Key Points
Fn-EDA+ promotes arterial thrombosis.
Platelet TLR4 mediates the prothrombotic effect of cellular Fn.
Abstract
Cellular fibronectin containing extra domain A (Fn-EDA+), which is produced in response to tissue injury in several disease states, has prothrombotic activity and is known to interact with Toll-like-receptor 4 (TLR4). The underlying mechanism and cell types involved in mediating the prothrombotic effect of Fn-EDA+ still remain unknown. Using intravital microscopy, we evaluated susceptibility to carotid artery thrombosis after FeCl3-induced injury in mice expressing Fn lacking EDA (Fn-EDA−/− mice) or Fn containing EDA (Fn-EDA+/+ mice). Fn-EDA−/− mice exhibited prolonged times to first thrombus formation and complete occlusion and a significant decrease in the rate of thrombus growth (P < .05 vs Fn-EDA+/+ mice). Genetic deletion of TLR4 reversed the accelerated thrombosis in Fn-EDA+/+ mice (P < .05) but had no effect in Fn-EDA−/− mice. Bone marrow transplantation experiments revealed that TLR4 expressed on hematopoietic cells contributes to accelerated thrombosis in Fn-EDA+/+ mice. In vitro studies showed that cellular Fn-EDA+ interacts with platelet TLR4 and promotes agonist-induced platelet aggregation. Finally, Fn-EDA+/+ mice specifically lacking platelet TLR4 exhibited prolonged times to first thrombus formation and complete occlusion (P < .05 vs Fn-EDA+/+ mice containing platelet TLR4). We conclude that platelet TLR4 contributes to the prothrombotic effect of cellular Fn-EDA+, suggesting another link between thrombosis and innate immunity.
Introduction
Fibronectins (Fn’s) are dimeric multidomain glycoproteins that are found in circulation and as fibrils in tissue extracellular matrix. Fn’s have multiple isoforms generated by alternative processing of a single primary transcript at 3 domains: extra domain A (EDA), extra domain B (EDB), and the type III homologies connecting segment.1 Two major Fn isoforms exist in humans and mice: (1) plasma Fn (pFn), which is synthesized by hepatocytes and does not contain the EDA or EDB segments; and (2) cellular Fn (cFn), which contains either the EDA or EDB segments, or both, and is deposited as fibrils in the extracellular matrix. cFn is synthesized locally by fibroblasts and other cells including macrophages and endothelial cells.1
Both pFn and cFn have an arginine-glycine-aspartate binding site for integrins, including the platelet integrins αIIbβ3, αvβ3, and α5β1, and they share the ability to interact with coagulation proteins and subendothelial matrix proteins such as collagen.2 A considerable amount of work has been done to explore the potential role of pFn in thrombosis and hemostasis.3-10 pFn levels in the circulation of healthy humans and mice are relatively high (230-650 μg/mL in humans; 150-530 μg/mL in mice), whereas cFn is normally present in plasma only in negligible amounts.11 Experiments done in pFn-deficient mice suggested that pFn is required to promote thrombus growth and stability in injured arterioles.3,12 Unexpectedly, however, deficiency of pFn enhanced platelet aggregation and thrombus formation in the absence of both fibrinogen and von Willebrand factor, 2 key molecules that are required for platelet adhesion and thrombus growth.8,10 This apparent discrepancy was explained recently by an elegant study by Wang et al, who demonstrated that pFn has dual functions in supporting early hemostasis and negatively regulating thrombosis at later stages of thrombus growth.9
In pathological disease states such as diabetes and ischemic stroke, plasma levels of cellular Fn containing extra domain A (Fn-EDA+) can become elevated.13,14 Previously, it was shown that inclusion of the EDA segment gives Fn a prothrombotic potential in vivo15 ; however, the underlying mechanisms of this effect still remain unknown. The EDA segment of cFn, but not other domains, has been shown to activate human Toll-like-receptor (TLR) 4 expressed in HEK293 cells.16 EDA activation of TLR4 requires myeloid differentiation-2 receptor participation.16 Using specific TLR4 inhibitors, we and others have demonstrated that cellular Fn-EDA+ activates TLR4 signaling.16-20 Several studies have suggested a role for TLR4 signaling in promoting thrombosis,21-24 particularly in the context of endotoxemia and sepsis.22,23 Recently, extracellular histones have been shown to induce platelet aggregation and thrombin generation through TLR4 and TLR2,21 suggesting an lipopolysaccharide (LPS)–independent role for TLR4 in thrombosis that is mediated by endogenous ligands. Although these aforementioned studies have shown an interaction of Fn-EDA+ and TLR4, none of the studies have explored the functional role of Fn-EDA+/TLR4 signaling in thrombosis.
Given that cellular Fn-EDA+ is produced in response to tissue injury in several disease states that are associated with exacerbated risk of thrombosis, the aim of this study was to test the novel hypothesis that cellular Fn-EDA+ promotes thrombus formation and growth through TLR4. Using several novel strains of mutant mice along with bone marrow transplantation (BMT) and adoptive transfer experiments, we demonstrate for the first time that cellular Fn-EDA+ promotes platelet aggregation and arterial thrombosis specifically through TLR4 expressed on platelets.
Methods
Animals
All the mice used in the present study were backcrossed for more than 15 generations to the C57BL/6J background. Fn-EDA+/+ and Fn-EDA−/− mice have been described previously25 and are reviewed briefly in the supplemental Methods (see the Blood Web site). To generate Fn-EDA+/+/TLR4−/− mice, Fn-EDA+/+ mice were crossed to TLR4−/− mice. Similarly, control Fn-EDA−/−/ TLR4−/− mice were generated by crossing Fn-EDA−/− and TLR4−/− mice. Both male and female mice of age ∼8 to 10 weeks, weighing 22 to 28 g, were used. Platelets for infusion were isolated from 4- to 6-month-old donor mice of the same genotype. The University of Iowa Animal Care and Use Committee approved all experiments.
Platelet preparation
Blood from anesthetized mice was harvested from the retro-orbital plexus and collected in 1.5-mL polypropylene tubes containing 300 µL of enoxaparin (0.3 mg/mL; Sanofi-Aventis, US LLC). The blood was centrifuged at 100 g for 5 minutes, and the platelet-rich plasma (PRP) was collected in a fresh tube. To prevent platelet activation, PRP was incubated with prostaglandin I2 (PGI2; 2 μg/mL PRP) at 37°C for 5 minutes. PRP was further centrifuged at 600 g for 5 minutes, and obtained pellets were resuspended in 1 mL modified Tyrode–N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (137 mM NaCl, 0.3 mM Na2HPO4, 2 mM KCl, 12 mM NaHCO3, 5 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5 mM glucose, 0.35% bovine serum albumin, pH 7.2) containing PGI2 (2 μg/mL) for 5 minutes at 37°C.26 The washing step was repeated twice to remove PGI2, and platelets were fluorescently labeled with calcein green, AM (2.5 µg/mL; Molecular Probes, Eugene, OR) for 10 minutes at room temperature.
BMT
BMT was performed in Fn-EDA+/+ mice (6 weeks in age) as described.27 In brief, recipient mice were irradiated with 2 doses of 6.5 Gy at an interval of 4 hours between the first and second irradiations. Bone marrow cells were sterilely extracted from excised femurs and tibias of euthanized Fn-EDA+/+, Fn-EDA+/+/TLR4−/−, or GPIbα/human interleukin-4 receptor (IL4R) donor mice. Bone marrow cells (8 × 106) were resuspended in sterile phosphate-buffered saline and injected into the retro-orbital venous plexus of lethally irradiated recipient Fn-EDA+/+ mice. Using this procedure, we generated chimeric Fn-EDA+/+ mice having Fn-EDA+/+/TLR4−/− or GPIbα/human IL4R hematopoeitic cells and control transplanted Fn-EDA+/+ mice with Fn-EDA+/+ marrow. Engraftment of Fn-EDA+/+/TLR4−/− bone marrow was confirmed by real-time polymerase chain reaction (PCR) after 4 weeks by genomic DNA extracted from peripheral blood cells of recipient mice. Diluted blood from chimeric Fn-EDA+/+ mice was stained with antibodies against GPIbα (phycoerythrin-anti-CD42b) and integrin α2b (fluorescein isothiocyanate–anti-CD41) to confirm successful transplantation of GPIbα/human IL4R bone marrow in chimeric Fn-EDA+/+ mice by using flow cytometry (supplemental Figure 3; FACSCalibur, BD Biosciences). Complete blood counts were obtained using an automated veterinary hematology analyzer (Sysmex).
Platelet depletion and transfusion
Anti-hIL4R (clone 25463; R&D Systems) antibody at a concentration of 2.5 μg/g body weight28 was infused through the retro-orbital plexus to deplete platelets from chimeric Fn-EDA+/+ mice having GPIbα/human IL4R transgenic bone marrow. After 1 hour, 1 × 109 platelets from Fn-EDA+/+ or Fn-EDA+/+/TLR4−/− mice was injected through the retro-orbital plexus, and mice were subjected to carotid artery thrombosis induced by FeCl3 injury.29
Intravital microscopy
Thrombus formation in the carotid artery after FeCl3 injury was assessed by intravital microscopy as described previously.29 Briefly, mice were anesthetized using 100 mg/kg ketamine and 10 mg/kg xylazine. An incision was made, and the right common carotid artery was carefully exposed and kept moist by superfusion with warm phosphate-buffered saline (37°C). Fluorescent platelets (2.5 × 109 platelets per kg) were infused through the retro-orbital plexus. Whatman paper saturated with ferric chloride (5%) solution was applied topically for 3 minutes, and thrombus formation in the injured vessel was monitored in real time by using a Nikon upright microscope with a Plan Fluor 4X/0.2 objective. Each injured vessel was recorded using a high-speed electron-multiplying camera for 30 minutes or until occlusion occurred. Videos were evaluated off-line using a Nikon computer-assisted image analysis program.29
Quantitative analysis of arteriolar thrombus formation
To study real-time thrombus formation in vivo, we monitored the following: (1) the time required to form the first thrombus >100 μm in diameter, which was taken as an index of platelet aggregation; (2) the thrombus growth rate, defined as the increase in thrombus diameter above 100 μm over a period of 2 minutes (fold increase in diameter was calculated by dividing the diameter of the thrombus at time n by the diameter of the same thrombus at time 0, defined as the time point at which the thrombus diameter first reached 100 μm); and (3) the time to form occlusive thrombus, which was considered as the time required for blood to stop completely flowing for >1 minute.
Immunoprecipitation and immunoblotting
Platelets (1 × 108) suspended in plasma were lysed on ice with radioimmunoprecipitation assay buffer and precleared by incubation with control immunoglobulin G (IgG; 1 μg) and protein A/G agarose beads (Santa Cruz) (1:50) for 45 minutes at 4°C. The beads were pelleted down by centrifugation at 15 000 g for 5 minutes, and the supernatant fraction was incubated with anti-TLR4 (Abcam) (1:50) antibody or anti-Fn (Abcam) (1:100) antibody or control IgG (Sigma) (1 μg) at 4°C for 5 hours and then overnight with protein A/G agarose beads (1:10). The beads were pelleted as described previously, washed 3 times with 2× RIPA buffer, lysed with Laemmli buffer, and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. In another set of experiments, washed platelets were subjected to thrombin-induced aggregation and then lysed with Laemmli buffer. Proteins in the lysate were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis using 4% to 20% or 10% polyacrylamide gels and electrotransferred onto polyvinylidene difluoride membranes (15 V, 1.5 hours). Membranes were blocked for 1 hour with 5% nonfat milk or 5% bovine serum albumin in Tris-buffered saline containing 0.05% Tween 20 and incubated overnight at 4°C with anti-TLR4 (Abcam) (1:500), anti phospho–nuclear factor κB (NF-κB) p65 (Ser536) (Cell Signaling Technology) (1:1000), anti phospho–IκB kinase (IKK) α/β (Ser176/180) (Cell Signaling Technology) (1:1000), or anti-β-actin (Sigma) antibodies (1:5000). Blots were subjected to 3 washes with Tris-buffered saline containing 0.05% Tween 20 for 5 minutes each and incubated with the appropriate horseradish peroxidase–conjugated secondary antibody (Dako) (1:1000) for 2 hours at room temperature. Proteins were detected using enhanced chemiluminescence substrate (Thermo Scientific) and exposure to photographic films.
Platelet aggregation
Blood was drawn from anesthetized mice and anticoagulated with trisodium citrate (1.9% weight to volume ratio) at 1:9 ratio. PRP and washed platelets were prepared as described previously. The final platelet count was adjusted to ∼3 × 108/mL with platelet-poor plasma or Tyrode’s buffer. Aggregation was initiated by adding 4 μM of adenosine 5′-diphosphate (Chrono-par) or 5 μg/mL collagen (Chrono-par) to PRP, and thrombin (0.02 U/mL) (Sigma) to washed platelet suspensions in the presence of either human cFn (# F2518, Sigma) or human pFn (# F2006, Sigma). Aggregation was recorded using a light transmittance aggregometer (Chrono-Log Corporation).
Statistical analysis
Results are reported as the mean ± standard error of the mean (SEM). The statistical significance of the difference between means was assessed using the unpaired Student t test (for the comparison of 2 groups) or by analysis of variance followed by Tukey's multiple comparisons test (for the comparison of more than 2 groups). P < .05 was considered significant.
Results
TLR4 contributes to cellular Fn-EDA+-mediated accelerated thrombosis
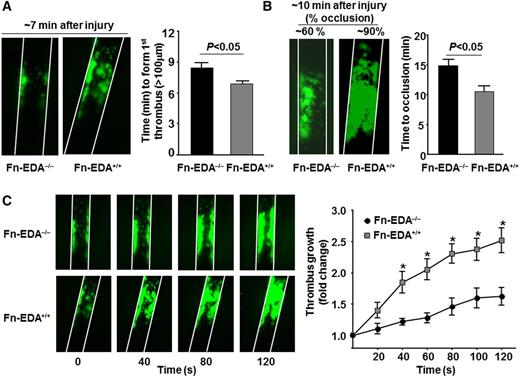
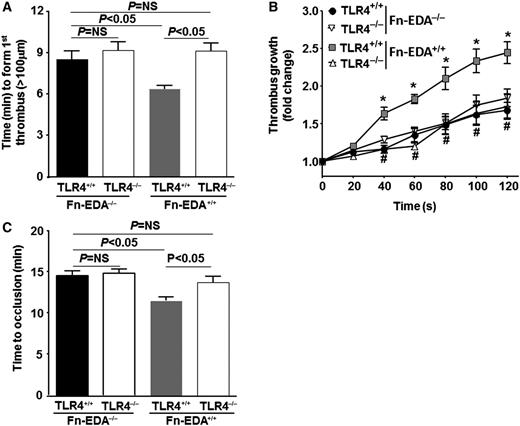
We first determined whether cellular Fn-EDA+ promotes arterial thrombosis in a large vessel (the carotid artery). Using intravital microscopy, we measured the time required to form a first thrombus (≥100 μm), thrombus growth kinetics, and the time required for complete occlusion in the FeCl3 injury–induced carotid artery thrombosis model. The mean time to form first thrombus and the mean time to occlusion were significantly accelerated in Fn-EDA+/+ mice compared with Fn-EDA−/− mice (P < .05; Figure 1A-B). Consistent with these results, the rate of thrombus growth was increased in Fn-EDA+/+ mice compared with Fn-EDA−/− mice (P < .05; Figure 1C). Several previous studies have demonstrated that cellular Fn-EDA+ interacts with the innate immune receptor TLR4, which has been shown to play a role in modulating thrombosis in the presence of its exogenous and endogenous ligands.21-24,30 To determine whether TLR4 contributes to cellular Fn-EDA+-mediated accelerated arterial thrombosis, we generated Fn-EDA+/+ mice on a TLR4-deficient background (Fn-EDA+/+/TLR4−/−). The time to form first thrombus and time to occlusion were significantly prolonged in Fn-EDA+/+/TLR4−/− mice compared with Fn-EDA+/+ mice (P < .05; Figure 2 A,C). In agreement with this finding, the rate of thrombus growth was significantly reduced in Fn-EDA+/+/TLR4−/− mice compared with Fn-EDA+/+ mice (P < .05; Figure 2B). To rule out the possibility that global deletion of TLR4 may have impaired thrombus formation independently of cellular Fn-EDA+ in Fn-EDA+/+ mice, we compared Fn-EDA−/− with Fn-EDA−/−/TLR4−/− mice. The mean time to form first thrombus, growth kinetics, and time to occlusion were similar in Fn-EDA−/− mice compared with Fn-EDA−/−/TLR4−/− mice (Figure 2A-C).
Cellular Fn-EDA+ promotes carotid artery thrombosis. Representative microphotographs and corresponding graphs showing quantitative analysis of time to first thrombus formation (A), time to occlusion (B), and thrombus growth (C) in FeCl3-injured carotid arteries as visualized by upright intravital microscopy. Platelets were labeled with calcein green. White lines delineate the arteries. *P < .05 compared with Fn-EDA−/− mice. Data are presented as mean ± SEM. N = 9 to 10 mice per group.
Cellular Fn-EDA+ promotes carotid artery thrombosis. Representative microphotographs and corresponding graphs showing quantitative analysis of time to first thrombus formation (A), time to occlusion (B), and thrombus growth (C) in FeCl3-injured carotid arteries as visualized by upright intravital microscopy. Platelets were labeled with calcein green. White lines delineate the arteries. *P < .05 compared with Fn-EDA−/− mice. Data are presented as mean ± SEM. N = 9 to 10 mice per group.
Cellular Fn-EDA+ promotes carotid artery thrombosis through TLR4. Graphs representing mean time to first thrombus formation (A), thrombus growth (B), and mean time to occlusion (C) in FeCl3-injured carotid arteries. *P < .05 compared with Fn-EDA−/−/TLR4+/+ mice. #P < .05 compared with Fn-EDA+/+/TLR4+/+ mice. Data are presented as mean ± SEM. N = 9 to 10 mice per group.
Cellular Fn-EDA+ promotes carotid artery thrombosis through TLR4. Graphs representing mean time to first thrombus formation (A), thrombus growth (B), and mean time to occlusion (C) in FeCl3-injured carotid arteries. *P < .05 compared with Fn-EDA−/−/TLR4+/+ mice. #P < .05 compared with Fn-EDA+/+/TLR4+/+ mice. Data are presented as mean ± SEM. N = 9 to 10 mice per group.
It was reported more than 20 years ago that cFn along with pFn can incorporate into fibrin clots,31 raising the possibility that cellular Fn-EDA+ may affect hemostasis as well as thrombosis. However, we found that bleeding times were normal in Fn-EDA+/+ mice when compared with Fn-EDA−/− mice (supplemental Figure 1). We found that TLR4−/− mice had normal bleeding times (supplemental Figure 1). Platelet GPIbα expression at baseline was comparable in Fn-EDA+/+, Fn-EDA−/−, and TLR4−/− mice (supplemental Figure 2). Thrombin-induced αIIbβ3 integrin activation and P-selectin exposure were similar in washed platelets from Fn-EDA+/+, Fn-EDA−/−, and TLR4−/− mice (supplemental Figure 3). No significant differences in total blood counts were observed among Fn-EDA+/+, Fn-EDA−/−, and TLR4−/− mice (not shown). Similarly to previous published studies,15 we found that Fn-EDA+/+ mice have significantly lower levels of plasma and platelet Fn when compared with Fn-EDA−/− mice (supplemental Figures 5 and 6). Deletion of TLR4 in Fn-EDA−/− or Fn-EDA+/+ mice did not alter plasma or platelet Fn levels, or fibrinogen level, when compared with Fn-EDA−/− or Fn-EDA+/+ mice, respectively (supplemental Figures 5-7).
TLR4 expressed on cells of hematopoietic origin promotes thrombosis in Fn-EDA+/+ mice
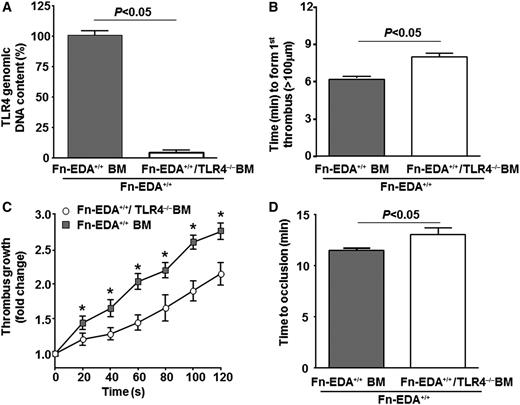
To investigate the cellular source of TLR4 that contributes to cellular Fn-EDA+-mediated accelerated arterial thrombosis, we transplanted irradiated Fn-EDA+/+ mice with bone marrow from either Fn-EDA+/+/TLR4−/− or Fn-EDA+/+/TLR4+/+ mice. This BMT protocol resulted in chimeric mice that express TLR4 on endothelial cells but lack TLR4 in cells of hematopoietic origin. Four weeks following BMT, real-time PCR analysis showed that ∼90% of circulating leukocytes from Fn-EDA+/+/TLR4−/−-BM→ Fn EDA+/+ mice lacked TLR4 in genomic DNA, whereas 100% of leukocytes from Fn-EDA+/+/TLR4+/+-BM→ Fn-EDA+/+ mice contained TLR4 (Figure 3A). The total blood cell counts were similar in Fn-EDA+/+/TLR4−/−-BM→ Fn-EDA+/+ and Fn-EDA+/+/TLR4+/+-BM→ Fn EDA+/+ mice (not shown). The time to form first thrombus (≥100 μm) and time to occlusion were significantly prolonged, and the rate of thrombus growth was significantly decreased in Fn-EDA+/+/TLR4−/−-BM→ Fn-EDA+/+ mice compared with Fn-EDA+/+/TLR4+/+-BM→ Fn-EDA+/+ mice (P < .05; Figure 3B-D).
TLR4 on cells of hematopoietic origin contributes to cellular Fn-EDA+-mediated accelerated thrombosis. (A) Real-time PCR analysis for TLR4 (normalized with glyceraldehyde-3-phosphate dehydrogenase) in genomic DNA from peripheral blood mononuclear cells after BMT as indicated. (B-D) Graphs representing mean time to first thrombus formation (B), thrombus growth (C), and mean time to occlusion (D) in FeCl3-injured carotid arteries. *P < .05 compared with Fn-EDA+/+ mice transplanted with bone marrow from Fn-EDA+/+/TLR4−/− mice. Data are presented as mean ± SEM. N = 9 to 10 mice per group.
TLR4 on cells of hematopoietic origin contributes to cellular Fn-EDA+-mediated accelerated thrombosis. (A) Real-time PCR analysis for TLR4 (normalized with glyceraldehyde-3-phosphate dehydrogenase) in genomic DNA from peripheral blood mononuclear cells after BMT as indicated. (B-D) Graphs representing mean time to first thrombus formation (B), thrombus growth (C), and mean time to occlusion (D) in FeCl3-injured carotid arteries. *P < .05 compared with Fn-EDA+/+ mice transplanted with bone marrow from Fn-EDA+/+/TLR4−/− mice. Data are presented as mean ± SEM. N = 9 to 10 mice per group.
Cellular Fn-EDA+ potentiates platelet aggregation through TLR4
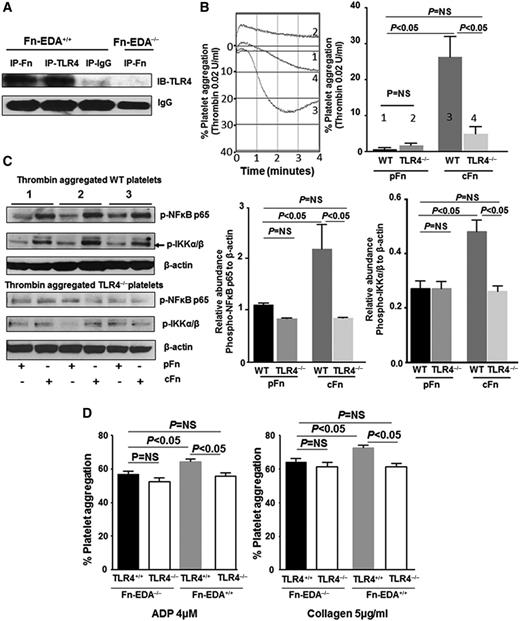
Platelets express functional TLR432 and its downstream signal transduction machinery.33 Platelet TLR4 has been shown to mediate microvascular thrombosis in endotoxemia23 and enhanced platelet aggregation with LPS34,35 and extracellular histones.21 We therefore determined if the prothrombotic effect of cellular Fn-EDA+ is mediated through platelet TLR4. Immunoprecipitation studies with platelet lysates confirmed that cellular Fn-EDA+ interacts with platelet TLR4 (Figure 4A). To determine whether cellular Fn-EDA+ directly mediates platelet aggregation, we examined the effect of human cFn (which contains EDA) on washed wild-type (WT) platelets. When used alone, cFn concentrations up to 100 μg/mL failed to induce platelet aggregation (not shown). Previously, it was shown that LPS alone up to 100 μg/mL does not induce platelet aggregation.34,35 However, LPS has been shown to potentiate platelet responses to subthreshold concentrations of agonists.34,35 Hence, we examined the effects of cFn on aggregation of washed platelets induced by low-dose thrombin (0.02 U/mL). Human pFn (which lacks EDA) was used as control for the potential confounding influence of Fn interactions with platelet integrins and fibrin. In the presence of cFn (40 μg/mL), stimulation with a subthreshold concentration of thrombin (0.02 U/mL) induced significant platelet aggregation (Figure 4B), whereas no aggregation response was observed with pFn (40 μg/mL) at the same dose of thrombin (Figure 4B). No differences in platelet responses were observed in the presence of cFn or pFn at higher thrombin concentrations (data not shown). To confirm that the proaggregatory effect of cFn is mediated through TLR4, we examined the effect of cFn on TLR4−/− platelets. In the presence of cFn (40 μg/mL), subthreshold thrombin (0.02 U/mL) induced significantly reduced platelet aggregation in TLR4−/− platelets compared with WT platelets (Figure 4B). This result strongly suggests that cFn promotes platelet aggregation directly through platelet TLR4.
Cellular Fn-EDA+ potentiates platelet aggregation through TLR4. (A) Representative immunoblots showing interaction between cellular Fn-EDA+ and TLR4. Proteins in platelet lysates from Fn-EDA+/+ or Fn-EDA−/− mice were immunoprecipitated with anti-Fn or anti-TLR4 antibodies or control IgG and immunoblotted using anti-TLR4 antibodies. (B) Representative tracings and corresponding bar diagrams showing aggregation responses of WT or TLR4−/− platelets to thrombin (0.02 U/mL) in the presence of 40 µg/mL of either human pFn or cFn. Tracing 1 represents WT washed platelets treated with pFn; tracing 2 represents TLR4−/− washed platelets treated with pFn; tracing 3 represents WT washed platelets treated with cFn; and tracing 4 represents TLR4−/− washed platelets treated with cFn. Data are presented as mean ± SEM. N = 4 to 5 mice per group. (C) Representative immunoblots and corresponding bar diagrams showing expression of phospho-NF-κB p65 and phospho-IKK α/β relative to β-actin in lysates of thrombin (0.02 U/mL) aggregated (4 minutes) WT and TLR4−/− platelets in the presence of either pFn or cFn. Data are presented as mean ± SEM. N = 3 mice per group. (D) Bar diagrams showing aggregation responses in PRP induced by adenosine 5′-diphosphate (ADP; 4 µM) or collagen (5 µg/mL). Data are presented as mean ± SEM. N = 5 to 6 mice per group.
Cellular Fn-EDA+ potentiates platelet aggregation through TLR4. (A) Representative immunoblots showing interaction between cellular Fn-EDA+ and TLR4. Proteins in platelet lysates from Fn-EDA+/+ or Fn-EDA−/− mice were immunoprecipitated with anti-Fn or anti-TLR4 antibodies or control IgG and immunoblotted using anti-TLR4 antibodies. (B) Representative tracings and corresponding bar diagrams showing aggregation responses of WT or TLR4−/− platelets to thrombin (0.02 U/mL) in the presence of 40 µg/mL of either human pFn or cFn. Tracing 1 represents WT washed platelets treated with pFn; tracing 2 represents TLR4−/− washed platelets treated with pFn; tracing 3 represents WT washed platelets treated with cFn; and tracing 4 represents TLR4−/− washed platelets treated with cFn. Data are presented as mean ± SEM. N = 4 to 5 mice per group. (C) Representative immunoblots and corresponding bar diagrams showing expression of phospho-NF-κB p65 and phospho-IKK α/β relative to β-actin in lysates of thrombin (0.02 U/mL) aggregated (4 minutes) WT and TLR4−/− platelets in the presence of either pFn or cFn. Data are presented as mean ± SEM. N = 3 mice per group. (D) Bar diagrams showing aggregation responses in PRP induced by adenosine 5′-diphosphate (ADP; 4 µM) or collagen (5 µg/mL). Data are presented as mean ± SEM. N = 5 to 6 mice per group.
To further characterize cFn-induced activation of TLR4 signaling in platelets, we looked at expression of phospho-IKK and phospho-NF-κB p65, which are components of the canonical signaling pathway downstream of TLR4 and have been reported to mediate potentiation of agonist-induced platelet responses by TLR4 agonists.35 Immunoblotting experiments showed significantly increased levels of phospho-IKK and phospho-NF-κB in low-dose thrombin–stimulated WT platelets in the presence of cFn compared with pFn (Figure 4C). These differences were not seen in TLR4−/− platelets. Together, these results suggest that cFn potentiates platelet aggregation and IKK/NF-κB signaling in thrombin-stimulated platelets.
To determine if cellular Fn-EDA+ present in plasma is able to promote platelet aggregation through TLR4, we used PRP from Fn-EDA+/+, Fn-EDA−/−, Fn-EDA+/+/TLR4+/+, and Fn-EDA−/−/TLR4−/− mice. PRP stimulated with low doses of adenosine 5′-diphosphate and collagen showed that Fn-EDA+ modestly but significantly increased platelet aggregation through TLR4 when compared with pFn found in Fn-EDA−/− mice (Figure 4D).
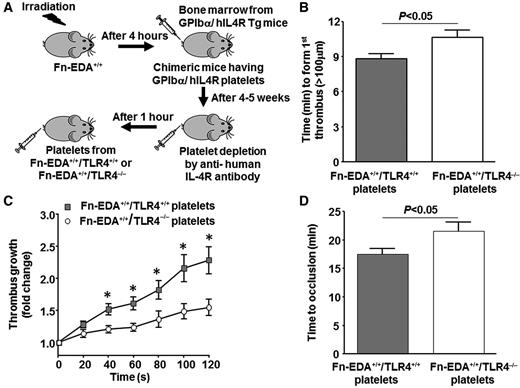
Platelet TLR4 contributes to accelerated thrombosis in Fn-EDA+/+ mice
Next, we determined whether cellular Fn-EDA+ present in plasma is able to promote arterial thrombosis in vivo through platelet TLR4. We used a recent elegant adoptive platelet transfer method,27,36 which allowed us to rapidly generate Fn-EDA+/+ mice lacking TLR4 only in platelets. We performed transplantation of bone marrow from GPIbα/human IL4R transgenic mice into Fn-EDA+/+ mice and characterized the hematopoietic phenotype by flow cytometry (supplemental Figure 4). GPIbα/human IL4R transgenic mice lack murine GPIbα but express the extracellular domain of the human IL4R fused to the transmembrane and cytoplasmic domains of human GPIbα.37 We then depleted circulating platelets using anti-hIL4R antibody as described36 and transfused platelets from either Fn-EDA+/+/TLR4−/− or Fn-EDA+/+/TLR4+/+ mice (Figure 5A). Using this strategy, we were able to induce endogenous platelet clearance without significantly affecting the function of transfused exogenous platelets in thrombus formation, growth, and subsequent occlusion of the injured vessels. Fn-EDA+/+ mice lacking platelet TLR4 showed significantly prolonged time to first thrombus formation and time to complete occlusion, as well as a decreased thrombus growth rate, compared with Fn-EDA+/+ mice containing platelet TLR4 (P < .05; Figure 5B-D).
Platelet TLR4 contributes to cellular Fn-EDA+-mediated accelerated thrombosis in injured arteries. (A) Schematic depicting the technique for generating chimeric mice with platelet-specific TLR4 deficiency. (B-D) Graphs representing mean time to first thrombus formation (B), thrombus growth (C), and mean time to occlusion (D) in FeCl3-injured carotid arteries. *P < .05 compared with mice infused with Fn-EDA+/+/TLR4−/− platelets. Data are presented as mean ± SEM. N = 9 to 10 mice per group.
Platelet TLR4 contributes to cellular Fn-EDA+-mediated accelerated thrombosis in injured arteries. (A) Schematic depicting the technique for generating chimeric mice with platelet-specific TLR4 deficiency. (B-D) Graphs representing mean time to first thrombus formation (B), thrombus growth (C), and mean time to occlusion (D) in FeCl3-injured carotid arteries. *P < .05 compared with mice infused with Fn-EDA+/+/TLR4−/− platelets. Data are presented as mean ± SEM. N = 9 to 10 mice per group.
Discussion
Platelets are now recognized to be important mediators of inflammation as well as thrombosis.38,39 Platelets express several innate immune receptors, including TLRs such as TLR4.32,33 The major new mechanistic finding of the current study is that TLR4 expressed on platelets mediates the prothrombotic effects of cellular Fn-EDA+ on platelet aggregation and arterial thrombosis.
Previous studies have suggested that inclusion of the EDA segment in Fn (cFn) promotes thrombosis in mice,15 but the underlying mechanism was not known. Recent studies have suggested a role for TLR4 signaling in promoting thrombosis,21-24 and the EDA segment of cFn, but not other domains, is known to activate human TLR4 expressed in HEK293 cells via myeloid differentiation-2.16 Interestingly, polymorphisms in human TLR4 have been associated with increased atherothrombotic risk.40,41 Additionally, LPS derived from gram-negative bacteria, a known ligand for TLR4, has been shown to induce platelet activation,34,42,43 platelet-neutrophil interaction,30 and microvascular thrombosis22,23 in a TLR4-dependent fashion. This prompted us to investigate the contribution of TLR4 signaling to cellular Fn-EDA+-mediated accelerated thrombosis using genetic approach in vitro and in vivo. Our results demonstrates that TLR4 signaling contributes to accelerated arterial thrombosis in mice expressing cellular Fn-EDA+. Of note, global deletion of TLR4 did not significantly affect arterial thrombosis in Fn-EDA−/− mice, which strongly suggests that the role of cellular Fn-EDA+ in promoting arterial thrombosis requires TLR4. Although our studies indicate that TLR4 plays an important role in accelerating thrombosis in Fn-EDA+/+ mice, it remains possible that other mechanisms that are TLR4 independent, such as an increased tendency of Fn-EDA+ to deposit as fibrils,44 or an alteration in arginine-glycine-aspartate motif present in the Fn type III-10 domain resulting in enhanced binding to platelet integrins αIIbβ3, ανβ3, and α5β1, may also contribute toward this process.1,44 Future complex studies will be required to define the role of these platelet integrins in Fn-EDA+-mediated accelerated thrombosis.
TLR4 is expressed on multiple cell types that might contribute to the prothrombotic effect of Fn-EDA+. By BMT experiments, we demonstrated that it is TLR4 on cells of hematopoietic origin, but not endothelial origin, that mediates the prothrombotic effects of cellular Fn-EDA+. Next, we investigated the specific effects of TLR4 expressed on platelets in contributing to prothrombotic effects of cellular Fn-EDA+. Platelets have functional TLR4,32 which is known to play a pathogenic role in microvascular thrombosis,23 thrombocytopenia,45 and NETosis30 associated with sepsis. LPS has been demonstrated to potentiate agonist-induced platelet aggregation and granule release.34,35,42 Recently, extracellular histones21 acting as endogenous ligands have been demonstrated to induce platelet aggregation in part through TLR4. These observations prompted us to determine the effects of cellular Fn-EDA+ on platelet aggregation because Fn-EDA+ has been reported to be a TLR4 ligand.16-18,20 Using commercially available human cFn (contains EDA), we determined in vitro platelet aggregation studies with washed platelets. Although cFn could not induce platelet aggregation on its own, even at relatively high concentrations (100 μg/mL), it potentiated aggregation induced by subthreshold thrombin stimulation in WT platelets. This potentiation effect was markedly decreased in TLR4−/− platelets, suggesting that cFn promotes platelet aggregation at least in part through TLR4. In concordance with these results, PRP containing cFn in Fn-EDA+/+ mice potentiated agonist-induced platelet aggregation to a small but significant extent compared with pFn present in PRP from Fn-EDA−/− mice, an effect not seen in Fn-EDA+/+ mice lacking TLR4, suggesting that cellular Fn-EDA+ requires TLR4 to promote in vitro platelet aggregation.
The precise signaling pathway that mediates the prothrombotic effects of TLR4 has not been defined. Platelets have the molecular machinery necessary for signal transduction downstream of TLR4, including the canonical MyD88/IKK/NF-κB pathway, which has been shown to be essential for platelet functional responses.46-48 The potentiation of platelet responses by LPS is known to be dependent on MyD8834 and possibly the IKK/NF-κB pathway.35 Consistent with these reports, we found that cellular Fn-EDA+ potentiated thrombin-induced IKK/NF-κB activation in WT but not TLR4−/− platelets. Platelet-specific IKK deficiency,49 as well as pharmacologic inhibition of the IKK/NF-κB pathway,46,47 leads to impairment of platelet responses. Based on these observations, we propose that elevated levels of cellular Fn-EDA+ present in pathological condition may potentiate platelet aggregation through platelet TLR4/IKK/NF-κB signaling. To further ascertain the role of platelet TLR4 in vivo, we performed platelet depletion and reinfusion (adoptive transfer) experiments. We found that Fn-EDA+/+ mice with Fn-EDA+/+/TLR4−/− platelets had significantly attenuated thrombosis compared with those with Fn-EDA+/+/TLR4+/+ platelets, establishing that platelet TLR4 contributes to Fn-EDA+-mediated accelerated arterial thrombosis in vivo, possibly by potentiation of agonist-induced platelet aggregation through IKK/NF-κB signaling.
There also are additional and/or alternative mechanisms that could underlie the role of platelet TLR4 in mediating the prothrombotic effects of cellular Fn-EDA+. First, platelet TLR4 activation can induce splicing of tissue factor messenger RNA and increased tissue factor-dependent platelet procoagulant activity.50 Secondly, platelet TLR4 can mediate the secretion of inflammatory cytokines particularly interleukin 1β, which can in turn activate platelets in an autocrine loop.42 Lastly, platelet-TLR4 can promote platelet-neutrophil interactions through an undefined mechanism and induce NETosis.30 The recruited neutrophils can contribute to arterial thrombosis by release of tissue factor51,52 and/or serine proteases, cathepsin G, and neutrophil elastase, which degrade tissue factor pathway inhibitor.53 The contribution of these alternate mechanisms to the observed prothrombotic effects of cellular Fn-EDA+ in vivo cannot be ruled out.
In summary, we demonstrated that cellular Fn-EDA+ has a prothrombotic role in thrombosis, which is distinct from pFn. Importantly, for the first time, we herein report a novel role for interaction between cellular Fn-EDA+ and platelet TLR4 in arterial thrombosis. Our findings suggest that elevated cellular Fn-EDA+ levels associated with clinical conditions including diabetes and atherosclerosis may result in enhanced thrombosis in these patients at high risk of cardiovascular events.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (R01 HL118246 and R01 HL118742 [A.K.C.] and HL062984 [S.R.L.]) and by a grant from the American Society of Hematology (S.R.L.).
Authorship
Contribution: P.P. and P.P.K. performed experiments, analyzed data, interpreted results, and cowrote the manuscript; S.R.L. contributed intellectually and helped to edit the manuscript; and A.K.C. directed the project, designed the study, interpreted results, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anil K. Chauhan, University of Iowa, Department of Internal Medicine, 25 S. Grand Ave, 3160 Medical labs, Iowa City, IA 52242; e-mail: anil-chauhan@uiowa.edu.