Key Points
CMV serostatus significantly influences chimerism levels after T-cell–depleted allogeneic transplantation.
CMV-specific T cells are exclusively of recipient origin after R+/D− T-cell–depleted transplants and appear to provide protective immunity.
Abstract
Cytomegalovirus (CMV) remains a significant cause of morbidity after allogeneic hematopoietic stem cell transplantation (HSCT). Clinical risk varies according to a number of factors, including recipient/donor CMV serostatus. Current dogma suggests risk is greatest in seropositive recipient (R+)/seronegative donor (D−) transplants and is exacerbated by T-cell depletion. We hypothesized that in the setting of reduced-intensity T-cell-depleted conditioning, recipient-derived CMV-specific T cells escaping deletion may contribute significantly to CMV-specific immunity and might therefore also influence chimerism status. We evaluated 105 recipients of alemtuzumab-based reduced-intensity HSCT and collated details on CMV infection episodes and T-cell chimerism. We used CMV-specific HLA multimers to enumerate CMV-specific T-cell numbers and select cells to assess chimerism status in a subset of R+/D− and R+/seropositive donor patients. We show that in R+/D− patients, CMV-specific T cells are exclusively of recipient origin, can protect against recurrent CMV infections, and significantly influence the chimerism status toward recipients. The major findings were replicated in a separate validation cohort. T-cell depletion in the R+/D− setting may actually, therefore, foster more rapid reconstitution of protective antiviral immunity by reducing graft-vs-host directed alloreactivity and the associated elimination of the recipient T-cell compartment. Finally, conversion to donor chimerism after donor lymphocytes is associated with clinically occult transition to donor-derived immunity.
Introduction
Cytomegalovirus (CMV) infection remains a major cause of morbidity and cost after allogeneic hematopoietic stem cell transplantation (HSCT) and continues to directly cause mortality in a small subset of patients. The vast majority of infection derives from the reactivation of latent virus in seropositive recipients (R+), with a smaller but significant contribution from primary infection of seronegative patients (R−) with seropositive donors (D+). The risk for infection episodes, generally detected as viral DNAemia by polymerase chain reaction-based methods, correlates inversely with the reconstitution of CMV-specific immunity after HSCT.1,2 In high-risk cases in which the recipient is seropositive, this is likely to be the most prolonged when the donor is seronegative (D−), as immunity is then reliant on the development of a primary immune response.3-5 After myeloablative conditioning, the establishment of protective immunity may be delayed still further by the incorporation of T-cell depletion strategies aimed at reducing the risk for graft-vs-host disease (GvHD).
The coexistence of elements of both donor and recipient hematopoiesis after HSCT is referred to as mixed chimerism. It can occur independently within any or all of the hematopoietic lineages but has been most extensively studied within the T-cell compartment. Mixed T-cell chimerism is more common with less intensive conditioning regimens, particularly after the incorporation of T-cell depletion, as recipient T cells can escape deletion mediated by either myeloablative conditioning or by the more intense alloreactive graft-vs-host activity associated with T-replete HSCT.
Extrapolating from prior work showing the extent to which viral infection can skew the reconstituting T-cell repertoire,6 we hypothesized that recipient-derived CMV-specific T cells that had escaped deletion during conditioning might be driven to expand during episodes of CMV infection, potentially then providing protective immunity and significantly influencing patterns of T-cell chimerism. We predicted that these influences would be most notable when either the donor or the recipient was virus-naive and the other was virus-experienced, and that such differences would be more evident in a setting in which the transplant conditioning was nonablative and in which mixed chimerism was engendered by T-cell depletion.
Methods
Patients undergoing HSCT at University College Hospital (London, United Kingdom) between January 2004 and August 2012, receiving conditioning with 1 of 2 regimens incorporating in vivo alemtuzumab (Genzyme, Cambridge, MA), were considered eligible for study inclusion. The 2 regimens have been previously described: fludarabine/melphalan/alemtuzumab and carmustine/etoposide/cytarabine arabinoside/melphalan/alemtuzumab.7,8 All patients received ciclosporin A as prophylaxis against GvHD. In the absence of GvHD, immunosuppression was tapered from 3 months after transplantation. Patients who were no longer receiving immunosuppression had no clinically significant GvHD, but those who had mixed chimerism or progressive disease/relapse were offered donor lymphocyte infusion (DLI) where donor availability permitted.
To evaluate the reproducibility of the initial findings, we identified a validation cohort consisting of patients undergoing HSCT between January 2004 and August 2012 at the Royal Free Hospital (London, United Kingdom). These patients underwent transplants using the same conditioning protocols and had chimerism analyses performed to the same schedule in the same laboratory as those in the primary study cohort.
Chimerism analysis
Multilineage chimerism analyses were performed every 3 months after transplantation until establishment of full donor chimerism. A semiquantitative method based on short tandem repeat region analyses was used as previously described.7,9,10 Percentage donor chimerism was calculated from informative short tandem repeat regions based on peak height measured in relative fluorescent units (RFUs) by the following formula: % donor chimerism = RFU donor-specific fragment/(RFU donor-specific fragment + RFU recipient-specific fragment) × 100.
Cell sorting
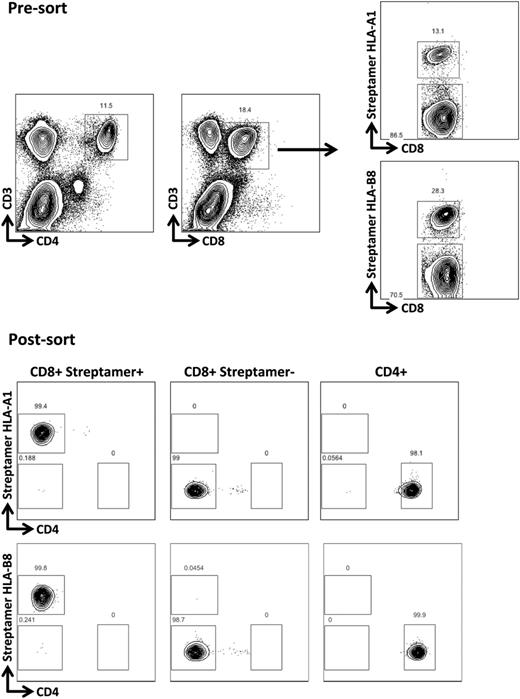
Peripheral blood mononuclear cells were separated by gradient centrifugation on Ficoll-Paque Plus (GE Healthcare, Sweden) and then labeled with anti-CD3-PECy7, anti-CD4-FITC, anti-CD8-e450 (eBiosciences, San Diego, CA) and relevant MHC-class I streptamers labeled with Strep-Tactin-APC flurochrome (IBA Life Sciences, Göttingen). HLA-A*0101, CMV-pp50:VTEHDTLLY; HLA-A*0201, CMV-pp65:NLVPMVATV; HLA-B*0702, CMV-pp65:TPRVTGGGAM; HLA-B*0801, CMV- IE-1:QIKVRVDMV, CD8+streptamer+, CD8+streptamer−, and CD4+ T cells were purified with a BD FACSAria II cell sorter before extraction of DNA for chimerism analyses.
Cytomegalovirus surveillance
CMV surveillance was performed weekly, using a semiquantitative polymerase chain reaction (limit of detection of 200 copies/mL). In patients without evidence of CMV DNAemia, surveillance was discontinued at day 100. In those with recurrent episodes of viremia, those with CMV disease, and those receiving systemic corticosteroids, monitoring was continued beyond 100 days. Preemptive treatment was given if viral titers exceeded 1000 copies/mL on one occasion, or for 2 consecutively rising titers. Systemic antiviral treatment consisted of ganciclovir where graft function permitted, or foscarnet in the presence of cytopenias. Treatment continued until CMV viremia fell below the limit of quantification.
Statistical analysis
The cumulative incidence of non-relapse-related mortality and relapse were calculated using the competing risks method.11 Comparisons of patient demographics between R+/D− and R−/D+ cohorts were made using Fisher’s exact test and χ-square test for trend. The frequency of CMV disease in groups with and without GvHD was calculated using Fisher’s exact test. Comparisons of percentage recipient chimerism, number of CMV reactivations, number of days receiving treatment of CMV, and lymphocyte number at 3 months were calculated using the Mann-Whitney U test. All tests were 2-tailed unless stated otherwise, with P < .05 considered to be statistically significant.
Results
Patients
Forty-four HSCT recipients with R+/D− or R−/D+ CMV serostatus were identified as the primary study cohort. Three (6.8%) died before chimerism analyses were performed. The characteristics of the remaining 41 are summarized in Table 1. There were no significant differences between the cohorts in terms of disease type, donor type, graft source, conditioning regimen, or incidence of clinically significant (grade II-IV acute or extensive chronic) GvHD. Unsurprisingly, the primary study cohort consisted mainly of sibling donor HSCT, as CMV-matched donors were chosen in preference and were more generally available for the unrelated donor HSCT cohort. All patients in the primary study cohort successfully engrafted (median time to neutrophils > 0.5 × 109/L was 11 days in both R−/D+ and R+/D−). The cumulative incidence of non-relapse-related mortality was 16% (R−/D+ = 15%; R+/D− = 18%), and the relapse incidence was 28% (R−/D+ = 27%; R+/D− = 28%). The median follow-up for the 33 primary cohort patients alive at the end of the study period was 46 months (range, 12-110 months). An additional 61 patients were identified for further comparison, where both recipient and donor were CMV seronegative (R−/D−; n = 32) or CMV seropositive (R+/D+; n = 29).
Recipient chimerism is significantly greater in R+/D− than R−/D+ HSCT
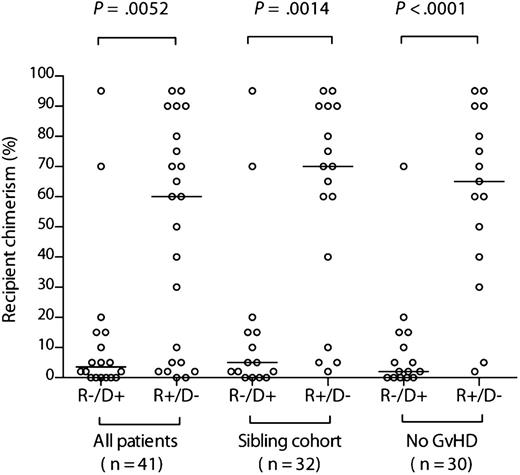
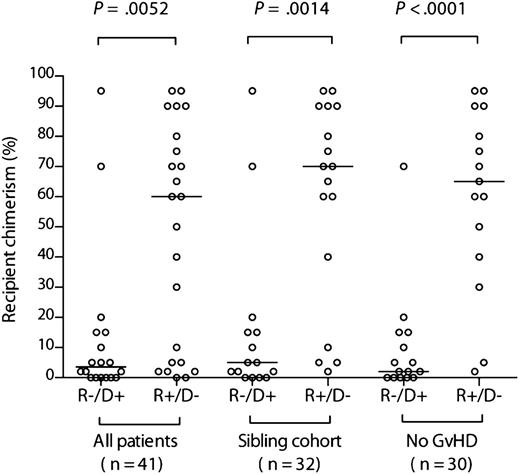
We first compared the levels of recipient chimerism before DLI between the 2 cohorts with mismatched CMV serostatus. There was a broad range of levels in both groups. However, recipient chimerism was significantly greater in the R+/D− cohort (median, 60% [range, 0%-95%] recipient vs median, 3.5% [range, 0%-95%], respectively; P = .0052) (Figure 1). Because donor type and GvHD potentially influence chimerism levels, we performed the same comparison but restricted it to either the sibling donor cohort (n = 32) or the cohort without clinically significant GvHD (n = 30). The differences were even more pronounced (Figure 1), illustrating that they were not driven by these confounding factors (median, 70% [range, 2%-95%] recipient vs 5% [range, 0%-95%] in the sibling cohort [P = .0014]; median, 60% [range, 2%-95%] recipient vs 2% [range, 0%-70%], respectively, in those without GvHD [P < .0001]). Unsurprisingly, recipient chimerism levels were significantly lower in those in the R+/D− cohort with GvHD (n = 8) than those without (n = 15) (median, 3.5% [range, 0%-90%) vs 65% [range, 2%-95%]; P = .02).
Recipient chimerism is greater after R+/D− compared with R−/D+ HSCT. Comparison of chimerism levels according to recipient/donor serostatus in the entire evaluable cohort, the sibling donor cohort, and the cohort without clinically significant GvHD. The horizontal bars indicate the respective medians. Comparisons between R−/D+ and R+/D− cohorts were performed using a 2-tailed Mann-Whitney U test.
Recipient chimerism is greater after R+/D− compared with R−/D+ HSCT. Comparison of chimerism levels according to recipient/donor serostatus in the entire evaluable cohort, the sibling donor cohort, and the cohort without clinically significant GvHD. The horizontal bars indicate the respective medians. Comparisons between R−/D+ and R+/D− cohorts were performed using a 2-tailed Mann-Whitney U test.
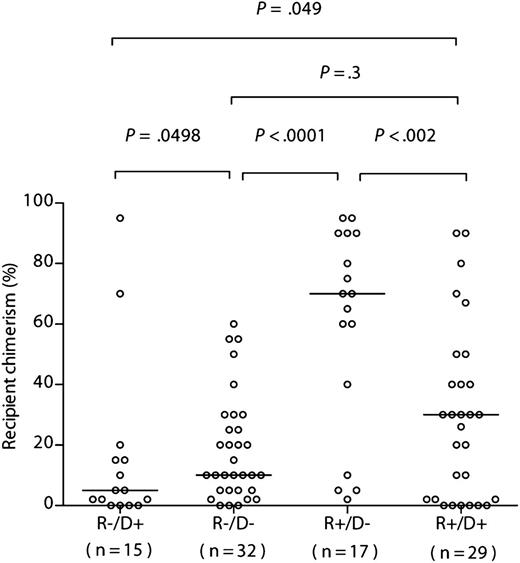
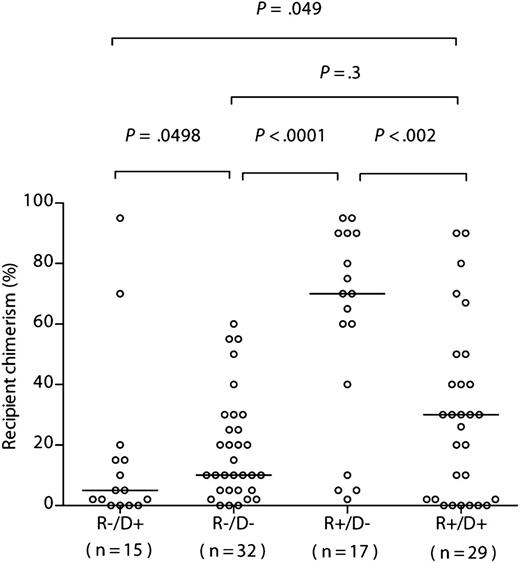
To assess the predicted bidirectionality of effect of CMV serostatus on chimerism, we compared the levels in the sibling cohorts with those of a contemporary sibling donor R−/D− cohort (n = 32) and R+/D+ cohort (n = 29) (Figure 2). Recipient chimerism was significantly greater in the R+/D− than in the R−/D− cohort (median, 70% [range, 2%-95%] recipient vs median, 10% [range, 0%-60%], respectively; P < .0001; 1-tailed Mann-Whitney), and significantly lower in R−/D+ vs the R−/D− cohort (median, 5% [range, 0%-95%] recipient vs median, 10% [range, 0%-60%], respectively; P = .0498; 1-tailed Mann-Whitney). Furthermore, recipient chimerism was significantly greater in the R+/D− than in the R+/D+ cohort (median, 70% [range, 2%-95%] recipient vs median, 30% [range, 0%-90%], respectively; P < .003; 1-tailed Mann-Whitney), and significantly lower in R−/D+ vs the R+/D+ cohort (median, 5% [range, 0%-95%] recipient vs median, 30% [range, 0%-90%], respectively; P = .049; 1-tailed Mann-Whitney) (Figure 2). Although there was a greater range of recipient chimerism levels in the R+/D+ cohort compared with in the R−/D− cohorts, there was no statistically significant difference between the groups (median, 30% [range, 0%-90%] vs median, 10% [range, 0%-60%] respectively; P = .3) (Figure 2).
Recipient chimerism after R−/D− HSCT is greater than that after R−/D+ HSCT, but significantly less than after R+/D− HSCT. Comparison of chimerism levels according to recipient/donor serostatus in the sibling donor cohort. The horizontal bars indicate the respective medians. Statistical comparisons (R−D+ vs R−D−; R−D− vs R+/D−; R−D+ vs R+D+; R+D+ vs R+/D) were performed using a 1-tailed Mann-Whitney U test. Statistical comparisons between R−/D− and R+/D+ were performed using a 2-tailed Mann-Whitney U test.
Recipient chimerism after R−/D− HSCT is greater than that after R−/D+ HSCT, but significantly less than after R+/D− HSCT. Comparison of chimerism levels according to recipient/donor serostatus in the sibling donor cohort. The horizontal bars indicate the respective medians. Statistical comparisons (R−D+ vs R−D−; R−D− vs R+/D−; R−D+ vs R+D+; R+D+ vs R+/D) were performed using a 1-tailed Mann-Whitney U test. Statistical comparisons between R−/D− and R+/D+ were performed using a 2-tailed Mann-Whitney U test.
To confirm the reproducibility of our initial findings, we identified an independent “validation” cohort consisting of patients at another institution who had undergone transplants using the same conditioning platforms (described in supplemental Table 1, available on the Blood Web site). This cohort contained fewer patients with R+/D− (n = 9) and R−/D+ (n = 4) serostatus. Nevertheless, the same pattern of skewing of chimerism was seen (R+/D− median, 79% [range, 0%- 98%] recipient vs R−/D+ median, 5% [range, 0%-13%]; P = .02) (supplemental Figure 1A). This effect was maintained when the R+/D− cohort was compared with all CMV-seronegative (R−) patients (n = 29; median, 79% [range, 0%-98%] recipient vs 2% [range, 0%-70%], respectively; P = .0003) (supplemental Figure 1B). Subgroup analyses within the R+/D− and R−/D+ validation set were limited by the low numbers, particularly within the R−/D+ group, so analyses restricted to the sibling donor cohort and the cohort without significant GvHD were performed comparing the R+/D− cohort with all R− patients. These recapitulated the findings in the primary study cohort, demonstrating large, statistically significant differences between the levels of recipient chimerism (supplemental Figure 1B) (for the sibling-only group, median, 66% [range, 26%-98%] vs median, 2% [range, 0%-70%], respectively; P = .0009; for the group without significant GvHD, median, 62% [range, 0%-98%] vs median, 6% [range, 0%-70%], respectively; P = .007).
Higher levels of recipient chimerism are associated with fewer CMV-related events in the R+/D− cohort
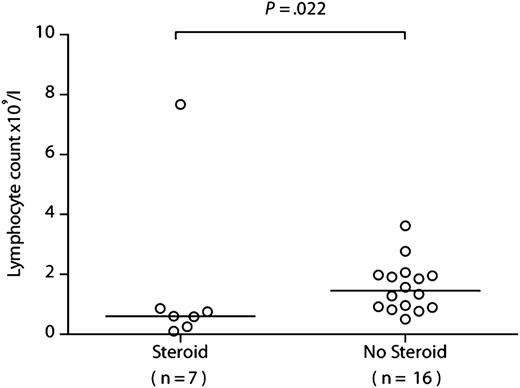
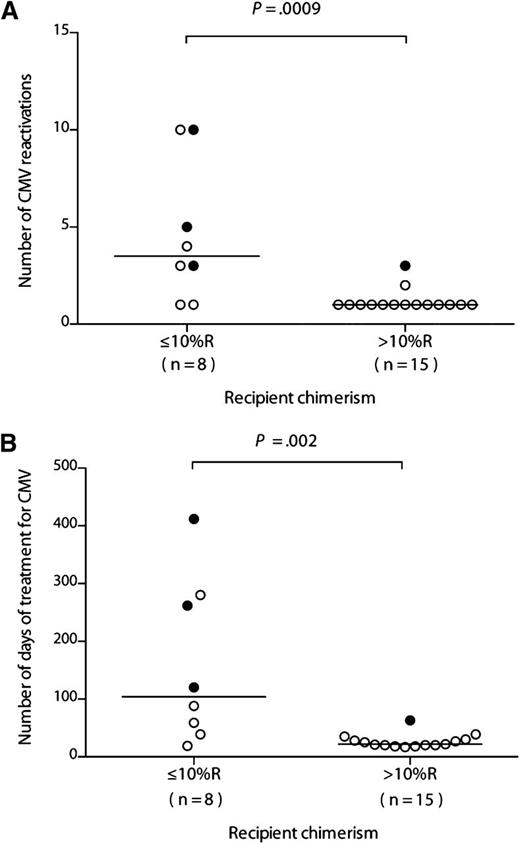
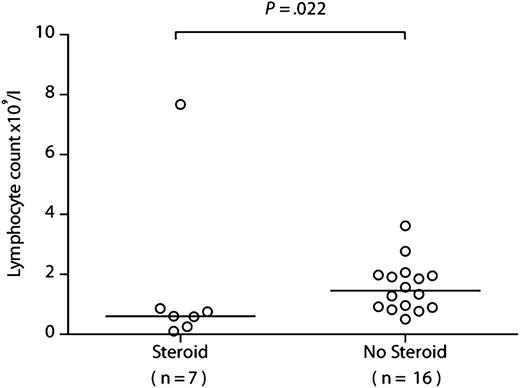
If recipient-derived CMV-specific T cells provide protective immunity, then patients with the lowest levels of recipient T-cell chimerism should have more problematic CMV infections. We therefore compared the prior clinical courses of patients within the R+/D− cohort according to their chimerism levels at 6 months after HSCT, choosing a cutoff of 10% (none had a level between 10% and 30%). All patients within the R+/D− cohort experienced at least 1 episode of CMV DNAemia requiring therapy, in keeping with our prior experience of alemtuzumab-based HSCT. The first occurrence of quantifiable CMV DNAemia occurred at a median of 28 days after transplant (range, 11-46 days), with a median of 32 days to first anti-CMV treatment (range, 12-50 days). The number of infection episodes and number of days receiving treatment were significantly lower for those with more than 10% (n = 15) vs those with 10% or less (n = 8) recipient chimerism (median, 1 episode [range, 1-3 episode] vs 3.5 episodes [range, 1-10 episodes]; P = .0009 [Figure 3A]; and median, 21 days [range, 17-63 days] vs 104 days [range, 19-412 days]; P = .002 [Figure 3B], respectively). Furthermore, CMV disease was documented in only 1/15 (6.7%) of those with more than 10% recipient chimerism (colitis) compared with 3/8 (37.5%) of those with 10% or less recipient chimerism (2 with retinitis, 1 with both pneumonitis and colitis; P = .1). R+/D− transplants were not, therefore, universally associated with problematic CMV infection, as 15/23 (65.2%) patients had only a single episode. Indeed, this scenario was largely confined to the group of patients developing GvHD, who suffered both from reduced absolute lymphocyte counts secondary to corticosteroids (7/8 with GvHD received steroids; median lymphocyte count at 3 months, 0.60 × 109/L [range, 0.10-7.67 × 109/L) vs 1.46 × 109/L [range, 0.25-3.62 × 109/L] in those not receiving steroids; P = .022) (Figure 4) and eradication of the recipient-derived T-cell compartment, including the CMV-specific memory T-cell pool. The number of infection episodes and number of days receiving treatment in those with GvHD were 3.5 episodes (range, 1-10 episodes) and 104 days (range, 25-412 days), respectively, compared with 1 episode (range, 1-2 episodes) and 22 days (range, 17-88 days) in those without GvHD (P = .0009 and P = .002 respectively). All 4 episodes of CMV disease occurred in patients with GvHD (P = .008).
CMV infection episodes and duration of antiviral therapy are lower in those with more than 10% recipient T-cell chimerism after R+/D− HSCT. The number of CMV reactivations (A) and total number of days of CMV treatment in the R+/D− cohort (B) are shown in relation to recipient chimerism in the T-cell compartment, 10% or less recipient (R) or more than 10% R. A filled circle indicates a patient with CMV disease. The horizontal bar marks the median for the respective cohort. Comparisons between 10% or less R and more than 10% R cohorts were performed using a 2-tailed Mann-Whitney U test.
CMV infection episodes and duration of antiviral therapy are lower in those with more than 10% recipient T-cell chimerism after R+/D− HSCT. The number of CMV reactivations (A) and total number of days of CMV treatment in the R+/D− cohort (B) are shown in relation to recipient chimerism in the T-cell compartment, 10% or less recipient (R) or more than 10% R. A filled circle indicates a patient with CMV disease. The horizontal bar marks the median for the respective cohort. Comparisons between 10% or less R and more than 10% R cohorts were performed using a 2-tailed Mann-Whitney U test.
Lymphocyte counts are significantly lower in patients receiving systemic corticosteroids. The lymphocyte counts are shown at 3 months after HSCT in patients from the R+/D− cohort who received corticosteroids for GvHD vs those who did not. Comparison was performed using a 2-tailed Mann-Whitney U test.
Lymphocyte counts are significantly lower in patients receiving systemic corticosteroids. The lymphocyte counts are shown at 3 months after HSCT in patients from the R+/D− cohort who received corticosteroids for GvHD vs those who did not. Comparison was performed using a 2-tailed Mann-Whitney U test.
Because CMV-specific T cells in the R+/D+ cohort could potentially derive from both donor and recipient memory pools, we predicted that chimerism levels would not have the same effect on CMV-related events in this subgroup. As there was a broad range of chimerism levels without the “bimodal” distribution observed in the R+/D− cohort, we performed the analyses according to above- and below-median recipient chimerism (30%). There were no differences in terms of CMV infection episodes for patients above compared with below the median (median, 1 reactivation [range, 0-3 reactivations] vs 1 reactivation [range, 0-2 reactivations], respectively; P = .98) or for total number of days of anti-CMV treatment (median, 21 days [range, 0-69 days] vs 20 days [range, 0-25 days], respectively; P = .3).
Although the validation cohort contained only 9 patients with R+/D− serostatus, we found a similarly low level of clinically problematic CMV infection in this group. The median number of treatment episodes was 1 (range, 0-9 episodes), and the median number of days receiving anti-CMV treatment was 34 (range, 0-322 days). With the small numbers of R+/D− patients, and marked skewing toward recipient chimerism, only 1 patient had recipient chimerism levels of 10% or less. It was therefore not possible to assess the apparent protective effect of high levels of recipient chimerism against CMV reactivation using the same chimerism cut points (≤10% and >10% recipient) used in the primary study cohort. However, it is noteworthy that the only patient who had multiple problematic infection episodes (n = 9), prolonged treatment episodes (322 days), and CMV disease (retinitis) was this single patient with recipient chimerism of 10% or less in whom all lineages were fully donor.
CMV-specific T cells are exclusively of recipient origin after R+/D− HSCT
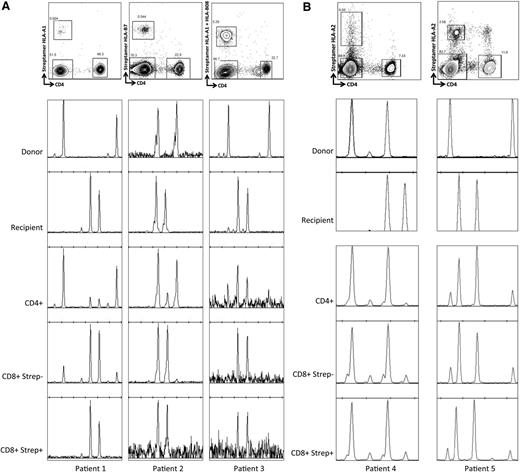
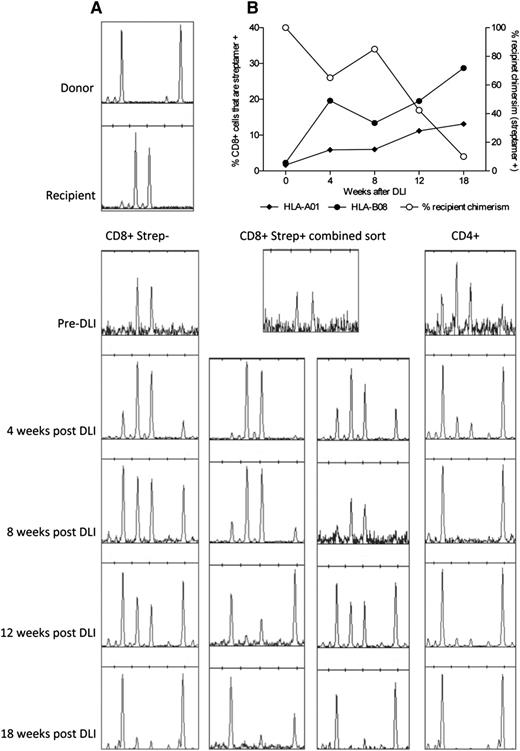
To further test the hypothesis that recipient-derived virus-specific T cells contributed to the differences in chimerism levels, we analyzed CMV-specific T cells after R+/D− HSCT. Because our routine clinical practice is to administer DLI for mixed chimerism from 6 months after HSCT, the majority of patients had already been converted to full donor chimerism. However, we were able to identify 3 patients in the R+/D− cohort who were 6 months post-HSCT, with stable levels of recipient chimerism (50%-90%), who had HLA types that allowed for labeling and selection of CMV pp65-, pp50-, or IE-1-specific CD8+ cells, using CMV-specific HLA-streptamers (Figure 5A). The CMV-specific streptamer-positive components of CD8+ T-cell compartments were 1.96% (HLA-*A0101 VTE), 0.75% (HLA-B*0702 TPR), and 3.93% (1.66% for HLA-*A0101 VTE and 2.27% for HLA-B*0801 QIK), respectively. Chimerism analyses were performed on flow-sorted T-cell subsets (Figure 5A). Although the total T-cell, CD4+, and CD8+streptamer− compartments showed varying levels of mixed chimerism, the CD8+streptamer+ CMV-specific T-cells appeared to be exclusively of recipient origin in all patients. Furthermore, chimerism was consistently more skewed toward recipient in the CD8+, as opposed to the CD4+ T-cell compartment, in keeping with larger clonal burst sizes of CD8+ vs CD4+ virus-specific T cells, and greater dependence of the CD4+ T-cell compartment on thymic repopulation after allogeneic HSCT.
CMV-specific T cells are of recipient origin after R+/D− HSCT but of both recipient and donor origin in the context of R+/D+ HSCT with mixed chimerism. (A) The upper panels illustrate the strategy used for sorting T-cell populations (pregated on CD3). Chimerism patterns are shown for donor and recipient, and subsequently for the selected T-cell populations at 6 months after HSCT. The CD8+ compartment is more skewed toward recipient chimerism than the CD4+ compartment, and the streptamer+ (CMV-specific) CD8+ cells show 100% recipient chimerism. (B) In contrast, in the setting of mixed chimerism after R+/D+ transplants, the CMV-reactive CD8+ T cells (streptamer+) come from both donor and recipient compartments, and the recipient chimerism levels were similar in the different cellular compartments (patient 4: CD4+ 4%, CD8+streptamer− 13%, CD8+streptamer+ 13%; patient 5: CD4+ 80%, CD8+streptamer− 86%, CD8+streptamer+ 85%).
CMV-specific T cells are of recipient origin after R+/D− HSCT but of both recipient and donor origin in the context of R+/D+ HSCT with mixed chimerism. (A) The upper panels illustrate the strategy used for sorting T-cell populations (pregated on CD3). Chimerism patterns are shown for donor and recipient, and subsequently for the selected T-cell populations at 6 months after HSCT. The CD8+ compartment is more skewed toward recipient chimerism than the CD4+ compartment, and the streptamer+ (CMV-specific) CD8+ cells show 100% recipient chimerism. (B) In contrast, in the setting of mixed chimerism after R+/D+ transplants, the CMV-reactive CD8+ T cells (streptamer+) come from both donor and recipient compartments, and the recipient chimerism levels were similar in the different cellular compartments (patient 4: CD4+ 4%, CD8+streptamer− 13%, CD8+streptamer+ 13%; patient 5: CD4+ 80%, CD8+streptamer− 86%, CD8+streptamer+ 85%).
We hypothesized that the lower levels of recipient chimerism seen in R+/D+ cohort compared with the R+/D− cohort were indicative of the ability of the preexisting donor-derived CMV-specific memory repertoire to contribute to CMV-specific immunity, abrogating the effect of an unbalanced expansion of recipient CMV-specific T cells. We were interested in evaluating whether there was a clear bias toward contribution of the donor or recipient in the setting of use of in vivo alemtuzumab during transplant conditioning. We therefore repeated the chimerism analyses of CMV-specific T cells previously outlined, but on R+/D+ patients with stable mixed chimerism at 6 months after HSCT. We identified 2 suitable cases to allow sorting using HLA-A*0201, CMV-pp65:NLV streptamers. In both cases, the CD4+, CD8+streptamer−, and CD8+streptamer+ populations revealed mixed chimerism, demonstrating that the CMV-reactive cells were derived from both donor and recipient (Figure 5B). Interestingly, within a given individual, the levels of recipient chimerism were much more similar across these 3 subpopulations than had been demonstrated in the R+/D− cohort, regardless of the absolute level of recipient chimerism, suggesting a balanced expansion and contraction of cells from both donor and recipient.
Donor lymphocytes promote conversion to donor-derived immunity without clinically occult CMV infection
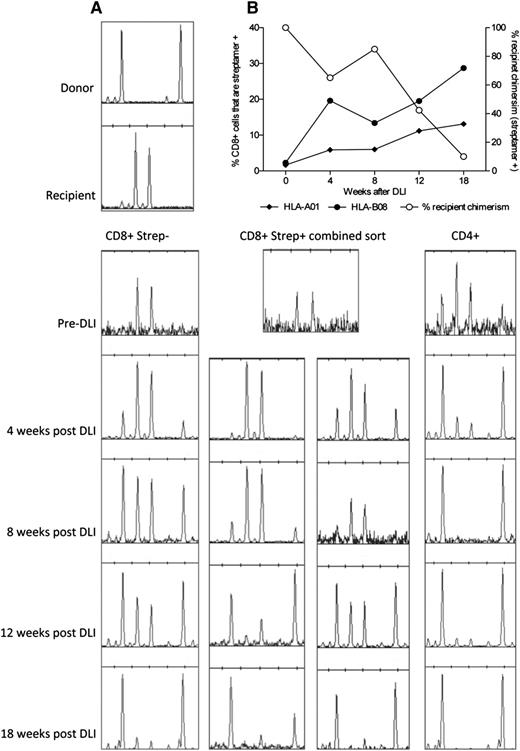
Eleven R+/D− patients in the primary study cohort had received DLI for persistent recipient chimerism (median, 75% recipient; range, 5%-95%) before data collection. All converted to less than 5% recipient (median, 0%). Although surveillance monitoring for CMV DNAemia had not been performed after DLI in these cases, no patients developed symptomatic CMV infection. Of the 3 patients who had not yet received DLI, patients 1 and 3 went on to receive DLI for mixed chimerism. Patient 2 did not receive DLI because of concerns of exacerbating GvHD. We performed serial chimerism analyses of highly purified flow-sorted T-cell subpopulations (Figure 6) after administration of DLI. We predicted that recipient-derived CMV-specific immunity would be eradicated, with the possibility of coincident development of CMV infection. Interestingly, in 1 patient (patient 3), we observed a gradual expansion of the CMV-specific T-cell compartment directed toward pp50 and IE-1, as measured with 2 HLA-streptamers, from a baseline level of 3.9% of the CD8+ compartment to a maximal level of 41% (Figure 7A). This occurred in the absence of quantifiable CMV viremia (polymerase chain reaction became positive below the limit of quantification; ie, <200 copies/mL). The increase in CMV-specific T-cell content was coincident with the emergence of donor-derived CMV-specific T cells restricted to both of the HLA alleles (Figure 7B), suggesting rapid priming of donor-derived CMV-specific immunity. By 18 weeks after infusion, the vast majority of these CMV-specific T cells were of donor origin. The conversion occurred without demonstrable GvHD. In the other patient (patient 1), there was also a shift toward donor chimerism in the CD8+streptamer+ (HLA-*A0101) compartment, suggesting the emergence of donor-derived CMV-reactive T cells. Again, this happened without quantifiable CMV viremia or disease, although without a significant expansion in the total number of CMV pp50-specific T cells (data not shown).
Expansion of CMV-specific T-cell populations after donor lymphocyte infusion. The upper panels demonstrate the expansion of CMV-reactive T cells colabeled with CD3, CD8, and CMV-specific HLA-streptamers occurring after DLI in patient 3. Flow cytometric sorting provided highly purified populations for subsequent chimerism analyses.
Expansion of CMV-specific T-cell populations after donor lymphocyte infusion. The upper panels demonstrate the expansion of CMV-reactive T cells colabeled with CD3, CD8, and CMV-specific HLA-streptamers occurring after DLI in patient 3. Flow cytometric sorting provided highly purified populations for subsequent chimerism analyses.
Expansion of donor-derived CMV-specific T cells after donor lymphocyte infusion. (A) Conversion to donor chimerism was associated with massive expansion of donor-derived CMV-specific T cells in the weeks after DLI. (B) Serial chimerism analyses demonstrating the shift to donor chimerism within the different T-cell compartments. Taken together, the results demonstrate effective priming and expansion of a primary immune response from the virus-naive donor.
Expansion of donor-derived CMV-specific T cells after donor lymphocyte infusion. (A) Conversion to donor chimerism was associated with massive expansion of donor-derived CMV-specific T cells in the weeks after DLI. (B) Serial chimerism analyses demonstrating the shift to donor chimerism within the different T-cell compartments. Taken together, the results demonstrate effective priming and expansion of a primary immune response from the virus-naive donor.
Discussion
We demonstrate that CMV significantly influences the pattern of chimerism after T-cell–depleted reduced-intensity HSCT. Furthermore, recipient-derived virus-specific T cells provide apparent protection from recurrent CMV infection in the majority of R+/D− HSCT. Our data suggest, rather paradoxically, that by reducing GvHD-directed alloreactivity and the associated elimination of the recipient T-cell compartment, T-cell depletion may actually foster more rapid reconstitution of protective antiviral immunity after HSCT in the R+/D− setting than would occur if GvHD were more prevalent. CMV acts as a good model pathogen in view of its relatively high seroprevalence and the very high incidence of viral DNAemia after alemtuzumab-based GvHD prophylaxis. Nevertheless, there is no a priori reason why other viral pathogens causing infections in the early posttransplant period should not have a similar effect.
Although these data do not inform the debate regarding the significance of persistent mixed vs full donor chimerism per se, they do suggest that the level of chimerism in stable mixed chimeras may be driven by factors that have little influence on propensity to relapse or reject, and that the level of recipient signal in mixed chimeras may be of little clinical significance. The development of GvHD, which in most cases would be expected to manifest in both the lymphohematopoietic system as well as classically involved tissue sites, clearly has an overriding influence on levels/incidence of mixed chimerism. As such, it will likely mask any effect of viral infection in settings where it is more common (eg, T-cell–replete transplants or with alternate T-depletion strategies such as antithymocyte globulin). This may explain the apparent discrepancy between our results and those in a large group of patients undergoing HSCT incorporating antithymocyte globulin.5 Although reconstitution of CMV-specific T-cell immunity was slower in those with CMV seronegative donors, with a coincident increase in CMV-related complications, almost 50% of the cohort developed clinically significant GvHD, and chimerism was fully donor in all evaluable R+/D− patients by 3 months post-HSCT.
Similar considerations apply to another study of T-cell–replete HSCT, in which acute GvHD of grade II or higher occurred in 72% and chronic GvHD in 76% of the R+/D− cohort.4 Furthermore, 60% of the cohort underwent myeloablative conditioning. Interestingly, our assertion that strategies that result in full donor chimerism might offer little antiviral protection in the context of R+/D− transplants is supported by the clinical outcome data of these studies,4,5 as well as by a smaller study that demonstrated persistence of recipient-derived CMV-specific T cells in T-deplete but not T-replete HSCT.12 The latter study used an in vitro T-cell depletion strategy with Campath-1M plus complement, which results in depletion of mainly donor T cells while leaving recipient T cells intact. Two cases were evaluated for the derivation of CMV-specific CD8+ T cell immunity, which was shown to be exclusively recipient in both cases. Notably, patients receiving enhanced immunosuppression including steroids for GvHD were excluded from the study. Rates of CMV DNAemia and disease were significantly lower than in patients receiving R+/D− T-replete transplants. Although the authors hypothesized that such a favorable outcome would not be expected using alemtuzumab in vivo because of the more profound recipient T-cell depletion, our data show that recipient CMV-reactive T cells can escape alemtuzumab-directed lysis, expand in the context of virus, and provide protective immunity. We acknowledge, therefore, that our results are specifically relevant to the T-deplete setting. Indeed, we predict that our results would not be replicated in the context of T-replete conditioning regimens. With this in mind, it remains crucial to develop effective antiviral treatments that can be used in the setting of virus-naive donors, and a number of strategies are being pursued in this regard, with encouraging preliminary results.13-18
Our data illustrate 2 further important observations that deserve consideration. First, given the apparent protective effect of recipient-derived CMV-specific T cells, it is reasonable to question the potential effect of subsequent DLIs on CMV-related complications. A shift toward donor chimerism might be associated with a loss of CMV-specific immunity and recurrent CMV infections. We observed, however, that the shift to donor chimerism in the absence of GvHD was associated with a coincident expansion of donor-derived CMV-specific T cells. This expansion occurred in the context of low levels of CMV DNAemia (<200 copies/mL). There was no requirement for anti-CMV treatment and no evidence of CMV disease. These data require confirmation but suggest that conversion to donor chimerism following DLI can be associated with clinically occult transition to donor-derived immunity. The findings are consistent with the demonstration that donor-derived CMV-specific immune responses are primed within 7 to 8 weeks of transplantation in R+/D− HSCT, where the donor graft is either conventional G-CSF-mobilized peripheral blood stem cells or umbilical cord blood.19 One notable difference, however, was that CMV-specific T cells failed to proliferate sufficiently in vivo to provide protective immunity early after transplantation in the prior study, whereas this was clearly not the case after DLI. It is possible that the difference relates to CD4 deficiency, immunosuppressive drugs, or other environmental factors that are either absent or present at earlier points after HSCT.
Second, our use of HLA-streptamers to quantify CMV-specific responses suggests that although CMV-specific T cells may influence the level of recipient chimerism later after HSCT, it is unlikely that T cells with CMV specificity make up the majority of the recipient T-cell population at these later points. It is important to acknowledge that approaches to detect CMV-specific T cells using only a few immunogenic epitopes vastly underestimate CD8+ T-cell responses to CMV.20-22 Nevertheless, the degree of discrepancy between the levels of CMV-specific T cells at baseline (0.75%-3.93% of the CD8+ T cells) and overall recipient chimerism levels (50%-90%) implies that the majority of recipient-derived T cells are not CMV-specific. We hypothesize that events early posttransplant might have a role in “imprinting” the level of recipient chimerism, which thereafter remains stable in the absence of GvHD.
In conclusion, these data illustrate the potential effect of viral infections, and more specifically CMV, on chimerism levels after HSCT. They also demonstrate that in the absence of GvHD, protective recipient-derived immunity is established after R+/D− HSCT. This is not to advocate that CMV seronegative donors are chosen for seropositive recipients in preference to seropositive donors, but it highlights the potential benefit of avoiding GvHD in terms of CMV-associated toxicities when the R+/D− combination is unavoidable. Finally, the data provide evidence that DLI-mediated conversion to full donor chimerism may not be associated with a loss of CMV-specific immunity, but rather, with an effective primary response in donor-derived cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Simon Thomas and Alka Stansfield (Cell Medica Limited, London, United Kingdom) for provision of Strep-Tactin-APC flurochrome and streptamers.
R.S.S. is a Cancer Research UK Clinical Research Training Fellow. F.A.V. is supported by Leukaemia and Lymphoma Research. S.A.Q. is funded by a Cancer Research UK Career Development Fellowship and a Cancer Research Institute Investigator Award. K.S.P. receives funding from Cancer Research UK, Leukaemia and Lymphoma Research, and the Medical Research Council UK. This work was undertaken at University College London Hospitals/University College London, which received support from the Department of Health and Cancer Research United Kingdom funding schemes for National Institute for Health Research Biomedical Research Centres and Experimental Cancer Medicine Centres.
Authorship
Contribution: R.S.S., S.A.Q., and K.S.P. designed the study; R.S.S., F.A.V., J.Y.H., and S.V. performed the experiments; R.S.S., S.C., B.B., R.C., and K.S.P. collected clinical data; R.S.S., K.J.T., S.M., and K.S.P. provided clinical care of patients; R.S.S. and K.S.P. analyzed the results; and R.S.S., F.A.V., S.V., and K.S.P. wrote the manuscript with input and approval from the coauthors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karl S. Peggs, Department of Haematology, University College London Cancer Institute, Paul O'Gorman Building, 72 Huntley St, London WC1E 6DD, United Kingdom; e-mail: k.peggs@cancer.ucl.ac.uk.