In this issue of Blood, Prakash et al provide compelling in vitro and in vivo evidence for a novel role of cellular fibronectin (cFn) containing extra domain A (Fn-EDA+) in arterial thrombosis.1
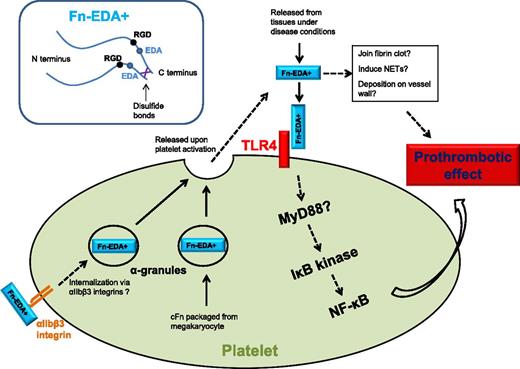
Fn-EDA+ interacts with platelet TLR4 to promote thrombosis, possibly through the platelet NF-κB signaling pathway. Platelet intracellular Fn-EDA+ is packaged from megakaryocytes and possibly also internalized via αIIbβ3 integrins, which are released upon activation from platelet α-granules. Fn-EDA+ may incorporate into fibrin clots, deposit on vessel wall, and induce NET formation.
Fn-EDA+ interacts with platelet TLR4 to promote thrombosis, possibly through the platelet NF-κB signaling pathway. Platelet intracellular Fn-EDA+ is packaged from megakaryocytes and possibly also internalized via αIIbβ3 integrins, which are released upon activation from platelet α-granules. Fn-EDA+ may incorporate into fibrin clots, deposit on vessel wall, and induce NET formation.
Fibronectins (Fn's) are large dimeric multidomain glycoproteins consisting of two 230- to 250-kDa monomers linked by disulfide bonds at their C terminus. Each Fn monomer contains collagen and fibrin binding sites in the N terminus and an Arg-Gly-Asp (RGD) integrin binding motif. The alternative splicing of Fn pre-messenger RNA (pre-mRNA) generates several Fn variants, which can be grouped into 2 main categories: plasma fibronectin (pFn) and cFn. In contrast to pFn, cFn contains either EDA or EDB or both.2 Fn's are widely distributed in extracellular matrix and blood circulation. They are required for embryogenesis, and are important for wound healing, infection, and malignant transformation.3
Using intravital microscopy models, the roles of both pFn and cFn in thrombosis have been established in the past decade.4-7 pFn is synthesized in the hepatocytes and accounts for almost all of the Fn's in the normal blood plasma. The depletion of pFn in mice resulted in a decrease of thrombus growth and stability.4 It was later found that pFn supports platelet aggregation and thrombosis only in the presence of fibrin, and the pFn-fibrin complex may be a prerequisite for its prothrombotic activities.5,6 In the absence of fibrin (eg, at the apical surface of the growing thrombi), pFn inhibits platelet aggregation, thus preventing excessive thrombosis and rescuing downstream blood supply.6 pFn also supports hemostasis through depositing on the injured vessel wall and strengthening the fibrin clot.6 cFn is synthesized by many cell types and joins the extracellular matrix locally. Although only minute amounts of cFn can be detected in blood plasma of healthy populations, plasma concentration of the EDA containing cFn, Fn-EDA+, is elevated in disease conditions such as atherosclerosis, ischemic stroke, and diabetes. To address the role of Fn-EDA+, Chauhan and colleagues previously studied thrombosis in a mouse model that constitutively expresses Fn-EDA+ but not pFn in the liver.7 Strikingly, despite a 70% to 80% decrease in total plasma Fn level, significantly enhanced thrombosis was observed in these mice as compared with wild-type mice with normal circulating pFn concentrations. This finding reveals an important fact: the extra domain in Fn-EDA+ does bring a higher thrombotic risk than pFn (ie, Fn's lack EDA). However, the mechanism for this prothrombotic function was previously unclear.
Here, Prakash and colleagues have addressed whether the observed prothrombotic effect of Fn-EDA+ can be mediated through its direct interaction with platelets.1 Using the FeCl3-induced carotid injury intravital microscopy model, they found that Fn-EDA+/+ mice exhibited significantly enhanced thrombosis compared with Fn-EDA−/− mice. However, this prothrombotic phenotype was abolished in the absence of Toll-like receptor 4 (TLR4). They further demonstrated that Fn-EDA+ targets TLR4 in hematopoietic lineage cells following bone marrow transplants with or without TLR4 expression (EDA+/+/TLR4+/+ or EDA+/+/TLR4−/−) into Fn-EDA+/+ mice. Finally, after depletion of endogenous platelets in Fn-EDA+/+ mice, mice transfused with EDA+/+/TLR4+/+ but not EDA+/+/TLR4−/− platelets showed enhanced thrombosis, thus localizing the downstream of Fn-EDA+ prothrombotic function to platelet TLR4. In addition, activation of a downstream nuclear factor κB (NF-κB) signaling pathway within the platelet was found following TLR4 activation by Fn-EDA+. This informative study reveals a novel Fn-EDA+ and platelet TLR4 interaction that contributes to thrombosis, suggesting a possible link between elevated Fn-EDA+, innate immunity, and thrombotic risk.
Is TLR4 the only explanation?8 One interesting finding of this article is that the prothrombotic effect of Fn-EDA+ in in vivo thrombosis models seems more striking than in vitro platelet aggregation assay.1 Could interaction between Fn-EDA+ and fibrin also contribute to the enhanced thrombosis? Fn's can be covalently linked with the fibrin network, and pFn was found to strengthen the fibrin clot.6 Because cFn possesses a greater tendency for polymerization than pFn, it is possible that Fn-EDA+ may enhance the clot stiffness even further. Bacterial lipopolysaccharide (LPS), a TLR4 ligand, has been reported to interact with platelet TLR4 to induce neutrophil extracellular trap (NET) formation.9 Could Fn-EDA+ induce NET formation through TLR4? As the link between NETs and thrombosis has been established recently, it is tempting to contemplate that Fn-EDA+ may also enhance thrombosis by promoting NET formation. These possibilities are especially appealing for the study of deep vein thrombosis, inflammation, and innate immunity, in which both fibrin and NETs play a vital role (see figure).
Platelets contain both pFn (∼80% of platelet Fn's) and cFn. pFn is internalized through platelet surface αIIbβ3 integrin,10 whereas cFn is synthesized in megakaryocytes and packaged in platelets. Although present at relatively small amounts, these Fn's can be released from α-granules upon platelet activation and boost the Fn concentration at the site of thrombosis (see figure). With an increase in Fn-EDA+ in blood plasma, the platelet Fn-EDA+ likely also increases via αIIbβ3 integrin internalization. Could the platelet endogenous Fn-EDA+ also contribute to thrombosis? Another interesting fact is that platelet aggregation and thrombus formation persist in the absence of both fibrinogen and von Willebrand factor (VWF), 2 factors previously considered essential for thrombosis. The platelet content of Fn in fibrinogen- and VWF-deficient animals is threefold to fivefold higher. Although pFn has been subsequently ruled out as the factor to mediate fibrinogen- and VWF-independent platelet aggregation, the prothrombotic Fn-EDA+ remains a possible candidate.5
In summary, the study from Prakash and colleagues is another solid step toward understanding how extra domains regulate Fn function. Fn's, either cFn or pFn, change their circulating concentration under many disease conditions. The low level of ED-containing cFn in normal blood circulation may be a result of natural selection to reduce thrombotic risk. Further investigations of circulating Fn-EDA+ in disease conditions may establish it as a potential therapeutic target for thrombotic disorders.
Conflict-of-interest disclosure: The authors declare no competing financial interests.