 patients, 94 of whom were eligible and evaluable. Their median age is 44 years (range, 20-60 years) and median WBC at presentation 10 x 109/L (range, 1 - 410 x 109/L). Among 93 patients with available data, 82 (88%) achieved CR or CR with incomplete count recovery (CRi). 34 patients were enrolled having received prior therapy, 16 in CR/CRi, 7 with unknown response status (5 achieved CR on the study), and 11 with refractory disease after 1 course of prior therapy (9 achieved CR on the study). Among the 60 untreated patients, 51 achieved CR, 2 achieved CRi, 2 had unconfirmed CR, 2 died early, 2 were refractory and 1 had missing data. 41 patients underwent study-related alloSCT in CR1 including 35 with TBI-based regimens and 4 with busulfan-based regimens; 2 had missing data. 8 patients had non-prescribed alloSCT off study. Median follow-up among all patients known to be alive is 26 months (range 9 - 57 months). For the overall population, overall survival (OS), event-free survival (EFS), and relapse-free survival (RFS) at 12 months were 88%, 74%, and 85%, respectively (Figure 1). The 12-month RFS and OS after transplant were 71% and 87%, respectively. Landmark analysis at 131 days from the time of CR/CRi (time to alloSCT in 75% of transplanted patients), showed a statistically superior advantage for RFS (p=0.03) but no difference in survival (p=0.07) for the transplanted patients (Figures 2a and 2b, respectively). MRD status at CR1 is available in 19 patients, 13 were positive (MRD+) and 6 were negative (MRD-). There is no difference in RFS by the MRD status at CR (p=0.52).
Conclusion- The addition of dasatinib to the treatment in younger patients with Ph+ ALL significantly improves the 12-month RFS for those who undergo alloSCT in CR1 and is associated with an improved OS compared to historical data prior to the introduction of TKIs.
Acknowledgments: Dr. Meir Wetzler is posthumously recognized for his important contributions to this study. Support from NIH/NCI/NCTN grants CA180888, CA180819, CA180821, CA180820; and in part by Bristol-Myers Squibb Company. Dasatinib was distributed by the NCI/DCTD/CTEP.
Uy:Novartis: Research Funding. Borate:Genoptix: Consultancy; Gilead: Speakers Bureau; Seattle Genetics: Research Funding; Novartis: Speakers Bureau; Alexion: Speakers Bureau; Amgen: Speakers Bureau. Erba:Seattle Genetics: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Celator: Research Funding; Astellas: Research Funding; Celator: Research Funding; Amgen: Consultancy, Research Funding; Incyte: Consultancy, Speakers Bureau; Pfizer: Consultancy; Sunesis: Consultancy; Novartis: Consultancy, Speakers Bureau; Ariad: Consultancy; Celgene: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Millennium/Takeda: Research Funding; Millennium/Takeda: Research Funding; Jannsen (J&J): Other: Data Safety and Monitoring Committees ; Seattle Genetics: Consultancy, Research Funding; Celgene: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Astellas: Research Funding; Daiichi Sankyo: Consultancy; Ariad: Consultancy; Sunesis: Consultancy; GlycoMimetics: Other: Data Safety and Monitoring Committees ; Pfizer: Consultancy; Jannsen (J&J): Other: Data Safety and Monitoring Committees ; Daiichi Sankyo: Consultancy; GlycoMimetics: Other: Data Safety and Monitoring Committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract