Key Points
Proteinuria and elevated markers of complement activation at TMA diagnosis are associated with poor outcome.
Clinical interventions should be considered in HSCT patients with these high-risk features at the time TMA is diagnosed.
Abstract
Transplant-associated thrombotic microangiopathy (TMA) leads to generalized endothelial dysfunction that can progress to multiorgan injury, and severe cases are associated with poor outcomes after hematopoietic stem cell transplantation (HSCT). Identifying patients at highest risk for severe disease is challenging. We prospectively evaluated 100 consecutive HSCT recipients to determine the incidence of moderate and severe TMA and factors associated with poor overall outcomes. Thirty-nine subjects (39%) met previously published criteria for TMA. Subjects with TMA had a significantly higher nonrelapse mortality (43.6% vs 7.8%, P < .0001) at 1 year post-HSCT compared with those without TMA. Elevated lactate dehydrogenase, proteinuria on routine urinalysis, and hypertension were the earliest markers of TMA. Proteinuria (>30 mg/dL) and evidence of terminal complement activation (elevated sC5b-9) in the blood at the time of TMA diagnosis were associated with very poor survival (<20% at 1 year), whereas all TMA subjects without proteinuria and a normal sC5b-9 serum concentration survived (P < .01). Based on these prospective observations, we conclude that severe TMA occurred in 18% of HSCT recipients in our cohort and propose an algorithm to identify the highest-risk patients who might benefit from prompt clinical interventions.
Introduction
Hematopoietic stem cell transplantation (HSCT)-associated thrombotic microangiopathy (TMA) is a severe complication of HSCT and may affect 20% to 30% of recipients.1 TMA is a multifactorial disease where generalized endothelial dysfunction leads to microangiopathic hemolytic anemia, intravascular platelet activation, and formation of platelet-rich thrombi within the microcirculation.2,3 These processes can contribute to end-organ injury and poor outcomes, especially involving the kidney.4-6
TMA can range from a mild, self-limited form to uncontrolled fulminant disease leading to death. The reasons behind the heterogeneity in disease severity are unknown but are likely related to factors involving the recipient, the donor, and/or triggering events such as infections. Multiorgan involvement typically manifests as pulmonary hypertension, polyserositis, gastrointestinal symptoms, central nervous system injury, and renal impairment.7-10
The mechanisms causing endothelial injury in patients with TMA are only beginning to be studied. We recently reported that activation of the complement system may be involved in the pathogenesis of TMA and have described the potential therapeutic benefit of terminal complement blockade by eculizumab.11-13
It remains a challenge to identify patients at highest risk for severe disease who may benefit from prompt clinical interventions.14-16 Current diagnostic, prognostic, and therapeutic recommendations for HSCT-TMA are based on retrospective studies.17-19 Therefore, we prospectively recruited children and young adults undergoing HSCT at our center to examine risk factors for the most severe TMA outcomes. Our objectives were to update the available diagnostic criteria and assess factors associated with a higher risk of death in patients diagnosed with TMA after HSCT. Reliable diagnostic and prognostic criteria will guide the use of expensive therapies such as complement blockade.
Methods
Study subjects
All patients from birth to 30 years of age scheduled to undergo HSCT at Cincinnati Children’s Hospital Medical Center (CCHMC) were approached to participate in this prospective study, after approval from the CCHMC institutional review board. The study was conducted in accordance with the Declaration of Helsinki. Our goal was to enroll 100 subjects to be followed until 1 year after transplant. Clinical data were collected starting 3 weeks prior to the conditioning regimen. Laboratory data were captured from the electronic medical record, and information was stored in a REDCap database.
Definition of TMA
TMA was diagnosed using published diagnostic criteria proposed by Cho et al (2010)17 that included (1) lactate dehydrogenase (LDH) elevated above the upper limit of normal for age; (2) de novo thrombocytopenia with a platelet count <50 × 109/L or a ≥50% decrease in the platelet count; (3) de novo anemia with a hemoglobin below the lower limit of normal or anemia requiring transfusion support; (4) microangiopathic changes defined as the presence of schistocytes in the peripheral blood or histologic evidence of microangiopathy on a tissue specimen; and (5) absence of a coagulopathy and a negative Coombs test. All laboratory criteria had to occur concurrently, and criteria 1 to 4 were documented on at least 2 consecutive tests to be classified as positive. ADAMTS13 activity was measured in subjects with TMA to exclude a diagnosis of thrombotic thrombocytopenic purpura. The date of TMA diagnosis was defined as the first date when all diagnostic criteria were fulfilled.
Laboratory monitoring
Complete blood count and electrolyte panels were monitored daily. Schistocytes were counted by direct examination of the peripheral blood smear and reported as 1+ or 2+ (1-4 or 5-8 cells per oil immersion field [100× objective], respectively). LDH was measured twice a week. Haptoglobin and urinalyses were performed weekly. Random urine protein and creatinine quantification was measured in subjects with proteinuria. Proteinuria was defined as a random urinalysis protein concentration of ≥30 mg/dL and separately examined by random urine protein to creatinine ratio (normal <0.2 mg/mg, nephrotic range >2 mg/mg).20,21 Serum concentration of the soluble membrane attack complex (sC5b-9) at the time of TMA diagnosis and in 20 consecutive time-matched HSCT recipients without TMA was tested by enzyme-linked immunosorbent assay at the CCHMC Hematology Clinical Laboratory (normal 72-244 ng/mL).13,22,23
Clinical monitoring
The total number of red cell and platelet transfusions were documented during the first 100 days after transplant. Transfusion criteria were a hemoglobin of <7 g/dL and a platelet count of <10 × 109/L. Platelet engraftment was defined as a platelet count of 20 × 109/L for 3 consecutive days without transfusions. All subjects had routine monitoring for cytomegalovirus and Epstein-Barr virus 1 or 2 times per week by polymerase chain reaction. Acute kidney injury (AKI) was defined as a doubling of the serum creatinine from each subject’s pre-HSCT baseline.20,21 Raw systolic and diastolic blood pressure values were transformed to a standardized blood pressure index where an index value of 1 indicates a blood pressure at the 95th percentile value for age, sex, and height. For subjects ≥18 years of age, hypertension was defined as a blood pressure ≥140/90 mm Hg. We documented the number of antihypertensive medications required by each patient to achieve hypertension index of <1.24
We recorded transplant complications and biopsy/autopsy data. There were no uniform guidelines for the treatment of TMA, so any TMA-directed interventions such as therapeutic plasma exchange, rituximab, or cyclosporine (CSA) withdrawal were recorded from the medical record.
Study outcomes
We examined risk factors for TMA by comparing subjects who developed TMA with the HSCT recipients who did not. To identify risk factors for death, we separately examined the subjects with TMA by whether they were alive or dead at 1 year post-HSCT. We analyzed the outcome of death as nonrelapse mortality (NRM) and overall survival (OS) at 1 year post-HSCT.
Statistical analysis
Continuous variables were reported as medians and interquartile ranges (IQR), and differences between groups were analyzed with Wilcoxon rank sums. Fisher’s exact tests were used to compare categorical variables. To assess factors associated with TMA diagnosis, all variables were analyzed based on their occurrence at any time during the first 100 days after HSCT. Acute graft-versus-host disease (GVHD) and infections were included as time-dependent exposures in that they were only considered prior to the diagnosis of TMA in those developing TMA and at any time in the first 100 days in subjects without TMA. Differences in CSA trough levels between the study groups were compared using a linear mixed model, which assumed an autoregressive correlation to account for the dependence of CSA measurements taken within a single subject.
In the TMA group, we plotted the time course of hematologic and kidney-specific markers relative to the TMA diagnosis day using the first date when the marker was present in the time-course analysis. Hypertension was defined as the first day when the systolic index was ≥1.
Finally, we examined risk factors for death in subjects with TMA by including the LDH, haptoglobin, and proteinuria values closest to TMA diagnosis, ±3 days. The lowest hemoglobin and platelet count were also included from ±3 days around the TMA diagnosis to account for the potential effect of transfusions. Systolic hypertension was recorded as the median index value for each subject for the 3 days prior to TMA. The concentration of sC5b-9 was measured on stored samples obtained at TMA diagnosis.
The Kaplan-Meier method was used to estimate the 1-year OS, comparing subjects with and without TMA. Gray’s competing risk method was used to compute the 1-year NRM cumulative incidence curve. Cox regression models were used to estimate the hazard ratios for death among subjects with TMA with respect to proteinuria >30 mg/dL and serum sC5b-9 concentrations, where OS time was the dependent variable and the binary predictors were included as independent variables. All tests conducted were 2-tailed, and P values < .05 were considered statistically significant. Statistical analyses were performed with R statistical software (version 3.0.1).
Results
Cohort characteristics
One hundred consecutive HSCT recipients were enrolled into this study from September 2010 until December of 2011. Overall, 39 out of 100 (39%) subjects met study criteria for a diagnosis of TMA. TMA was not diagnosed in any of the 10 autologous transplant recipients, so analyses were restricted to the 90 allogeneic recipients. TMA was diagnosed a median of 32 days [IQR 17-43] posttransplant (day 0), with 36 out of 39 (92.3%) diagnosed within the first 100 days and the remaining 3 subjects diagnosed before 1 year posttransplant.
The demographic and transplant characteristics of the allogeneic transplant recipients are shown in Table 1. Most of the subjects were white children, with a higher proportion of males. Nonmalignant diseases were the most common underlying diagnosis (71%). Sixty-five recipients (72.2%) received an unrelated donor graft, and the most common stem cell source was bone marrow. A similar number of subjects received myeloablative and reduced-intensity regimens (53% vs 47%). Nearly all (95.6%) of the HSCT recipients received CSA for GVHD prophylaxis. Only subjects with Fanconi anemia (FA) received ex vivo T-cell–depleted peripheral blood stem cells (PBSCs) in addition to CSA. Uniform drug monitoring was used to maintain a CSA trough of 250 to 350 ng/mL.
Risk factors for TMA
Among the 90 HSCT recipients, subjects with TMA were more likely to have a nonmalignant disorder (84.6% vs 60.7%, P < .01) and receive a PBSC graft (25.6% vs 2.0%, P < .01). Although subjects without TMA were more likely to have received total body irradiation (P = .06), this was confounded by the fact that it was only used for subjects with malignancy, who themselves had a lower risk of TMA. CSA combined with ex vivo T-cell depletion was associated with a higher risk of TMA (20.5% vs 2.0%, P = .01) but again was used as GVHD prophylaxis only in subjects with FA. We were not able to evaluate CSA as an independent risk factor for TMA because nearly all subjects (95.6%) received CSA for GVHD prophylaxis. On average, subjects had 19 CSA measurements during the first 100 days posttransplant. CSA level was 251.6 and 247.2 ng/mL for the subjects with TMA and without TMA, respectively (P = .65).
Subjects with TMA required almost twice as many platelet and red cell transfusions during the first 100 days and took longer to achieve platelet engraftment (Table 2). Both the presence of ≥30 mg/dL of protein on urinalysis (P < .01) and persistence of proteinuria for >2 weeks were significantly more common in subjects with TMA (P < .01). Subjects with TMA also had a higher degree of proteinuria as defined by a random urine protein to creatinine ratio, with the median level of proteinuria in the nephrotic range (ie, > 2 mg/mg). The risk of AKI (measured by serum creatinine) was slightly higher in the TMA group (46.2% vs 33.3%), but this difference was not statistically significant (P = .28). More subjects with TMA had elevated sC5b-9 concentrations at the time of TMA diagnosis (71.7% vs 20%, P < .01), as compared with the 20 HSCT subjects without TMA who were tested at approximately 30 days after HSCT (median time to TMA diagnosis). Serum sC5b-9 concentrations in subjects with TMA as compared with those without TMA were 332.9 ng/mL (IQR 274.45-445.05 ng/mL) and 201.4 ng/ mL (IQR 171.7-273.9 ng/mL), respectively (P = .03).
Acute GVHD of any grade was not a risk factor for the subsequent development of TMA (P = .61). Infections documented prior to TMA diagnosis were also not associated with an increased risk of TMA. Subjects with TMA required more medications to maintain a systolic blood pressure index <1 and were more likely to be admitted to the intensive care unit and develop respiratory failure, gastrointestinal bleeding, and pulmonary hypertension (Table 2). We explored several multivariable models to examine independent risk factors for the development of TMA, but none identified significant associations.
Time course of TMA diagnosis
We plotted the time-course of diagnostic markers for TMA (Figure 1). Systolic hypertension, an elevated LDH, and proteinuria occurred 10 to 14 days prior to TMA diagnosis. A decreased haptoglobin lagged the first elevation in LDH by almost 2 weeks, and AKI, defined as doubling of the serum creatinine, did not occur until almost a month after TMA diagnosis.
Time course of clinical and laboratory markers in relation to date of TMA diagnosis. The date of TMA diagnosis is marked as day 0 (black vertical line) and represents the posttransplant day when study diagnostic criteria for TMA were fulfilled, including elevated LDH and schistocytosis. We additionally included the timing of proteinuria (≥30 mg/dL on random urinalysis), hypertension (defined as a systolic blood pressure >95th percentile for age, sex, and height), AKI (doubling of the pretransplant serum creatinine), and a haptoglobin less than the lower limit of normal. For each variable, the white vertical lines represent the median day the criterion became positive and the gray area the IQR. Hypertension (day −14) and an elevated LDH (day −13) were the first markers of TMA, followed by proteinuria (day −10). Schistocytosis occurred on day 0, because this was typically the last criterion to turn positive and thus defined the TMA diagnosis date. A decreased haptoglobin lagged the first elevation in LDH by almost 2 weeks. AKI, defined using serum creatinine, occurred a median of almost 28 days after TMA was diagnosed.
Time course of clinical and laboratory markers in relation to date of TMA diagnosis. The date of TMA diagnosis is marked as day 0 (black vertical line) and represents the posttransplant day when study diagnostic criteria for TMA were fulfilled, including elevated LDH and schistocytosis. We additionally included the timing of proteinuria (≥30 mg/dL on random urinalysis), hypertension (defined as a systolic blood pressure >95th percentile for age, sex, and height), AKI (doubling of the pretransplant serum creatinine), and a haptoglobin less than the lower limit of normal. For each variable, the white vertical lines represent the median day the criterion became positive and the gray area the IQR. Hypertension (day −14) and an elevated LDH (day −13) were the first markers of TMA, followed by proteinuria (day −10). Schistocytosis occurred on day 0, because this was typically the last criterion to turn positive and thus defined the TMA diagnosis date. A decreased haptoglobin lagged the first elevation in LDH by almost 2 weeks. AKI, defined using serum creatinine, occurred a median of almost 28 days after TMA was diagnosed.
Risk factors for death in those diagnosed with TMA
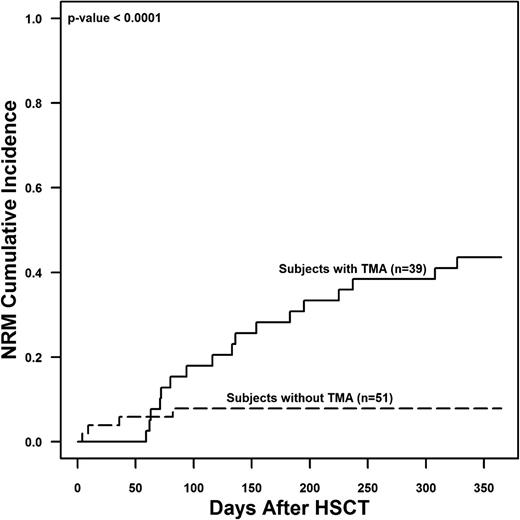
Of the 39 subjects with TMA, 18 (46.2%) died by 1 year posttransplant in comparison with only 5 of 51 (9.8%) without TMA (P < .01). Subjects with TMA had a significantly higher risk of 1-year NRM (43.6% ± 8% vs 7.8% ± 3.8%, P < .0001) compared with those without TMA (Figure 2). In the 18 subjects that died with active TMA, the median time to death was 116 days after HSCT (IQR 71-195 days).
NRM among study subjects with and without TMA at 1 year after HSCT. Gray’s competing risk method was used to obtain the cumulative incidence of NRM. The 1-year NRM was 43.6% ± 8% in subjects with TMA and 7.8% ± 3.8% in HSCT subjects without TMA (P < .0001).
NRM among study subjects with and without TMA at 1 year after HSCT. Gray’s competing risk method was used to obtain the cumulative incidence of NRM. The 1-year NRM was 43.6% ± 8% in subjects with TMA and 7.8% ± 3.8% in HSCT subjects without TMA (P < .0001).
All subjects who died after a TMA diagnosis had active laboratory or histologic signs of microangiopathy at the time of death. The cause of death in these 18 subjects was listed as follows: TMA/multiorgan failure (n = 4), TMA/pulmonary hypertension (n = 3), TMA/sinusoidal obstructive syndrome (n = 1), acute GVHD/multiorgan failure (n = 6), infection (n = 1), posttransplant lymphoproliferative disorder (n = 1), acute myeloid leukemia relapse (n = 1), and necrotizing encephalopathy (n = 1). Two subjects had an autopsy showing severe multiorgan TMA involving the kidney, lung, bowel, and heart. Both subjects had clinical and histologic evidence of pulmonary hypertension and histologic evidence of TMA in mesenteric arterioles and denuded bowel mucosa. Laboratory studies at TMA diagnosis showed elevated serum sC5b-9 concentrations and proteinuria >30 mg/dL.
Factors associated with a higher risk of death among those diagnosed with TMA are shown in supplemental Table 1 available at the Blood Web site. Subjects with proteinuria ≥30 mg/dL (P = .01), proteinuria persisting >2 weeks (P = .04), higher median urine protein to creatinine ratio (1.8 vs 0.8 mg/mg, P = .03), and elevated sC5b-9 above normal at TMA diagnosis (P = .005) were at higher risk of death. There were no significant differences in demographic and HSCT characteristics in subjects with elevated vs normal sC5b-9 at TMA diagnosis, but overall outcomes were significantly worse in subjects with elevated sC5b-9. Of the 28 subjects with elevated sC5b-9, 17 died by 1 year after HSCT, whereas of the 11 subjects with TMA and normal sC5b-9, only 1 died (60.7% vs 9%, P = .004). Subjects with TMA who died had a median sC5b-9 concentration of 498.2 ng/mL (IQR 304.8-713.2 ng/mL) as compared with 289 ng/mL (IQR 143.8-360.4 ng/mL) in surviving subjects with TMA (P < .001). The overall incidence of acute GVHD was similar in both groups (27.8% vs 23.8%). Viral infections or bacteremia were not associated with a higher risk of death in those with TMA. Subjects with TMA who died required a greater number of antihypertensive medications to control their blood pressure and were more likely to develop gastrointestinal bleeding (55.6% vs 0%, P < .01). More nonsurvivors required renal replacement therapy (22.2% vs 5.6%), but this difference was not statistically significant (P = .16). Finally, there was no difference in treatments received between nonsurvivors and survivors, including therapeutic plasma exchange, rituximab, or CSA withdrawal.
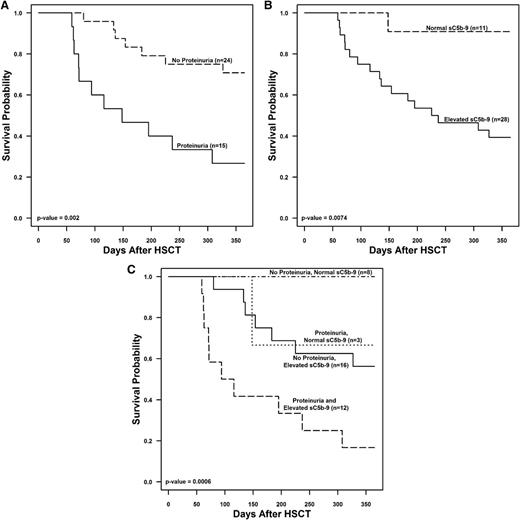
To further examine factors associated with death by 1 year after transplant, we compared the following laboratory and clinical markers within ±3 days of TMA diagnosis between subjects with TMA who died and those who survived: (1) an LDH 1.5 times above the upper limit of normal for age; (2) a platelet count nadir <50 ± 109/L; (3) a hemoglobin nadir <8 g/dL; (4) haptoglobin below normal; (5) proteinuria >30 mg/dL; (6) a random urine protein/creatinine ratio >2 mg/mg; (7) a systolic hypertension index ≥1; and (8) a sC5b-9 concentration above normal indicating terminal complement activation. Schistocytosis was present in all subjects at TMA diagnosis so was not included in the models. Figure 3 shows the association between each marker and the risk of death by 1 year after HSCT. At the time of TMA diagnosis, greater anemia, proteinuria, and activation of terminal complement were associated with an increased risk of death. Haptoglobin was higher in subjects who died, likely representing tissue injury as a nonspecific inflammatory marker.25 For further analysis, we focused on the prognostic significance of proteinuria and the sC5b-9 concentration as the hemoglobin value may be confounded by transfusions. Subjects who had either proteinuria ≥30 mg/dL or elevated sC5b-9 at TMA diagnosis had worse 1-year posttransplant survival (26.7% and 39.3%) than those who did not (70.8% and 91%) (P < .001). Subjects with either proteinuria or elevated sc5b-9 at TMA diagnosis had a significantly increased hazard ratio for death of 4.06 (95% confidence interval [CI], 1.56-10.55; P = .002) and 9.5 (95% CI, 1.2-71.8; P = .0074), respectively, as compared with subjects without proteinuria or with normal sC5b-9. Subjects with both proteinuria and an elevated sC5b-9 at the time of TMA diagnosis had the lowest 1-year survival (16.7% ± 10.8%), whereas subjects with a normal sC5b-9 concentration and no proteinuria had a 1-year survival of 100% (P < .01). There were no death events in the no proteinuria and normal sC5b-9 group, thus the hazard ratio could not be estimated. Subjects with no proteinuria and elevated sC5b-9 and with proteinuria and normal sC5b-9 both had reduced hazard ratios of 0.3 (95% CI, 0.1-0.8, P = .01) and 0.22 (95% CI, 0.03-1.73, P = .15), respectively, although this did not reach statistical significance in the proteinuria and normal sC5b-9 group (Figure 4).
Association between markers at the time of TMA diagnosis and death by 1 year after HSCT. At the time of TMA diagnosis, a hemoglobin ≤8 g/dL, proteinuria (defined either as ≥30 mg/dL of protein on a random urinalysis or a random urine protein/creatinine [Ur prot/creat] ratio >2 mg/mg), and a soluble membrane attack complex (sC5b-9) concentration above the upper limit of normal were all significantly associated with an increased risk of death. Haptoglobin was higher in patients who died, likely representing an acute inflammatory state.
Association between markers at the time of TMA diagnosis and death by 1 year after HSCT. At the time of TMA diagnosis, a hemoglobin ≤8 g/dL, proteinuria (defined either as ≥30 mg/dL of protein on a random urinalysis or a random urine protein/creatinine [Ur prot/creat] ratio >2 mg/mg), and a soluble membrane attack complex (sC5b-9) concentration above the upper limit of normal were all significantly associated with an increased risk of death. Haptoglobin was higher in patients who died, likely representing an acute inflammatory state.
TMA risk stratification. The figure displays Kaplan-Meier survival curves for subjects with TMA (n = 39) examining proteinuria ≥30 mg/dL and serum sC5b-9 concentrations at the time of TMA diagnosis in (A) subjects with proteinuria of ≥30 mg/dL vs no proteinuria, (B) subjects with elevated serum sC5b-9 concentration vs normal sC5b-9 concentration, and (C) subjects with no proteinuria and normal sC5b-9, proteinuria ≥30 mg/dL and normal sC5b-9, no proteinuria, and elevated sC5b-9 and both proteinuria ≥30 mg/dL and elevated sC5b-9 at the time of TMA diagnosis.
TMA risk stratification. The figure displays Kaplan-Meier survival curves for subjects with TMA (n = 39) examining proteinuria ≥30 mg/dL and serum sC5b-9 concentrations at the time of TMA diagnosis in (A) subjects with proteinuria of ≥30 mg/dL vs no proteinuria, (B) subjects with elevated serum sC5b-9 concentration vs normal sC5b-9 concentration, and (C) subjects with no proteinuria and normal sC5b-9, proteinuria ≥30 mg/dL and normal sC5b-9, no proteinuria, and elevated sC5b-9 and both proteinuria ≥30 mg/dL and elevated sC5b-9 at the time of TMA diagnosis.
Discussion
To our knowledge, this is the first prospective study examining risk factors for TMA and providing risk stratification in HSCT recipients diagnosed with this complication. Our study cohort was composed of complex transplant recipients including a high proportion of unrelated donors, young children, and subjects receiving HSCT for nonmalignant disorders. We observed a TMA incidence of 39% based on currently published diagnostic criteria, with 92.3% of cases occurring within 100 days of transplant and a median time to presentation of 32 days. Although we previously observed a high risk of TMA after autologous transplantation, especially in children with neuroblastoma, none of the 10 autologous transplant recipients in our cohort developed TMA.13,26
The overall incidence of TMA that we observed is higher than what has been reported by others, likely because we prospectively monitored all recipients in a uniform manner, in contrast to retrospective reports that likely captured only the most severe cases.17,25,27 Consistent with our previous retrospective studies, we observed that proteinuria and hypertension were early markers of TMA and were present in some subjects even before the full hematologic criteria became apparent.26 Specifically, proteinuria >30 mg/dL as measured by routine dipstick and hypertension >95th percentile were the earliest signs of TMA, along with an elevation in the LDH. In contrast, kidney dysfunction assessed by serum creatinine was a very late marker, highlighting its limitations, as it remains an insensitive marker to detect impaired renal function in HSCT recipients, who potentially have low muscle mass and thus low creatinine generation rates.17,25,26,28,29
These observations suggest that a diagnosis of TMA should be considered in HSCT recipients with an acute elevation in LDH, proteinuria, and hypertension out of proportion to what would be expected from calcineurin inhibitor and steroid therapy. Importantly, these markers were apparent up to 2 weeks before schistocytes were detected. The haptoglobin level was actually higher among subjects with TMA who died, possibly indicating its reflection as a marker of inflammation.30-32 Therefore, the absence of a low haptoglobin should not exclude consideration of the diagnosis of TMA in HSCT recipients. These factors may be due to an earlier recognition of the TMA symptoms in this study given the systematic and careful monitoring of subjects, but it could also indicate that HSCT-associated TMA is a different and distinct disease from atypical hemolytic uremic syndrome and other disorders that share features of complement dysregulation. Future research should be targeted at identifying novel markers of endothelial injury to aid in the earlier diagnosis of TMA after HSCT, because the available laboratory markers appear over a period of 2 to 3 weeks, possibly delaying diagnosis and prompt intervention.
Examining risk factors associated with TMA after allogeneic transplant, we found a higher incidence of TMA among subjects with nonmalignant disorders and those receiving PBSCs or GVHD prophylaxis including CSA with ex vivo T-cell depletion. Only children with FA in our study, 89% of whom developed TMA, received a treatment including T-cell–depleted PBSCs and CSA, so it is impossible to tell whether the increased risk of TMA reflected increased susceptibility in persons with FA or was associated with the treatment protocol. We were unable to evaluate CSA as an independent risk factor, as almost all of our allogeneic subjects received CSA as part of their GVHD prophylaxis. However, CSA serum levels were similar in subjects with and without TMA during first 100 days after transplantation.
Acute GVHD and infections have been associated with the development of TMA in retrospective studies.10,19,33 However, in our time-dependent analyses, we were unable to detect an association between acute GVHD or infections preceding the diagnosis of TMA. Whereas GVHD is typically associated with damage to the epithelial tissues of the skin, lungs, and gastrointestinal systems, some have speculated that TMA represents a form of “endothelial GVHD,” a hypothesis that deserves further study as we learn more about specific biomarkers of endothelial dysfunction.34
Subjects who fulfilled the diagnostic criteria for TMA had a higher risk of NRM and a far worse OS at 1-year posttransplant. This supports that TMA remains a significant complication of HSCT. Subjects who were diagnosed with TMA had multiple clinically significant complications, including severe hypertension requiring a median of at least 3 antihypertensive medications to control their blood pressure. Additionally, pulmonary hypertension was diagnosed exclusively in the TMA group, and severe gastrointestinal bleeding occurred only in subjects with TMA who did not survive transplant. This supports prior observations that TMA can present as a multiorgan disease that may coexist or may be attributed to other HSCT complications, such as GVHD, as demonstrated by the autopsy findings.7,35-40
The severity of TMA in our cohort ranged from self-limiting to acute multiorgan failure and death. One of our objectives was to examine factors associated with a higher risk of death among subjects with TMA to identify those who may benefit from therapeutic interventions. Subjects who died after TMA diagnosis had a greater degree of anemia, higher risk of proteinuria, and were more likely to have evidence of terminal complement activation at their time of TMA diagnosis. Elevated levels of sC5b-9 were present in nearly all subjects with TMA who died but in only about half of those who survived. These data suggests that complement activation plays a significant role in the pathogenesis of severe TMA after HSCT.
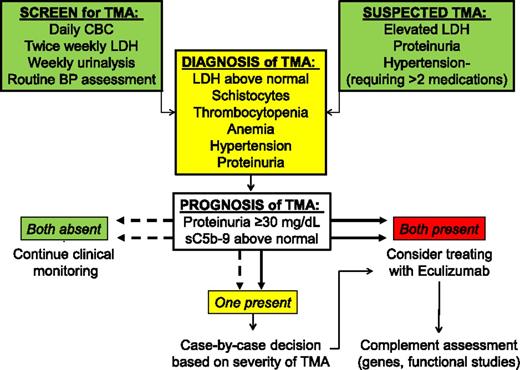
Hemoglobin concentration can be more difficult to interpret in subjects requiring frequent transfusions, so we focused on proteinuria and an elevated sC5b-9 in our final risk-stratification model. Subjects with both proteinuria and signs of terminal complement activation at TMA diagnosis had a 1-year survival <20%, whereas subjects with normal sC5b-9 and no proteinuria had a survival of 100%. Accordingly, we propose a diagnostic and prognostic algorithm for the evaluation of TMA after HSCT (Figure 5). In subjects with evidence of terminal complement activation measured by elevated serum sC5b-9 concentration, we also suggest an evaluation of the terminal complement cascade, including factor H–related genes, as reported previously.12 Future prospective trials are needed to test the hypothesis that blockade of the complement cascade, with eculizumab or other therapies under development, will improve outcomes among the highest-risk HSCT recipients.
Algorithm for the evaluation of TMA after HSCT. Screening for TMA includes monitoring LDH, complete blood count, and routine urinalyses. TMA should be suspected in HSCT recipients with an acute elevation of LDH, proteinuria >30 mg/dL, and hypertension more severe than expected with calcineurin or steroid therapy, usually requiring >2 antihypertensive medications. Clinical interventions should be considered for patients with both proteinuria >30 mg/dL and elevated sC5b-9.
Algorithm for the evaluation of TMA after HSCT. Screening for TMA includes monitoring LDH, complete blood count, and routine urinalyses. TMA should be suspected in HSCT recipients with an acute elevation of LDH, proteinuria >30 mg/dL, and hypertension more severe than expected with calcineurin or steroid therapy, usually requiring >2 antihypertensive medications. Clinical interventions should be considered for patients with both proteinuria >30 mg/dL and elevated sC5b-9.
We recognize limitations of our analysis. Importantly, opportunities for histologic diagnosis of TMA are limited, so we were unable to determine if all the subjects with clinical TMA diagnosis would have demonstrated evidence of TMA in tissue samples. The few autopsy samples we had available did show TMA with multiorgan involvement. Kidney biopsy remains a high-risk procedure in HSCT recipients, especially in the early stages of transplant when bleeding risk is high from thrombocytopenia and hypertension, precluding a tissue evaluation of all subjects.5,41,42 We also acknowledge that TMA was not listed as the primary cause of death in the majority of study subjects. Nevertheless, the combination of markers we examined did identify a subset of HSCT recipients at very high risk of death compared with those without these TMA markers. Only randomized, therapeutic trials powered to examine the outcome of death from TMA would be able to provide a direct causal link between TMA and poor outcomes.
In conclusion, we observed that signs of TMA were present in a large number of HSCT recipients, with approximately 20% of subjects having clinically significant disease, which potentially impacted their survival. Patients with proteinuria and evidence of complement activation as measured by elevated sC5b-9 serum concentrations should be considered for prompt interventions. TMA may present as a systemic disease, damaging other organs in addition to the kidney. Terminal complement activation may have a significant impact on the severity of TMA and therefore represents a novel therapeutic target in selected patients with high-risk features of TMA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the physicians, nurses, care managers, transplant coordinators, and other care providers and staff at CCHMC and especially the patients and their families participating in this study. The authors also thank Gretchen Radloff, Teresa Kinsella, Rebekah Kennedy, Thelma Kathman, Mary Block, and Bradley P. Dixon for their assistance with the study data and sample management.
The REDCap database is supported by a Cincinnati Children’s Hospital Center for Clinical and Translational Science and Training grant (UL1-RR026314-01 from the National Institutes of Health National Center for Research Resources). B.L.L. is supported by an American Society for Blood and Marrow Transplantation/Genentech New Investigator award.
Authorship
Contribution: S.J., B.L.L., and S.M.D. designed the study, performed research, and wrote the paper; A.L. and J.K. performed statistical analyses and prepared figures; and C.D., J.G., K.M., M.G., J.B., J.E.-B., G.W., R.S.C., and Z.P. provided vital conceptual insights for study design, assisted with study subject accrual and data collection, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sonata Jodele, Division of Bone Marrow Transplantation and Immune Deficiency, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 11027, Cincinnati, OH 45229; e-mail: sonata.jodele@cchmc.org.


![Figure 3. Association between markers at the time of TMA diagnosis and death by 1 year after HSCT. At the time of TMA diagnosis, a hemoglobin ≤8 g/dL, proteinuria (defined either as ≥30 mg/dL of protein on a random urinalysis or a random urine protein/creatinine [Ur prot/creat] ratio >2 mg/mg), and a soluble membrane attack complex (sC5b-9) concentration above the upper limit of normal were all significantly associated with an increased risk of death. Haptoglobin was higher in patients who died, likely representing an acute inflammatory state.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/4/10.1182_blood-2014-03-564997/4/m_645f3.jpeg?Expires=1769629121&Signature=X-Ey0zwUXl68oukXOwZBDGQAqvXTxN0sTaulCnHnT6xtxfYUOG3yq8tE5Wwx3R18F-FK9lwtWCOYwa0xHmSn6iV9dlk6EUp6lm5tsoNrBilLfPobHFyuMxpo2N9m2XFDiRg2HDarQxJ9NucGYlxwKwMNRQkYxSwsrvC9KWS9bZUA1FgHo0-zwNHwKc3eb2Psrzhl9nBcULKHz-Ed2JMIEfEEQOYvAbNfWmfTi6J5a2v0FDzFZ9Uidzs1kitS2e9t8TsMGb0s4v9LRmose7Finp5m8Z5~x3A5LZvFg9PrpnlrkUpU-5xjUlgzft~j8kNcQHDfhrhVzQ~K3x3g0u-B6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)