Key Points
Jak2 activation-loop–defective mutation results in partial interferon γ signaling, but Jak2 mutant mice die due to abolished EpoR signaling.
Jak2 scaffold function mediates IFNGR complex integrity, activity, and physiological Jak1 localization.
Abstract
Janus kinases (Jak) play essential roles in cytokine and growth factor signaling. Conventional gene targeting of Jak2, creating a null allele, leads to a block in definitive erythropoiesis as a result of failing signal transduction at the homomeric erythropoietin receptor (EpoR) and at the heteromeric interferon γ receptor (IFNGR). To investigate the in vivo relevance of the activation loop of Jak2, a Jak2-YY1007/1008FF knockin mutation was introduced into the germline of mice. The phenotype of the Jak2FF/FF mouse line reveals that tyrosine residues 1007/1008 are absolutely essential for kinase function and signal transduction at the homomeric EpoR. Detailed studies using the Jak2 activation loop mutant uncover an essential scaffolding function of Jak2 within the IFNGR receptor complex and reveal that Jak1 can mediate a semiredundant function for IFNGR signal transduction. These studies are highly important for the molecular understanding of cytokine and growth factor signaling and provide new insights for future strategies in the design of pharmacological blockers of Jak2.
Introduction
Janus kinase 2 (Jak2) is a nonreceptor tyrosine kinase that is crucial for signal transduction of a set of cytokines and growth factors.1-3 Genetic inactivation of Jak2 leads to embryonic lethality due to a lack of definitive erythropoiesis, whereas an increased activity of Jak1, Jak2, and Jak3 leads to hemopoietic malignancies.4-7 The 4 Jak family members Jak1, Jak2, Jak3, and Tyk2 are preassociated with their cognate receptor chains, which dimerize upon ligand binding, an event that leads to activation of tyrosine kinase activity and phosphorylation of intracytoplasmic receptor chain tyrosine residues. These serve as docking sites of signal transducers and activators of transcription (Stats), which are subsequently phosphorylated by Jaks before leaving the receptor and inducing gene transcription.3,8 Recently, a mutational hot spot in the pseudokinase domain of Jak2 (Jak2V617F) was identified in patients suffering from myeloproliferative disorders.9-12 The V617F mutation leads to a constitutively active form of Jak2 that lacks the dominant-negative regulation ability of the pseudokinase domain and is additionally characterized by persistent phosphorylation of the tyrosine residues 1007/1008 within the kinase activation loop.4,7,13 This activity promotes oncogenic transformation and uncontrolled blood cell proliferation.4,7 Consistently, tyrosine 1007 phosphorylation was identified to be required for erythropoietin receptor (EpoR) activation.14 Furthermore, in vitro kinase studies addressing the autophosphorylation capacity of purified Jak2 reveal that the tyrosines 1007 and 1008 are essential for the high-activity catalytic state but dispensable for basal activity.15 In this context it is noteworthy that some autophosphorylation activity of Jak2 was shown to reside within the JH2 pseudokinase domain.16
Jak2 is the only Jak that transmits signals of homomeric receptor complexes (EpoR, TpoR, GHR, PrlR). Moreover, Jak2 is critically involved in signaling of heteromeric receptor complexes (interferon γ receptor [IFNGR], gp130, interleukin [IL] 12R, IL-23R)2,7,17 with the participation of other Jak family members. We and others demonstrated that genetic deletion of Jak2 leads to an absence of the Jak2 protein and results in a complete loss of signaling of homomeric (eg, EpoR) and heteromeric (eg, IFNGR) receptors.5,6 Similarly, Jak1 is also essential for IFNGR signaling,18 indicating nonredundant roles of Jak1 and Jak2 at the heteromeric IFNGR.
Adenosine triphosphate (ATP)-competitive kinase inhibitors of Jak2 are of high clinical interest, but Jak2 inhibitors appear not to completely fulfill the expectations in reducing disease burden of myeloproliferative neoplasms.19 Cells persistently incubated with Jak2 kinase inhibitors unexpectedly reveal an increase in Jak2 activation-loop phosphorylation,13,20 indicating a fundamental discrepancy between genetic ablation and pharmacologic inhibition of Jak2 activity. It is tempting to speculate that noncanonical kinase-independent functions of Jak2 are responsible for this observation. Because no information is available on the in vivo functions of the tyrosines 1007/1008 in Jak2 signaling, we generated a novel mouse model, Jak2-YY1007/1008FF, by substituting tyrosines 1007/1008 with phenylalanines to address the biological functions of the activation loop at homomeric and heteromeric receptor signaling.
Analyses of the Jak2FF/FF mouse line revealed a crucial importance of the tyrosines 1007/1008 for homomeric EpoR signaling and demonstrated that kinase-inactive Jak2 mediates biologically relevant activation of interferon γ (IFN-γ) signaling. Additionally, we show that the kinase-deficient Jak2 protein contributes to the assembly of the IFNGR complex. Thus, these data show for the first time in vivo that Jak2 can act as a scaffold protein independent of its kinase function at the IFN-γ receptor and that Jak1 has a semiredundant role in the IFN-γ–initiated phosphorylation of Stat1.
Methods
Antibodies and reagents
The antibodies used for western blotting were Jak2 (D2E12), phospho-Jak2 Y1007/1008 (C80C3), Jak1 (6G4), phospho-Jak1 Y1022/1023, phospho-Stat1 Y701 (58D6), phospho-Stat1 S727, Stat1, phospho-Stat5, Stat5, β-actin (8H10D10), IRF-1 (D5E4), caspase-3 (8G10), NaK-ATPase, PARP (46D11), and horseradish peroxidase (HRP)-conjugated goat anti–rabbit immunoglobulin G (all obtained from Cell Signaling, Danvers, MA). IFNGR1 (E-7) was obtained from Santa Cruz (Santa Cruz, CA). GBP2 was obtained from Pfeffer Laboratory (Düsseldorf, Germany). IFNGR1-PE, erythropoietin (Epo), and IFN-γ were bought from R&D Systems (Minneapolis, MN). Anti-CD11b (M1/70), HRP-conjugated goat anti–mouse immunoglobulin G, and major histocompatibility complex class I (MHC-I) (H-2Kb, AF6-88.5) were obtained from Becton Dickinson (Heidelberg, Germany). Anti-F4/80 (BM8) was obtained from BioLegend (San Diego, CA). Flag (M2) antibody, MG-132, and chlorpromazine were obtained from Sigma-Aldrich (Steinheim, Germany). We used the hemagglutinin (HA) antibody (12CA5) from Roche (Mannheim, Germany). Anti-V5, phalloidin, and Alexa-Fluor 405, 488, and 633 secondary antibodies were obtained from Life Technologies (Grand Island, NY). Methyl-β-cyclodextrin was obtained from Merck Millipore (Darmstadt, Germany).
Cell culture, transfection, and virus infection
Jak2+/+, Jak2FF/FF, and Jak2−/− mouse embryonic fibroblasts (MEFs) were generated according to standard protocols at embryonic day 12.5 (E12.5) and immortalized by transfection of a plasmid encoding the SV40 large T antigen. All MEFs were cultured in Dulbecco’s modified Eagle’s medium (high glucose; Life Technologies) supplemented with 10% fetal calf serum [vol/vol] (Pan-Biotech, Passau, Germany) and 0.1 mg/mL of each penicillin and streptomycin (Biochrom AG, Berlin, Germany). Cells were transfected with JetPei or JetPrime (Peqlab, Erlangen, Germany) according to the manufacturer’s instructions. Virus infection and titration were performed as described previously.21 Stimulation with IFN-γ was performed with a concentration of 50 U/mL unless otherwise stated.
Immunoblotting
Immunoblotting was performed as described previously.22 Briefly, protein samples were electrophoresed on a 4% to 12% Bis-Tris gel (Life Technologies) according to the manufacturer’s instructions, blotted to a nitrocellulose membrane (GE Healthcare, Buckinghamshire, United Kingdom), and sequentially probed with primary antibodies. HRP-conjugated secondary antibodies were detected by autoradiography using enhanced chemoluminescence (Thermo Scientific, Rockford, IL). Membranes were cleaned from antibodies with stripping solution (Merck Millipore) according to the manufacturer’s instructions and subjected to further immunoblot analysis.
Results
Generation of a Jak2-YY1007/1008FF (Jak2-FF) activation-loop mutant mouse line
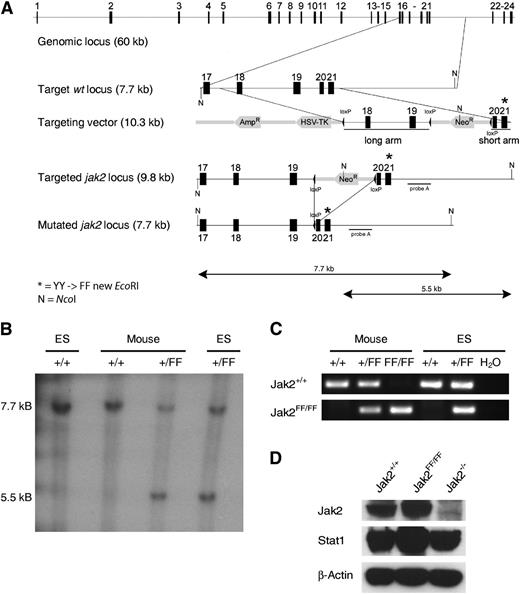
In order to investigate the biological functions of the Jak2 activation loop in vivo, we established a transgenic knockin line expressing an activation-loop mutant allele (YY1107/1008FF) of Jak2 in mice. Because it has been shown in vitro that the tandem tyrosines 1007/1008 in the activation loop of the kinase domain are required for the kinase activity of Jak2, we substituted the tyrosine residues 1007 and 1008 within the kinase domain of Jak2 with phenylalanines (Jak2-FF; Figure 1A).14,15 Gene targeting was performed in E14.1 embryonic stem (ES) cells as described elsewhere.5 Mutated ES cell lines were screened by polymerase chain reaction (PCR) and Southern blot analysis after NcoI digestion of ES cell DNA. Homologous recombination events were verified by hybridization with the flanking probe (Figure 1B). Homozygous Jak2FF/FF embryos (E12.5) could be detected by PCR analysis in the expected Mendelian ratio (Figure 1C). Next, we established MEFs at day E12.5 from Jak2+/+, Jak2FF/FF, and Jak2−/− embryos and analyzed Jak2 protein expression. This analysis revealed the presence of comparable amounts of the Jak2 protein in Jak2+/+ and Jak2FF/FF MEFs (Figure 1D). This clearly indicates that the mutant Jak2-FF protein is physiologically expressed.
Generation of a novel Jak2-YY1007/1008FF activation-loop mutant mouse model. (A) The murine Jak2 gene is depicted schematically at the top. The Ω-replacement vector was designed to replace exon 21 of the wild-type gene with a mutated version (YY1007/1008/FF). For positive selection, a neo cassette was used, whereas an HSV-Tk cassette was used for negative selection. The 5′ short arm was cloned using a XbaI restriction site and the 3′ short arm was inserted via BamHI. The targeting vector was electroporated into E14.1 ES cells.5 The neomycin resistance cassette was deleted by crossing Jak2-FF mice with a deleter mouse strain (N = NcoI). (B) For Southern blot analysis, DNA was digested with NcoI. Wild-type and Jak2-FF–targeted ES cells (lanes 1 and 4) and embryos after germline transmission of the Jak2 mutation (lane 2) and wild-type (lane 3) were analyzed. The expected fragment size after hybridization with the flanking probe is approximately 7.7 kb for the wild-type and approximately 5.5 kb for the targeted allele. Lane 1, Jak2+/+ ES cell; lane 2, Jak2+/+ mouse; lane 3, targeted Jak2+/FF mouse; lane 4, targeted Jak2+/FF ES cell clone 92/46. (C) Screening PCR of targeted ES cell clones and mice after deletion of the neo cassette via cross with a cre-deleter mouse line. Genomic DNA of ES cells and mice was used in a PCR with primers 1 und 2 (see Methods). Lane 1, Jak2+/+ control; lane 2, targeted Jak2+/FF mouse; lane 3, targeted Jak2FF/FF mouse; lane 4, Jak2+/+ control ES; lane 5, Jak2+/FF ES cell clone 92/46; lane 6, H2O control without template DNA. (D) Western blot analyses of protein extracts from MEFs generated from E12.5 with the genotype Jak2+/+, Jak2FF/FF, and Jak2−/−. Blots were incubated with antibodies directed against Jak2, Stat1, and β-actin.
Generation of a novel Jak2-YY1007/1008FF activation-loop mutant mouse model. (A) The murine Jak2 gene is depicted schematically at the top. The Ω-replacement vector was designed to replace exon 21 of the wild-type gene with a mutated version (YY1007/1008/FF). For positive selection, a neo cassette was used, whereas an HSV-Tk cassette was used for negative selection. The 5′ short arm was cloned using a XbaI restriction site and the 3′ short arm was inserted via BamHI. The targeting vector was electroporated into E14.1 ES cells.5 The neomycin resistance cassette was deleted by crossing Jak2-FF mice with a deleter mouse strain (N = NcoI). (B) For Southern blot analysis, DNA was digested with NcoI. Wild-type and Jak2-FF–targeted ES cells (lanes 1 and 4) and embryos after germline transmission of the Jak2 mutation (lane 2) and wild-type (lane 3) were analyzed. The expected fragment size after hybridization with the flanking probe is approximately 7.7 kb for the wild-type and approximately 5.5 kb for the targeted allele. Lane 1, Jak2+/+ ES cell; lane 2, Jak2+/+ mouse; lane 3, targeted Jak2+/FF mouse; lane 4, targeted Jak2+/FF ES cell clone 92/46. (C) Screening PCR of targeted ES cell clones and mice after deletion of the neo cassette via cross with a cre-deleter mouse line. Genomic DNA of ES cells and mice was used in a PCR with primers 1 und 2 (see Methods). Lane 1, Jak2+/+ control; lane 2, targeted Jak2+/FF mouse; lane 3, targeted Jak2FF/FF mouse; lane 4, Jak2+/+ control ES; lane 5, Jak2+/FF ES cell clone 92/46; lane 6, H2O control without template DNA. (D) Western blot analyses of protein extracts from MEFs generated from E12.5 with the genotype Jak2+/+, Jak2FF/FF, and Jak2−/−. Blots were incubated with antibodies directed against Jak2, Stat1, and β-actin.
The Jak2-FF mutant abolishes signaling at the homomeric EpoR
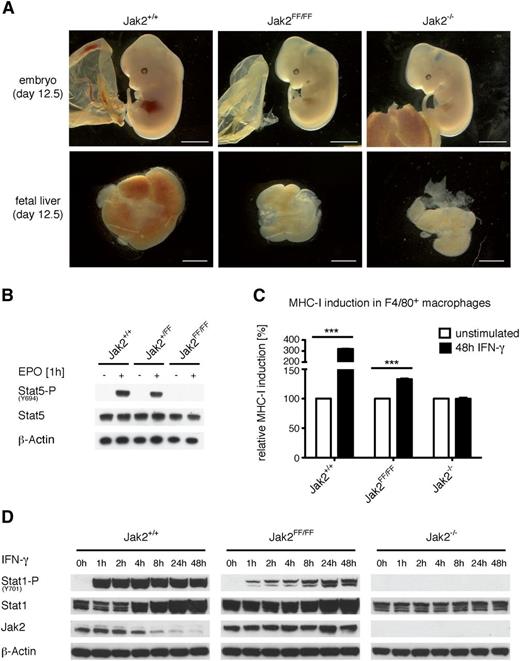
As shown previously, Jak2-deficient mice die at E12.5 due to a lack of definitive erythropoiesis.5,6 Interestingly, homozygous Jak2FF/FF mice exhibit a comparable phenotype and also lack definitive erythropoiesis (data not shown) and die at E12.5 (Figure 2A). Due to the lack of definitive erythropoiesis, the embryos appear pale and the liver mass of Jak2FF/FF mice is reduced. This observation is similar to the liver phenotype emerging in Jak2-deficient mice. No significant differences were observed when comparing wild-type and heterozygous littermates (data not shown). It is well established that Jak2 plays a crucial role for definitive erythropoiesis through the activation of Stat5 after Epo stimulation. To investigate whether Epo signaling in Jak2FF/FF fetal livers is functional, we prepared fetal liver cells from embryos at E12.5. The cells were stimulated ex vivo with Epo and Stat5 phosphorylation was analyzed via western blot. We observed a complete lack of Stat5 phosphorylation in homozygous Jak2FF/FF liver cells (Figure 2B). In contrast, wild-type and heterozygous littermates showed intact Stat5 phosphorylation (Figure 2B). We conclude that the Jak2 tyrosines 1007/1008 are essential for EpoR signaling and that the mutation leads to a kinase-inactive Jak2 protein in vivo.
Jak2FF/FFleads to partial IFN-γ signaling in primary macrophages and MEFs. (A) Jak2+/+, Jak2FF/FF, and Jak2−/− embryos (upper panel) and embryonic livers (lower panel) derived at E12.5. (B) Embryonic liver cells from Jak2+/+, Jak2+/FF, and Jak2FF/FF embryos were prepared at E12.5 and stimulated for 1 hour with Epo or left untreated. Cellular lysates were prepared and analyzed by immunoblotting using the indicated antibodies. (C) Fetal liver cells from Jak2+/+, Jak2FF/FF, and Jak2−/− embryos were prepared at E12.5 and were incubated with macrophage colony-stimulating factor for 6 days. FLDM were then stimulated with IFN-γ for 48 hours or left untreated. Cells were stained for F4/80 as a marker for mature macrophages. F4/80-positive cells were analyzed for MHC-I (H-2Kb) surface expression as a marker for IFN-γ signaling. One representative of 3 independent experiments run in triplicate is shown. The MHC-I expression of each genotype without stimulation was set as 100%. The standard deviation was below 3.7% of the detected values. ***P < .001. (D) MEFs from Jak2+/+, Jak2FF/FF, and Jak2−/− mice were stimulated in vitro with IFN-γ for the indicated time points. Subsequently, cellular lysates were prepared and analyzed by immunoblotting for expression of phospho-Stat1 (Y701), Stat1, and Jak2. β-actin served as a loading control. Results shown are representative of 3 independent experiments.
Jak2FF/FFleads to partial IFN-γ signaling in primary macrophages and MEFs. (A) Jak2+/+, Jak2FF/FF, and Jak2−/− embryos (upper panel) and embryonic livers (lower panel) derived at E12.5. (B) Embryonic liver cells from Jak2+/+, Jak2+/FF, and Jak2FF/FF embryos were prepared at E12.5 and stimulated for 1 hour with Epo or left untreated. Cellular lysates were prepared and analyzed by immunoblotting using the indicated antibodies. (C) Fetal liver cells from Jak2+/+, Jak2FF/FF, and Jak2−/− embryos were prepared at E12.5 and were incubated with macrophage colony-stimulating factor for 6 days. FLDM were then stimulated with IFN-γ for 48 hours or left untreated. Cells were stained for F4/80 as a marker for mature macrophages. F4/80-positive cells were analyzed for MHC-I (H-2Kb) surface expression as a marker for IFN-γ signaling. One representative of 3 independent experiments run in triplicate is shown. The MHC-I expression of each genotype without stimulation was set as 100%. The standard deviation was below 3.7% of the detected values. ***P < .001. (D) MEFs from Jak2+/+, Jak2FF/FF, and Jak2−/− mice were stimulated in vitro with IFN-γ for the indicated time points. Subsequently, cellular lysates were prepared and analyzed by immunoblotting for expression of phospho-Stat1 (Y701), Stat1, and Jak2. β-actin served as a loading control. Results shown are representative of 3 independent experiments.
The Jak2-FF mutant unmasks kinase independent/noncanonical function at the heteromeric IFNGR
In order to investigate putative kinase-independent functions of Jak2, we speculated that the Jak2-FF mutant, being preassociated at heteromeric receptors with another Jak, might be able to provide partial signaling after receptor stimulation. We chose the IFN-γ receptor as a prototype heteromeric receptor for further investigation. It is well known that Jak1 and Jak2 are pre-associated with the IFNGR1 and IFNGR2, respectively, and that the presence of either kinase is essential for IFN-γ signaling.5,23,24 We prepared embryonic livers at E12.5 from Jak2+/+, Jak2FF/FF, and Jak2−/− mice and differentiated fetal liver cells into macrophages (FLDM). A hallmark of IFN-γ signaling is the pronounced upregulation of surface MHC-I.25 Therefore, we monitored MHC-I surface expression after IFN-γ treatment in Jak2+/+, Jak2FF/FF, and Jak2−/− FLDMs via fluorescence-activated cell sorter analysis (Figure 2C). As expected, wild-type FLDM showed the highest increase of MHC-I surface expression after IFN-γ stimulation. Strikingly, we observed a significant increase of surface MHC-I expression in Jak2FF/FF macrophages in comparison with Jak2-deficient FLDM but lower expression compared with the wild-type cells (Figure 2C). To verify these results, we used Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs, stimulated them with IFN-γ, and analyzed Stat1 Y701 phosphorylation as a hallmark of IFN-γ signaling. A strong increase of Stat1 expression and phosphorylation in wild-type MEFs was observed, whereas the Jak2FF/FF MEFs showed a delayed and lower phosphorylation rate of Stat1 in comparison with the Jak2+/+ MEFs but nevertheless a significant upregulation of Stat1 phosphorylation and expression in comparison with the Jak2-deficient cells, where no Stat1 phosphorylation was detected. Wild-type Jak2 levels decreased upon IFN-γ treatment, reaching their minimum at 48 hours (Figure 2D). This observation is in agreement with published data demonstrating that phosphorylation of Jak2 at tyrosine residue 1007 is responsible for SOCS1 binding and induction of proteasomal Jak2 degradation.26 In contrast to wild-type Jak2, the Jak2-FF protein stayed stable during stimulation (Figure 2D). To corroborate our finding that Jak2 fulfills a kinase-independent function during IFN-γ–induced Stat1 activation, we additionally tested the kinase-inactive variant Jak2-K882E,27 which is catalytically inactive due to a mutation of the critical lysine to glutamic acid in the ATP-binding site. We transiently reconstituted Jak2−/− MEFs with expression plasmids encoding wild-type Jak2, Jak2-YY1007/8FF, and Jak2-K882E. The reconstituted cells were stimulated with IFN-γ and analyzed for Jak2 protein expression as well as for Stat1 activation. Indeed, both Jak2-YY1007/8FF- and Jak2-K882E-reconstituted MEFs showed comparable Stat1 activation (supplemental Figure 1A). This clearly demonstrates that the noncanonical activation of Stat1 is a common feature of kinase-inactive Jak2 and suggests that the presence of Jak2 is required for heteromeric receptor complex integrity.
Jak2-FF activates target genes of IFNGR signaling
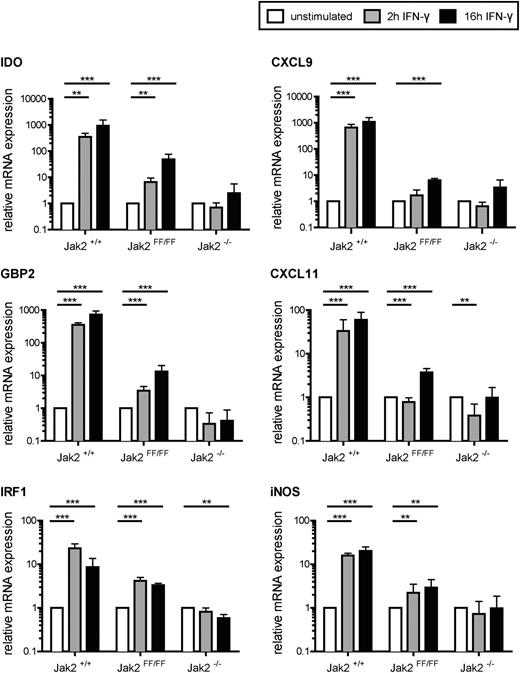
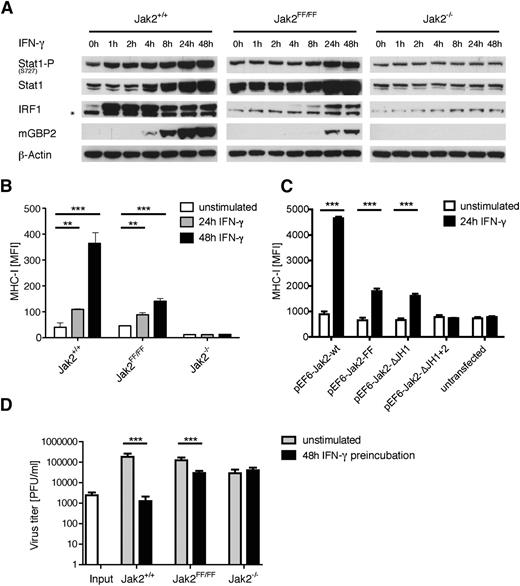
To investigate whether the kinase-independent function of Jak2 is biologically relevant, we investigated several steps of IFN-γ signal transduction, starting with messenger RNA expression of selected target genes. A significant upregulation of IDO, CXCL9, GBP2, CXCL11, IRF1, and iNOS could be detected in Jak2FF/FF MEFs upon IFN-γ stimulation, which was lower compared with the wild-type cells and virtually undetectable in Jak2-deficient MEFs (Figure 3). To further characterize the activation status of Stat1, we analyzed phosphorylation at serine 727. Serine 727 phosphorylation of Stat1 is required, but not essential, for maximal transcriptional activity.28-31 No obvious difference between the wild-type and the mutant variant of Jak2 concerning Stat1-S727 phosphorylation could be detected (Figure 4A). In contrast, Jak2-deficient MEFs showed no increase in Stat1 S727 phosphorylation upon IFN-γ incubation (Figure 4A). This underscores that the presence of Jak2, but not its kinase function, is required for the upregulation of Stat1 S727 phosphorylation. We next analyzed the protein expression levels of selected Stat1 target genes. After IFN-γ treatment of Jak2FF/FF MEFs, both IRF1 and mGBP2 protein levels were increased, but with a delayed expression kinetic in comparison with control cells (Figure 4A). In line with our previous results obtained with IFN-γ–stimulated Jak2FF/FF FLDM (Figure 2A), Jak2FF/FF MEFs also showed intermediate surface expression of MHC-I, whereas Jak2+/+ MEFs expressed the highest amount of MHC-I on the cell surface after IFN-γ stimulation (Figure 4B). Interestingly, MHC-I on the surface of Jak2-deficient MEFs is not upregulated after IFN-γ stimulation (Figure 4B). To confirm the noncanonical function of Jak2-YY1007/8FF, we cotransfected Jak2−/− MEFs with wild-type Jak2, Jak2-YY1007/8FF, Jak2-K882E, and a green fluorescent protein (GFP) expression plasmid, which allowed positive selection for GFP/Jak2 cotransfected cells. Analysis of MHC-I surface expression of IFN-γ stimulated Jak2 reconstituted cells showed significant and comparable MHC-I upregulation in both Jak2-YY1007/8FF- and Jak2-K882E-expressing cells (supplemental Figure 2). To identify the functional domain of Jak2 that is responsible for the noncanonical function, we generated 2 different C-terminal truncation mutants (Jak2-ΔJH1 lacks the kinase domain and additionally Jak2-ΔJH1+2 lacks the pseudokinase domain). We cotransfected Jak2−/− MEFs with wild-type Jak2, Jak2-YY1007/8FF, Jak2-ΔJH1, Jak2-ΔJH1+2, and a GFP expression plasmid. Analysis of MHC-I surface expression of IFN-γ–stimulated reconstituted cells showed significant and comparable MHC-I upregulation in both Jak2-YY1007/8FF- and Jak2-ΔJH1 expressing cells. In contrast, cells expressing a Jak2 lacking both the pseudokinase domain and the kinase domain displayed no MHC-I upregulation (Figure 4C).
The Jak2 noncanonical function is sufficient for IFN-γ–responsive target gene expression. MEFs from Jak2+/+, Jak2FF/FF and Jak2−/− mice were stimulated with IFN-γ for the indicated time. Subsequently, RNA was prepared and analyzed by quantitative reverse-transcription PCR for expression of IDO, CXCL9, GBP2, CXCL11, IFR1, and iNOS. Each data point represents the arithmetic means of 2 independent experiments of triplicate samples (n = 2 × 3). ***P < .001, **P < .01. The standard deviation is indicated for each data point.
The Jak2 noncanonical function is sufficient for IFN-γ–responsive target gene expression. MEFs from Jak2+/+, Jak2FF/FF and Jak2−/− mice were stimulated with IFN-γ for the indicated time. Subsequently, RNA was prepared and analyzed by quantitative reverse-transcription PCR for expression of IDO, CXCL9, GBP2, CXCL11, IFR1, and iNOS. Each data point represents the arithmetic means of 2 independent experiments of triplicate samples (n = 2 × 3). ***P < .001, **P < .01. The standard deviation is indicated for each data point.
The partial Jak2-FF signaling is biological relevant. (A) Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs were stimulated with IFN-γ for the indicated time points. Subsequently, cellular lysates and protein extracts were prepared and analyzed by immunoblotting. One representative experiment out of 3 independent experiments is shown. The asterisk indicates a nonspecific band detected by the IRF1 antibody. (B) Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs were stimulated with IFN-γ for the indicated time points or left untreated. As a readout for IFN-γ signaling, MHC-I (H-2Kb) surface expression was measured via flow cytometry. One representative experiment of 3 independent experiments run in triplicate is shown. The standard deviation is indicated for each data point. ***P < .001, **P < .01. (C) Jak2−/− MEFs were transiently transfected with Jak2-wt, Jak2-FF, Jak2-ΔJH1, Jak2-ΔJH1+2, and a GFP expression plasmid construct. Then 24 hours postcotransfection, GFP-positive and GFP-negative cells were analyzed via flow cytometry for MHC-I surface expression. One representative experiment of 3 independent experiments run in triplicate is shown. The standard deviation is indicated for each data point. ***P < .001. (D) Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs were either mock treated or incubated with 500 IU/mL IFN-γ for 48 hours before infection with VACV Western Reserve at 0.05 PFU/cell. VACV progeny titers were determined at 48 hours postinfection by plaque assay on CV-1 cells. One representative experiment of 3 independent experiments is shown. Each data point represents the arithmetic mean of 4 duplicate samples titrated at least in duplicate (n = 4 × 2). The standard deviation is indicated for each data point. ***P < .001.
The partial Jak2-FF signaling is biological relevant. (A) Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs were stimulated with IFN-γ for the indicated time points. Subsequently, cellular lysates and protein extracts were prepared and analyzed by immunoblotting. One representative experiment out of 3 independent experiments is shown. The asterisk indicates a nonspecific band detected by the IRF1 antibody. (B) Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs were stimulated with IFN-γ for the indicated time points or left untreated. As a readout for IFN-γ signaling, MHC-I (H-2Kb) surface expression was measured via flow cytometry. One representative experiment of 3 independent experiments run in triplicate is shown. The standard deviation is indicated for each data point. ***P < .001, **P < .01. (C) Jak2−/− MEFs were transiently transfected with Jak2-wt, Jak2-FF, Jak2-ΔJH1, Jak2-ΔJH1+2, and a GFP expression plasmid construct. Then 24 hours postcotransfection, GFP-positive and GFP-negative cells were analyzed via flow cytometry for MHC-I surface expression. One representative experiment of 3 independent experiments run in triplicate is shown. The standard deviation is indicated for each data point. ***P < .001. (D) Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs were either mock treated or incubated with 500 IU/mL IFN-γ for 48 hours before infection with VACV Western Reserve at 0.05 PFU/cell. VACV progeny titers were determined at 48 hours postinfection by plaque assay on CV-1 cells. One representative experiment of 3 independent experiments is shown. Each data point represents the arithmetic mean of 4 duplicate samples titrated at least in duplicate (n = 4 × 2). The standard deviation is indicated for each data point. ***P < .001.
IFN-γ has been shown to elicit its protective effect against vaccinia virus (VACV) replication in a Jak2-dependent manner.21,32 We therefore used the VACV infection model to probe the biological relevance of noncanonical functions of Jak2. IFN-γ preincubation of Jak2+/+ MEFs completely abrogated VACV replication, whereas treatment of Jak2FF/FF MEFs resulted in a significant 6-fold reduction of VACV replication (Figure 4D). In contrast, IFN-γ stimulation of Jak2−/− MEFs had no impact on VACV growth (Figure 4D). Thus, Jak2-YY1007/8FF is able to orchestrate the complete IFN-γ signal transduction cascade despite its lack of its kinase function. Hence, we propose that Jak2 acts as a scaffold protein at the IFNGR complex and that Jak1 can partially compensate for the loss of Jak2 kinase activity.
Subcellular localization of Jak1 and Jak2
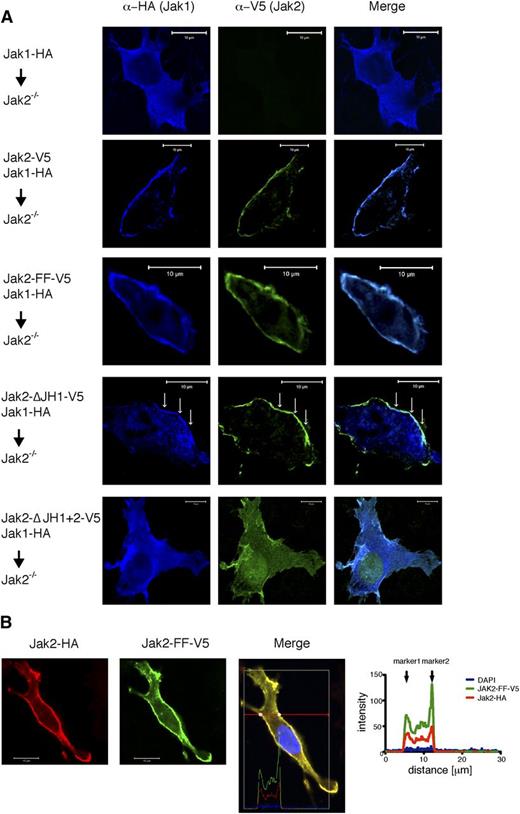
If Jak2 exerts a scaffold function in addition to its canonical function, one might expect a defective localization of IFNGR-associated proteins in cells lacking Jak2. To visualize Jak1 in Jak2-deficient MEFs, we transiently transfected Jak2−/− MEFs with Jak1-wt alone or together with Jak2-wt, Jak2-FF, Jak2-ΔJH1, and Jak2-ΔJH1+2 plasmids. Strikingly, we observed a profound delocalization of the Jak1-wt protein in Jak2−/− MEFs. The cotransfection of Jak2-wt or Jak2-FF revoked the delocalization of Jak1 (Figure 5A; supplemental Figure 3B). Jak1 and Jak2-ΔJH1 show partial colocalization at the plasma membrane, indicating that the JH1 domain is not required to maintain Jak2 scaffold function. Jak1 is not fully localized at the plasma membrane, probably due to the fact that the JH1 domain of Jak2 mediates to some extent the physiological localization of Jak1. Coexpression of Jak1-wt and Jak2-ΔJH1+2 in Jak2-deficient MEFs revealed a complete delocalization of both proteins (Figure 5A). Therefore, we conclude that the JH2 domain is necessary for maintaining the scaffold function. To proof whether the localization was identical in Jak2-wt and Jak2-FF, we cotransfected Jak2−/− MEFs with Jak2-wt and Jak2-FF expression plasmids. Colocalization analysis of both proteins revealed identical intracellular distribution patterns, indicating that the Jak2 kinase function is dispensable for correct localization (Figure 5B). Taken together, these results demonstrate that Jak2, independent of its kinase function, significantly shapes the subcellular localization of Jak1, highlighting the role of Jak2 as an important scaffold protein for the assembly of the IFN-γ receptor complex.
Subcellular distribution of Jak1 and Jak2. (A) Jak2−/− MEFs were transiently transfected with plasmid constructs that encode for HA-tagged Jak1-Wt and V5-tagged Jak2-Wt, Jak2-FF, Jak2-ΔJH1, or Jak2-ΔJH1+2. Then 24 hours posttransfection, the cells were fixed and stained with anti-HA (blue), and anti-V5 (green) antibodies. Subsequently, samples were analyzed by confocal microscopy. Bars, 10 µm. (B) Jak2−/− MEFs were transiently transfected with HA-tagged Jak2-Wt and V5-tagged Jak2-FF expression plasmid constructs. Then 24 hours posttransfection, the cells were fixed and stained with anti-HA (red), anti-V5 antibodies (green), and DAPI (blue). Subsequently, samples were analyzed by confocal microscopy. Bars, 10 µm. The histogram depicts fluorescence values the cross selection through the cell as indicated.
Subcellular distribution of Jak1 and Jak2. (A) Jak2−/− MEFs were transiently transfected with plasmid constructs that encode for HA-tagged Jak1-Wt and V5-tagged Jak2-Wt, Jak2-FF, Jak2-ΔJH1, or Jak2-ΔJH1+2. Then 24 hours posttransfection, the cells were fixed and stained with anti-HA (blue), and anti-V5 (green) antibodies. Subsequently, samples were analyzed by confocal microscopy. Bars, 10 µm. (B) Jak2−/− MEFs were transiently transfected with HA-tagged Jak2-Wt and V5-tagged Jak2-FF expression plasmid constructs. Then 24 hours posttransfection, the cells were fixed and stained with anti-HA (red), anti-V5 antibodies (green), and DAPI (blue). Subsequently, samples were analyzed by confocal microscopy. Bars, 10 µm. The histogram depicts fluorescence values the cross selection through the cell as indicated.
Turnover of the IFNGR is dependent on the Jak2 kinase activity
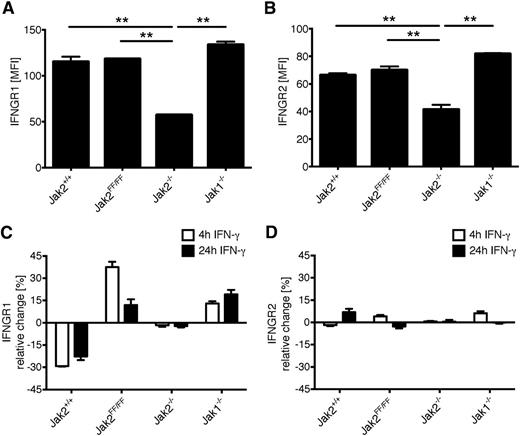
To further investigate IFN-γ receptor integrity, we analyzed the surface expression of IFNGR1 and IFNGR2. Unstimulated Jak2−/− MEFs showed a very low abundance of surface IFNGR1 and IFNGR2 in clear contrast to MEFs expressing Jak2-wt or Jak2-FF, which exhibit a comparable basal amount (Figure 6A-B). Analysis of IFNGR1 expression after IFN-γ stimulation revealed a striking decrease of the IFNGR1 protein level exclusively in Jak2+/+ MEFs. This is in contrast to MEFs that exhibit only partial or no IFN-γ signaling (ie, Jak2FF/FF or Jak2−/−), where IFNGR1 protein accumulates or is unaltered (Figure 6C). Of note, we found that IFNGR2 surface expression is not regulated by IFN-γ stimulation compared with IFNGR1, where a distinct regulation pattern could be observed (Figure 6D). A decrease of IFNGR1 surface expression has been correlated with internalization of the IFN-γ receptor complex, providing a regulatory mechanism to balance signal transduction.33 IFNGR1 and IFNGR2 are significantly reduced in Jak2−/− MEFs compared with MEFs expressing Jak2-wt or Jak2-FF (Figure 6B-C). To investigate how Jak2-FF enables the cell surface localization of the IFNGR1, we blocked basal endocytosis via different endocytosis inhibitors (methyl-β-cyclodextrin, chlorpromazine). Blocking the endocytosis pathway does not alter the turnover of the IFNGR complex in all cell lines. In contrast, inhibition of proteasomal degradation by MG-132 does not lead to a decreased surface expression of the IFNGR complex upon IFN-γ stimulation in Jak2+/+ MEFs (supplemental Figure 4). Taken together, these results suggest that the canonical Jak2 function appears to be essential for downregulation of IFNGR1 expression and that the noncanonical function of Jak2 is responsible for steady-state IFNGR1 expression.
Jak2 kinase activity is essential for IFN-γ complex internalization and degradation. (A-B) Unstimulated MEFs from Jak2+/+, Jak2FF/FF, Jak2−/−, and Jak1−/− mice were analyzed for IFNGR1 and IFNGR2 surface expression by flow cytometry. One representative experiment of 3 independent experiments run in triplicates is shown. The standard deviation is indicated for each data point. (C-D) MEFs from Jak2+/+, Jak2FF/FF, Jak2−/−, and Jak1−/− mice were stimulated with IFN-γ for the indicated time points or left untreated. IFNGR1 and IFNGR2 surface expression was measured via fluorescence-activated cell sorter analysis. One representative experiment of 3 independent experiments run in triplicate is shown. The standard deviation is indicated for each data point.
Jak2 kinase activity is essential for IFN-γ complex internalization and degradation. (A-B) Unstimulated MEFs from Jak2+/+, Jak2FF/FF, Jak2−/−, and Jak1−/− mice were analyzed for IFNGR1 and IFNGR2 surface expression by flow cytometry. One representative experiment of 3 independent experiments run in triplicates is shown. The standard deviation is indicated for each data point. (C-D) MEFs from Jak2+/+, Jak2FF/FF, Jak2−/−, and Jak1−/− mice were stimulated with IFN-γ for the indicated time points or left untreated. IFNGR1 and IFNGR2 surface expression was measured via fluorescence-activated cell sorter analysis. One representative experiment of 3 independent experiments run in triplicate is shown. The standard deviation is indicated for each data point.
The noncanonical function of Jak2 requires Jak1 activity
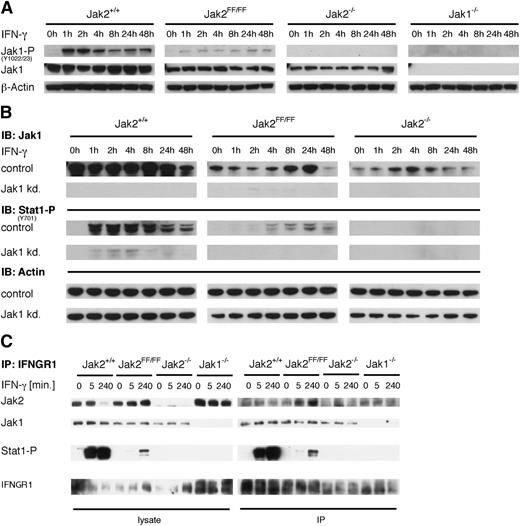
Because our previous results demonstrate that the Jak2 scaffold function is sufficient and crucial for IFN-γ–induced signal transduction, we addressed the question as to which kinase is responsible for Stat1 phosphorylation in Jak2FF/FF cells. Because of its presence in the heteromeric IFNGR complex, the most likely candidate is Jak1. To address this issue, we analyzed the status of the activation loop of Jak1 after IFN-γ treatment in Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs. As shown in Figure 7A, stimulation with IFN-γ leads to an immediate increase of Jak1 phosphorylation in Jak2+/+ MEFs, whereas a diminished but clearly observable phosphorylation of the activation loop of Jak1 in Jak2FF/FF MEFs was detectable. This indicates that Jak1 is activated in Jak2FF/FF cells after IFN-γ treatment and is presumably responsible for Stat1 activation. Additionally, we analyzed phosphorylation of Jak2 at tyrosines 1007/1008 in Jak1−/− MEFs after IFN-γ stimulation. As shown in supplemental Figure 5, there is a similar Jak2 phosphorylation kinetic in Jak1−/− MEFs compared with Jak1+/+ MEFs, but phosphorylation of tyrosine 1007/1008 appear diminished. Taken together, these results demonstrate that transphosphorylation of the 2 kinases is crucial for full Jak1 and Jak2 activation (Figure 7A). Next, we performed a stable knockdown of Jak1 in Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs to verify the role of Jak1 in Jak2FF/FF MEFs after IFN-γ stimulation. As shown in Figure 7B, almost no Stat1 phosphorylation after IFN-γ treatment was observed in Jak2+/+ and Jak2FF/FF cells when Jak1 expression is significantly reduced. Furthermore, we analyzed MHC-I surface expression in Jak1 knockdown MEFs and observed a strongly reduced upregulation of MHC-I surface expression in the Jak2+/+and Jak2FF/FF MEFs after IFN-γ treatment (supplemental Figure 6). Residual Stat1 phosphorylation and upregulation of MHC-I surface expression can be attributed to a knockdown efficiency of ∼95% of Jak1 in Jak2+/+ and Jak2FF/FF MEFs (data not shown). These results clearly indicate that delayed and diminished Stat1 phosphorylation and target gene expression after IFN-γ treatment required Jak1 and its kinase activity within the IFNGR complex in Jak2FF/FF MEFs. To analyze the interaction of Jak1, Jak2, and IFNGR, immunoprecipitation experiments were performed (Figure 7C). The data show that Stat1 is recruited to Jak2-wt– and Jak2-FF–containing receptor complexes as a prerequisite for phosphorylation within the complex. Moreover, only reduced amounts of Jak1 could be precipitated with IFNGR1 in Jak2−/− MEFs. Precipitated Jak1 in Jak2−/− MEFs presumably represents Jak1 molecules that are preassembled with IFNGR1 chains within the cytoplasm (see Figure 5).
Jak1 is responsible for partial signaling of Jak2-FF within the IFNGR complex. (A) Jak2+/+, Jak2FF/FF, Jak2−/−, and Jak1−/− MEFs were stimulated with IFN-γ for the indicated time points. Subsequently, cellular lysates were prepared and analyzed by immunoblotting for expression of phospho-Jak1 (Y1022/1023) and Jak1 protein. β-actin served as a loading control. Results shown are representative of 3 independent experiments. (B) Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs stably expressing either a scrambled small hairpin RNA (shRNA) (mock) or shRNAs directed against 2 different target sequences of Jak1 (shRNA 1, shRNA 2) were stimulated for the indicated times with IFN-γ. Cellular lysates were prepared and analyzed by immunoblotting for expression of phospho-Stat1 (Y701). To verify the knockdown, cellular lysates were analyzed for Jak1 protein expression. β-actin served as a loading control. (C) Jak2+/+, Jak2FF/FF, Jak2−/− and Jak1−/− MEFs were stimulated with IFN-γ for the indicated time points. Cellular lysates were prepared and immunoprecipitation (IP) with an antibody against IFNGR1 was performed. Subsequently, the indicated proteins were analysed by immunoblotting.
Jak1 is responsible for partial signaling of Jak2-FF within the IFNGR complex. (A) Jak2+/+, Jak2FF/FF, Jak2−/−, and Jak1−/− MEFs were stimulated with IFN-γ for the indicated time points. Subsequently, cellular lysates were prepared and analyzed by immunoblotting for expression of phospho-Jak1 (Y1022/1023) and Jak1 protein. β-actin served as a loading control. Results shown are representative of 3 independent experiments. (B) Jak2+/+, Jak2FF/FF, and Jak2−/− MEFs stably expressing either a scrambled small hairpin RNA (shRNA) (mock) or shRNAs directed against 2 different target sequences of Jak1 (shRNA 1, shRNA 2) were stimulated for the indicated times with IFN-γ. Cellular lysates were prepared and analyzed by immunoblotting for expression of phospho-Stat1 (Y701). To verify the knockdown, cellular lysates were analyzed for Jak1 protein expression. β-actin served as a loading control. (C) Jak2+/+, Jak2FF/FF, Jak2−/− and Jak1−/− MEFs were stimulated with IFN-γ for the indicated time points. Cellular lysates were prepared and immunoprecipitation (IP) with an antibody against IFNGR1 was performed. Subsequently, the indicated proteins were analysed by immunoblotting.
Taken together, the Jak2-FF mutation unveils a scaffold function of Jak2 within the IFNGR complex that is required for (1) the maintenance of the integrity of the functional IFNGR complex at the cell surface, (2) physiological Jak1 localization within the cell, and (3) the subsequent Stat1 phosphorylation.
Discussion
To dissect the role of Jak2 kinase function (canonical) and kinase-independent functions (noncanonical) in vivo, we generated for the first time a knockin mouse line carrying Jak2 alleles (Jak2-YY1007/1008FF) harboring point mutations within the activation loop. Consistent with the finding from Jak2-deficient mice lacking definitive erythropoiesis, the Jak2-FF mice also show embryonic lethality due to severe anemia at E12.5. Despite the indispensable role of the canonical function at the homomeric EpoR, this novel mouse model allowed unmasking of noncanonical functions of Jak2 at the heteromeric IFNGR.
In vitro data15 showed that the autophosphorylation ability of Jak2-YY1007/1008FF is independent of receptor-induced stimulation. Our results indicate that this basal catalytic state is not sufficient to induce biologically relevant signaling at the homomeric EpoR, or, more likely, does not exist in vivo. In contrast to homomeric EpoR, heteromeric IFNGR signaling is not completely dependent on Jak2 kinase activity shown here for the first time in vivo. Jak2-FF MEFs reveal partial Stat1 phosphorylation, subsequently diminished IFN-γ target gene induction, and establishment of an antiviral state. Furthermore, IFNGR expression is mediated by noncanonical Jak2 function and the physical presence of Jak2, independent of its kinase function, is required for physiological Jak1 localization. Therefore, we conclude that Jak2 acts as a scaffold protein at the heteromeric IFNGR. In line with these findings, different human Jak2 mutants in the αH domain exhibiting decreased or no autophosphorylation at YY1007/1008 have been reported. These mutations also lead to partial activation of Stat1 after IFN-γ stimulation.34 Moreover, no Stat5 activation was observed after Epo treatment.34 In a previous study, a mouse line harboring a dominant-negative Jak2 allele (W1038G/E1046R) has been generated. This mutation in the αG domain, being previously characterized as essential for kinase activity, also leads to failure of definitive erythropoiesis.35 Lack of definitive erythropoiesis in the Jak2-YY1007/1008FF and Jak2-W1038G/E1046R mice clearly indicates a complete absence of kinase activity at the homomeric EpoR. Of note, Stat5 activation is a hallmark of definitive erythropoiesis. No Stat5 activation was observed in Jak2-FF mice corroborating the complete abrogation of homomeric EpoR signaling. These results emphasize the importance of tyrosine 1007/1008 phosphorylation within the activation loop for Jak2-mediated signaling in vivo.
Of note, heteromeric receptors exhibit a more complex interplay of receptor chains and Jaks than homomeric receptors. Active Jak1 is required for partial IFN-γ signal transduction in Jak2-FF cells, indicating that Jak1 is able to directly phosphorylate Stat1. This postulate leads to a concept that activated Jak1 can directly mediate Stat1 activation and autophosphorylation of Jak1 requires the kinase-independent presence of Jak2 at the IFNGR. Accordingly, Jak2 scaffold function might contribute to the balancing of signal transduction by maintaining IFNGR complex integrity. To solve the question as to which extent a putative Jak1 scaffold function contributes to the regulation of signaling transduction, a new mouse model expressing a kinase inactive Jak1 would be of importance. Briscoe et al demonstrated in Jak1-deficient human-derived fibroblasts reconstituted with a kinase inactive Jak1 a partial activation of IFN-γ target genes, suggesting that Jak1 also harbors a scaffold function at the human IFNGR.36 In contrast to our study, Briscoe et al claimed that kinase inactive Jak2 cannot sustain IFN-γ inducible gene expression, although they found low-level Stat1 DNA binding.36 Their findings might result from the use of γ2A and U4A fibroblast cells, which were held in long-term culture, were derived from undirected mutagenesis, and might contain additional mutations. The Jak2-FF knockin mouse line generated in this study and its primarily derived cells harbor a defined genetic mutation avoids this issue. However, there might be subtle differences for the expression in IFNGR between mice and humans that have to be addressed in the future. Interestingly, for Tyk2-null human cells a lack of cell surface expression of the IFN-α receptor37 due to unmasking of an endocytic motif that is missing in the mouse receptor has been reported.38
To prove that our mouse model is a suitable model for the investigation of kinase-independent Jak2 functions, we further investigated the role of Jak2-K882E. Jak2-K882E is a kinase-inactive Jak2 variant due to an amino acid substitution at the ATP-binding pocket within the kinase domain.36,39 Jak2-K882E and Jak2-FF show comparable IFN-γ signal transduction, demonstrating that scaffold function is also independent of ATP binding. Recently, a kinase-inactive Tyk2 mouse line was published in which noncanonical, kinase-independent functions could not be observed with regard to type I IFN response and antiviral defense.40 However, in line with our data, noncatalytic functions of human Jak1 and Tyk2 in the context of IFNAR1, IL-6, and IL-10 stimulation were reported, suggesting that 1 catalytically competent Jak is sufficient for signaling provided that its partner behaves as scaffold, even if inactive.41,42 Investigation of a Jak3 activation-loop–defective mutant, YY1980/981FF, demonstrates that activation-loop tyrosines are not absolutely required for Jak3 catalytic function, whereas lysine mutation (K885A) in the ATP-binding pocket completely abrogates catalytic activity.43 Moreover, Jak3-K885A exhibits no kinase-independent function.43 In contrast to this, an activation-loop–defective Jak1 mutant exhibits no catalytic activity and putatively has partial activation capacity at heteromeric receptors.36,44 Taken together, Jak1, Tyk2, and Jak2 harbor kinase-independent functions, whereas the biological function of Jak3 seems to be restricted to its catalytic activity.
The newly discovered scaffold function of Jak2 for heteromeric receptor signaling could be of high value for the development of new effective Jak2 inhibitors. Studies with clinically approved Jak2 inhibitors disclose problems with the efficacy of Jak2 inhibition.19 Recently, a new class of Jak2 inhibitors that prevent persistent Jak2 phosphorylation of the activation loop was investigated.13,20 Further development of new Jak2-targeting drugs should take the biological relevance of the scaffold function into account. A double-mutant mouse harboring Jak2-V617F / YY1007/1008FF alleles would be of high value. Our study emphasizes the important role of tyrosine residues 1007/1008 for full activation of Jak2 signaling and may provide a novel model system for the development of Jak2 inhibitors that completely abrogate Jak2 function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Buchholz, N. Küpper, and V. Kuhl for their excellent technical assistance; H. Hengel (Freiburg, Germany), T. Bruhn, and T. Decker (Vienna, Austria) for critically reading the manuscript; and D. Degrandi and S. Scheu for fruitful discussions. Jak1−/− MEFs were a kind gift from R. Schreiber (St. Louis, MO). This study was generously supported by the FoKo Medical Faculty HHU Düsseldorf grants SFB455 and FOR729 (E.K.), the Leibniz-Prize (D.F.G.), and the Jürgen Manchot Foundation (K.P.). M.M. and B.S. were supported by the Austrian Science Fund (grant FWF SFB-F28).
Authorship
Contribution: K.P. and E.K. designed and planned experimental approach; D.F., E.K., C.W., M.T., P.L., B.S., and M.M. performed experiments and analyzed data; and E.K., D.F., and K.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Klaus Pfeffer, Institute of Medical Microbiology and Hospital Hygiene, Heinrich-Heine-University of Düsseldorf, Universitätsstraße 1, 40225 Düsseldorf, Germany; e-mail: klaus.pfeffer@hhu.de.
References
Author notes
E.K. and D.F. contributed equally to this study.