To the editor:
Endothelial cell protein C receptor (EPCR) plays a critical role in downregulating blood coagulation by promoting the activation of anticoagulant protein C by the thrombin:thrombomodulin (TM) complex.1 Recent studies from our laboratory and others showed that prohemostatic clotting factor, factor VII (and factor VIIa [FVIIa]) also binds EPCR with the same affinity as that of protein C and activated protein C (APC).2-4 At present, the pathophysiological significance of the EPCR interaction with FVIIa in hemostasis is unclear. We postulated earlier that FVIIa binding to EPCR may augment the hemostatic effect of FVIIa in therapeutic conditions where high concentrations of FVIIa were used to restore hemostasis.2,5,6 In these conditions, FVIIa concentration in plasma may reach as high as that of plasma protein C, and therefore, effectively competes with protein C for limited EPCR on the endothelium. This would result in the downregulation of protein C/APC-mediated anticoagulant pathway, allowing FVIIa-induced thrombin generation without impediment. If this hypothesis is correct, then blockade of EPCR-mediated anticoagulant pathway by other means should also augment FVIIa’s hemostatic effect in therapeutic conditions. Recently, Pavani et al7 showed that modified mouse FVIIa that binds EPCR exhibited superior hemostatic activity compared with wild-type mouse FVIIa and suggested that FVIIa tethering to EPCR on the endothelium may provide an extended locale of procoagulant reactions that is responsible for the procoagulant effect of FVIIa. It is conceviable that both the downregulation of APC generation and EPCR-dependent FXa generation contribute to the hemostatic effect of FVIIa in therapeutic conditions.
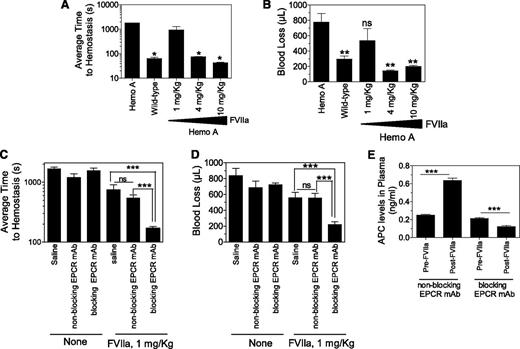
To determine the potential mechanism of EPCR-dependent FVIIa’s hemostatic effect in hemophilia, we investigated here the effect of mouse EPCR blocking (monoclonal antibody [mAb] 1560) and nonblocking antibodies (mAb 1567) on FVIIa-induced hemostasis in the hemophilia A mouse using the saphenous vein bleeding model.8 Both the blocking and nonblocking antibody bind to EPCR; the blocking antibody inhibits thrombin:TM-mediated protein C activation, whereas the nonblocking antibody does not.9 Neither antibody binds to mouse APC.9 The blocking antibody inhibits human FVIIa binding to mouse EPCR, whereas the nonblocking antibody does not.10 Saline or 3 different concentrations of human recombinant FVIIa were administered to hemophilia A mice via the tail vein 5 minutes before inducing saphenous vein injury. Following the injury, the time to hemostasis for each bleeding episode was recorded over a 30-minute time period, and the average time to achieve hemostasis (ATH) was calculated. Volume of blood loss was determined by measuring hemoglobin levels (see Figure 1 for details). Hemophilia A mice failed to achieve hemostasis within the 30-minute experimental time frame, whereas wild-type mice had an ATH of 64 seconds (Figure 1A). Administration of 1 mg/kg of FVIIa to hemophilia A mice had no or only a minimal effect on restoring hemostasis. Hemophilia A mice injected with 4 and 10 mg/kg of FVIIa had an ATH of 75 and 42 seconds, respectively. Both 4 and 10 mg/kg of FVIIa completely prevented the excessive blood loss in hemophilia A mice following the injury, whereas 1 mg/kg of FVIIa had no significant effect (Figure 1B).
EPCR blocking antibody augments hemostatic activity of a low dose of FVIIa in hemophilia A mice. (A) Average hemostatic times of hemophilia A and wild-type mice following saphenous vein injury and the effect of varying doses of FVIIa in restoring hemostasis in hemophilia A mice. Saline or varying doses of FVIIa (1, 4, and 10 mg/kg body weight) in 100 µL saline were given to hemophilia A mice via the tail vein. Five minutes after FVIIa administration, mice were subjected to saphenous vein incision following the procedure described by Buyue et al8 with a few modifications. Briefly, mice were anesthetized with ketamine (100 mg/kg ketamine and 8.5 mg/kg xylazine, ∼100 µL volume, intraperitoneally) and placed in the supine position on a heating mat. Saphenous vein from the ventral hind limb of the right leg of the mouse was exposed by dissecting the skin lengthwise over the saphenous neurovascular bundle. The exposed vein was overlaid with warm saline. Then, at the midway point of the exposed vein, an entry hole in the vein was made by inserting the tip of a 23-gauge needle into the vein. Blood was absorbed on a Kimwipe by gently touching the blood drop away from the puncture site. Immediately following the initial hemostasis, an ∼1-mm longitudinal distal cut was made using a Student Vannas spring scissors by inserting 1 blade into the vessel using the needle hole as the entry. Bleeding was observed for 30 minutes from this cut. After each hemostasis incident, the clot was disrupted gently by stroking the clot with a blunted 30-gauge needle in the direction of the blood flow to reinitiate a new bleeding episode. Duration of each bleeding episode in the 30-minute time period was noted, and average time to ATH was calculated. (B) Same as in (A), but volume of blood loss in 30-minute experimental time frame was determined. Throughout the 30-minute experimental time period, blood was adsorbed on a Kimwipe at every 20 seconds. Hemoglobin was extracted by soaking the wipes in 20 mL of solution of ABX Lysebio for 2 hours or more. Hemoglobin was also extracted from known volumes of freshly collected mouse blood to generate a standard curve for calculating the volume of blood loss (n = 4-6 mice per group). *Average clot time to hemostasis in these experimental groups was significantly shorter than that which was noted in hemophilia A mice not subjected to FVIIa treatment or given 1 mg/kg of FVIIa (P < .05). ns, not statistically significant; **P < .001 compared with values noted for hemophilia A mice not subjected to FVIIa treatment. (C-D) Effect of EPCR antibodies on hemostasis in hemophilia A mice. Hemophilia A mice were injected with EPCR nonblocking or blocking antibodies intraperitoneally (4 mg/kg body weight) 35 minutes prior to saphenous vein incision. Five minutes before saphenous vein incision, either saline (none) or a low dose of FVIIa (1 mg/kg) was administered via the tail vein. The indicated EPCR mAbs (4 mg/kg) were included with the saline and FVIIa injections. (C) Average time to hemostasis and (D) blood loss in the 30-minute experimental time frame were determined as described above. ns, not statistically significant; **P < .001; and ***P < .0001 (n = 8-9 mice per group). (E) Plasma levels of APC. EPCR nonblocking and EPCR blocking antibodies were given to hemophilia A mice as described in (C) and (D). Blood (100 µL) was collected from the mice via mandibular vein prior to giving FVIIa (1 mg/kg body weight) and the saphenous vein incision. At the end of 30-minute bleeding, blood was collected from the heart by cardiac puncture. In both cases, blood was collected in 0.38% sodium citrate and 0.01 M benzamidine hydrochloride (final concentrations). APC levels in plasma were assayed as described earlier.9 (n = 6 mice per group). ***P < .0001.
EPCR blocking antibody augments hemostatic activity of a low dose of FVIIa in hemophilia A mice. (A) Average hemostatic times of hemophilia A and wild-type mice following saphenous vein injury and the effect of varying doses of FVIIa in restoring hemostasis in hemophilia A mice. Saline or varying doses of FVIIa (1, 4, and 10 mg/kg body weight) in 100 µL saline were given to hemophilia A mice via the tail vein. Five minutes after FVIIa administration, mice were subjected to saphenous vein incision following the procedure described by Buyue et al8 with a few modifications. Briefly, mice were anesthetized with ketamine (100 mg/kg ketamine and 8.5 mg/kg xylazine, ∼100 µL volume, intraperitoneally) and placed in the supine position on a heating mat. Saphenous vein from the ventral hind limb of the right leg of the mouse was exposed by dissecting the skin lengthwise over the saphenous neurovascular bundle. The exposed vein was overlaid with warm saline. Then, at the midway point of the exposed vein, an entry hole in the vein was made by inserting the tip of a 23-gauge needle into the vein. Blood was absorbed on a Kimwipe by gently touching the blood drop away from the puncture site. Immediately following the initial hemostasis, an ∼1-mm longitudinal distal cut was made using a Student Vannas spring scissors by inserting 1 blade into the vessel using the needle hole as the entry. Bleeding was observed for 30 minutes from this cut. After each hemostasis incident, the clot was disrupted gently by stroking the clot with a blunted 30-gauge needle in the direction of the blood flow to reinitiate a new bleeding episode. Duration of each bleeding episode in the 30-minute time period was noted, and average time to ATH was calculated. (B) Same as in (A), but volume of blood loss in 30-minute experimental time frame was determined. Throughout the 30-minute experimental time period, blood was adsorbed on a Kimwipe at every 20 seconds. Hemoglobin was extracted by soaking the wipes in 20 mL of solution of ABX Lysebio for 2 hours or more. Hemoglobin was also extracted from known volumes of freshly collected mouse blood to generate a standard curve for calculating the volume of blood loss (n = 4-6 mice per group). *Average clot time to hemostasis in these experimental groups was significantly shorter than that which was noted in hemophilia A mice not subjected to FVIIa treatment or given 1 mg/kg of FVIIa (P < .05). ns, not statistically significant; **P < .001 compared with values noted for hemophilia A mice not subjected to FVIIa treatment. (C-D) Effect of EPCR antibodies on hemostasis in hemophilia A mice. Hemophilia A mice were injected with EPCR nonblocking or blocking antibodies intraperitoneally (4 mg/kg body weight) 35 minutes prior to saphenous vein incision. Five minutes before saphenous vein incision, either saline (none) or a low dose of FVIIa (1 mg/kg) was administered via the tail vein. The indicated EPCR mAbs (4 mg/kg) were included with the saline and FVIIa injections. (C) Average time to hemostasis and (D) blood loss in the 30-minute experimental time frame were determined as described above. ns, not statistically significant; **P < .001; and ***P < .0001 (n = 8-9 mice per group). (E) Plasma levels of APC. EPCR nonblocking and EPCR blocking antibodies were given to hemophilia A mice as described in (C) and (D). Blood (100 µL) was collected from the mice via mandibular vein prior to giving FVIIa (1 mg/kg body weight) and the saphenous vein incision. At the end of 30-minute bleeding, blood was collected from the heart by cardiac puncture. In both cases, blood was collected in 0.38% sodium citrate and 0.01 M benzamidine hydrochloride (final concentrations). APC levels in plasma were assayed as described earlier.9 (n = 6 mice per group). ***P < .0001.
Next, either EPCR nonblocking or blocking antibodies (4 mg/kg) were given to hemophilia A mice 35 minutes before the saphenous vein incision. Administration of either antibody alone had no significant effect on the bleeding time or the amount of blood loss (Figure 1C-D). However, a low dose of FVIIa (1 mg/kg) effectively restored hemostasis in mice pretreated with EPCR blocking antibody. In contrast, administration of EPCR nonblocking antibody failed to augment the poor hemostatic effect of 1 mg/kg of FVIIa. Plasma levels of APC in hemophilia A mice was increased following FVIIa infusion in mice pretreated with EPCR nonblocking antibody but not in mice pretreated with EPCR blocking antibody. Overall, the data presented herein provide a proof of concept to the hypothesis that blockade of endogenous protein C binding to EPCR enhances the hemostatic effect of FVIIa in therapeutic conditions. These data also fit with the hypothesis that the hemostatic effect achieved with high concentrations of FVIIa may come not only from FVIIa activation of factor X but also from the downregulation of the protein C/APC anticoagulant pathway by curtailing protein C binding to EPCR. However, blocking protein C binding to EPCR alone, in the absence of FVIIa administration, was insufficient to provide a hemostatic effect in hemophilia A mice. Recent studies of Pavani et al7 suggested that FVIIa binding to EPCR on the endothelium enhances its procoagulant effect in vivo, and this may be responsible for the hemostatic effect of FVIIa. This seems an unlikely explanation for our present observation since EPCR blocking antibody used in this study also blocks FVIIa binding to EPCR.10
Authorship
Acknowledgments: The authors thank Naomi Esmon for editing the letter.
This work was supported by National Institutes of Health, National Heart, Lung and Blood Institute grants HL107483 (L.V.M.R.) and UM1 HL120877 (C.T.E.).
Contribution: J.S. performed all experiments; L.V.M.R. conceived and designed the research and wrote the letter; U.R.P. contributed to the design of experiments; C.T.E. provided the reagents; and all authors contributed to the editing of the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: L. Vijaya Mohan Rao, Department of Cellular and Molecular Biology, The University of Texas Health Science Center at Tyler, Tyler, TX 75708-3154; e-mail: vijay.rao@uthct.edu.