Abstract
Heat shock factor 1 (HSF1) is best known as a key sensor of proteotoxic stress, but accumulating evidence also supports a major role for this transcriptional regulator in cancer biology. In a variety of human solid tumor cells, downregulation of HSF1 inhibits growth, induces cell death and limits metastatic potential. In breast cancers, nuclear accumulation of HSF1 and a tumor-specific gene expression signature reflecting HSF1 activation were found to be strongly associated with poor outcome (Mendillo et al, Cell 2012). In addition, we have recently reported, as a counter-intuitive reversal of the central dogma, that inhibition of protein translation represses the constitutive activation of HSF1 in cancers, and that HSF1 inhibition induced by the potent eIF4a inhibitor rohinitib (RHT) exerts profound, far-ranging anti-tumor effects (Santagata et al, Science 2013). Review of public databases supports targeting of HSF1 and eIF4a in AML: mRNA levels of HSPA8, one of the primary HSF1 targets, are correlated with poor prognosis in AML (Prognoscan, data from Metzeler et al, Blood 2008) and eIF4a mRNA levels were highest in AML among 12 cancer types (Oncomine, data from Ramaswamy et al, PNAS 2001). Here, we demonstrate that inactivation of HSF1 in acute myeloid leukemias (AMLs) by RHT exerts pronounced apoptogeniceffects with preferential activity against FLT3-ITD mutant cells in cell culture and in mice.
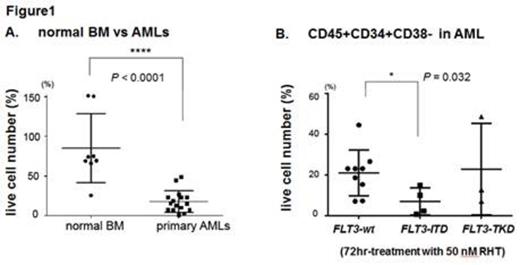
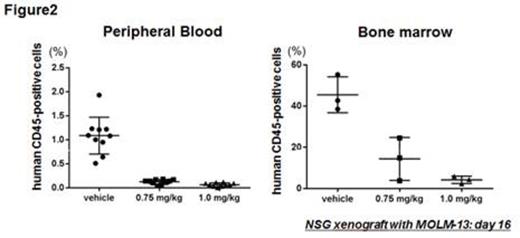
First, we confirmed our previous finding of inactivation of HSF1 by RHT in AML. In OCI-AML3, MOLM-13 and MV4;11 cells, mRNA levels of HSPA8 were reduced by 70% after RHT treatment compared to untreated controls. OCI-AML3 cells were then infected with lentivirus encoding a reporter GFP-luciferase fusion protein the expression of which is driven by promoter elements from either the HSPA1A or HSPA6 genes; an approximately 50% reduction of reporter induction by heat shock was observed after RHT treatment compared to untreated controls. Next, treatment of 7 human AML cell lines in culture showed that RHT induces marked anti-leukemia effects at low nanomolar concentrations (LD50s; 9.5 to 99.5 nM, IC50s; 4.7 to 8.8 nM, based on AnnexinV/PI-positivity as determined by flow cytometry at 72hr). The most pronounced cytotoxic effects were observed in FLT3-ITD+ cell lines (LD50s < 10 nM in MOLM13 and MV4;11 cells). Using two sets of isogenic cell lines (Ba/F3 and OCI-AML3 cells with FLT3-ITD or wild-type (wt) FLT3), we confirmed that RHT more potently kills FLT3-ITD cells (LD50s; 65.3 vs 20.1 nM in Ba/F3 cells). Furthermore, the combination of FLT3 inhibitor sorafenibwith RHT showed synergistic effects in cell culture (Combination Index: ED50 0.85, ED75 0.86, ED90 0.89). Immunoblot analysis showed higher phospho-HSF1 (Serine 326) in FLT3-ITD Ba/F3 cells than FLT3-wt cells, suggesting greater dependence of FLT3-ITD cells on HSF1 activation for survival. We also tested primary samples from 17 AML patients and bone marrow (BM) samples from 8 healthy donors. RHT potently induced apoptosis in AML cells, while relatively sparing normal BM cells (Figure 1A). Importantly, a similarly significant difference in sensitivity was also observed between AML and normal stem cells (CD45+CD34+CD38-). Moreover, the activity of RHT against the leukemic population was significantly higher in FLT3-ITD than in FLT3-wt cells (Figure 1B). We also evaluated the activity of RHT in a FLT3 mutant AML xenograft model using GFP-luciferase labeled MOLM-13 cells. Significantly decreased luciferase activity was detected by bioluminescence imaging and a dose-dependent reduction in GFP+ leukemic cells was seen in peripheral blood and BM by day 16 (Figure 2). Survival of the treatment groups was significantly prolonged (median; 18 vs 22.5 vs 24 days respectively, p < 0.0001).
In conclusion, HSF1 function provides an attractive therapeutic target in AML. The eIF4a inhibitor RHT down-regulates HSF1 transcriptional function and exerts robust anti-leukemia activity in cell culture and in mice. Although the relative contributions of HSF1 inactivation and translation inhibition to the net anti-leukemic activity of RHT remain to be defined, promising features of this approach include its activity against AML stem cells, while sparing normal stem cells and its particularly potent cytotoxicity for poor-prognosis FLT3-ITD AMLs. Taken together, these preclinical findings strongly support further development of eIF4a inhibitors in the treatment of AML.
Ishizawa:Karyopharm: Research Funding. Konopleva:Novartis: Research Funding; AbbVie: Research Funding; Stemline: Research Funding; Calithera: Research Funding; Threshold: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.