Key Points
TPO specifically activates Erk and NF-κB pathways in hematopoietic stem and progenitor cells.
Erk and NF-κB cooperate to trigger their common target, Iex-1, and DNA-PK-dependent NHEJ activation in HSPCs upon irradiation.
Abstract
Loss of hematopoietic stem cell (HSC) function and increased risk of developing hematopoietic malignancies are severe and concerning complications of anticancer radiotherapy and chemotherapy. We have previously shown that thrombopoietin (TPO), a critical HSC regulator, ensures HSC chromosomal integrity and function in response to γ-irradiation by regulating their DNA-damage response. TPO directly affects the double-strand break (DSB) repair machinery through increased DNA-protein kinase (DNA-PK) phosphorylation and nonhomologous end-joining (NHEJ) repair efficiency and fidelity. This effect is not shared by other HSC growth factors, suggesting that TPO triggers a specific signal in HSCs facilitating DNA-PK activation upon DNA damage. The discovery of these unique signaling pathways will provide a means of enhancing TPO-desirable effects on HSCs and improving the safety of anticancer DNA agents. We show here that TPO specifically triggers Erk and nuclear factor κB (NF-κB) pathways in mouse hematopoietic stem and progenitor cells (HSPCs). Both of these pathways are required for a TPO-mediated increase in DSB repair. They cooperate to induce and activate the early stress-response gene, Iex-1 (ier3), upon DNA damage. Iex-1 forms a complex with pERK and the catalytic subunit of DNA-PK, which is necessary and sufficient to promote TPO-increased DNA-PK activation and NHEJ DSB repair in both mouse and human HSPCs.
Introduction
Maintenance of genomic stability is crucial for preservation of hematopoietic stem cell (HSC) self-renewal throughout life. Bone marrow (BM), and more particularly HSC, injury is one of the most serious limiting factors of therapies with DNA-damaging agents used to treat otherwise-curable cancers. Indeed, exposure to ionizing radiation or chemotherapeutic drugs induces residual loss of HSC functions1-3 and a high risk of developing secondary acute myeloid leukemia or myelodysplastic syndromes (MDS). Finding ways to reduce the toxicity of anticancer DNA agents through the analysis of HSC response following DNA damage would have a major beneficial impact on cancer-treatment–related mortality.
Double-strand breaks (DSBs) are the most deleterious DNA lesions. In mammalian cells, DSBs are removed by 2 main repair pathways: homologous recombination and nonhomologous end-joining (NHEJ). NHEJ is regarded as the predominant mechanism for DSB repair in vertebrates in response to irradiation (IR).4 The DNA-PK complex, composed of the DNA-PK catalytic subunit and the Ku80/Ku70 heterodimer, is a major component of classical NHEJ. When this pathway is impaired, the cells use an alternative and slower DNA-PK–independent NHEJ that has been shown to generate DSB-induced translocations and deletions.4-6 Quiescent HSCs preferentially use NHEJ to repair IR-induced DSBs.7 DSB repair through NHEJ is necessary for HSC maintenance.8,9 NHEJ is intrinsically error-prone, and this has been shown to be responsible for the high vulnerability of HSCs to mutagenesis.7 We have recently shown that the fidelity and efficiency of HSC-intrinsic NHEJ-mediated repair can be improved by thrombopoietin (TPO).10 TPO is a main regulator of both megakaryocytopoiesis and HSCs.11-13 TPO promotes NHEJ DNA repair in human and mouse HSCs by directly stimulating DNA-PK activity.10 This effect is critical to preserve HSC genomic stability and function in mice subjected to total body IR (TBI). However, mutations resulting in constitutive activation of MPL occur in myeloproliferative neoplasms,14 and prolonged TPO administration may cause complications such as myelofibrosis and thrombosis.15 Finding a way to specifically enhance TPO-desirable effects in HSCs requires the identification of the TPO-induced signaling pathways involved in DNA repair.
Although many studies have examined signaling downstream of TPO/Mpl in megakaryocytes, little is known about the pathways evoked by this cytokine in HSCs. Our previous data showed that the effect of TPO on HSC DNA repair is unique because it cannot be replaced by other cytokines acting on HSCs.10 In agreement with the fact that DNA-PK/NHEJ complexes form quickly after IR, kinetics analysis indicates that the protective effect of TPO requires the presence of TPO shortly before IR and is abolished when TPO is added to the medium after IR. This suggests that TPO triggers a specific signal in HSCs that acts as a priming event facilitating DNA-PK activation upon treatment with DNA-damaging agents. TPO has been shown to activate several signaling cascades in HSCs, including Stat5, Erk, and p38.16-19 However, to our knowledge, no data have been reported concerning selective TPO/Mpl signaling pathways activated in HSCs and their role under genotoxic stress. Whether these pathways are different from those involved in TPO-mediated HSC maintenance is unknown.
We and others have shown that TPO induces a strong and sustained Erk MAPK activity in megakaryocytes that regulates their proliferation/differentiation balance.20-23 We have identified IEX-1 (IER3) as a TPO-induced Erk substrate.24,25 IEX-1 is a ubiquitous early-response gene induced by various stress stimuli, including IR and inflammatory cytokines.26 Cellular functions attributed to the IEX-1 protein include regulation of apoptosis, proliferation, and the activity of various signaling pathways.24,27-31 We have recently reported a role of IEX-1 in the DNA-damage response.32 We show here that upon IR, TPO, but not other cytokines, induces IEX-1 expression in hematopoietic stem and progenitor cells (HSPCs) through its unique ability to trigger sustained Erk and nuclear factor κB (NF-κB) activation. IEX-1 then connects specifically TPO/Mpl-induced phosphorylated Erks to DNA-PK when DNA damage occurs.
Methods
Animals and cell culture
C57BL/6 (CD45.2) Mpl−/−, Iex-1−/−, and Erk1−/− mice were previously described.33-35 Eight- to 10-week-old mice were used. Procedures were approved by the Animal Care Committee (registered P2.MG.137.10, 2010) and the department director of veterinary services of Paris (agreement 75-1064). Mouse TBI was performed in an IBL637 cesium irradiator. BM lineage-negative (Lin−), Lin−Sca+Kit+ (LSK), LSK-Flt3−, and LSK-CD34− cells were isolated and stained as described previously.10 Cells were cultured in StemSpan SFEM (StemCell Technologies) complete medium containing Flt3-ligand (FL; 100 ng/mL), interleukin-3 (IL-3; 10 ng/mL), IL-6 (10 ng/mL), stem cell factor (SCF; 100 ng/mL), and 50 ng/mL TPO (Peprotech, Rocky Hill, NJ) or in the same medium without TPO. Cord blood CD34+ HSPCs were isolated and cultured as previously described.10,25 Inhibitors were added 30 minutes before treatments. Cells were irradiated in vitro using a Biobeam 8000 irradiator (Gamma Service Medical GmbH, Leipzig, Germany).
PLA
For the proximity ligation assay (PLA), cells were cytocentrifugated on poly-l-lysine–coated slides and labeled with mouse anti–DNA-PKc and rabbit anti-pErk1/2 antibodies. Cells were then treated with the Duolink kit according to the manufacturer’s instructions and revealed with the Duolink far-red detection reagent (Olink Bioscience, Uppsala, Sweden). Slides were mounted using Dapi-Fluoromount-G (SouthernBiotech, Birmingham, AL) and visualized at room temperature using a Leica DMI 6000 (Leica Microsystems, Wetzlar, Germany) microscope equipped with a 63 × 1.6 oil-immersion objective and a MicroMAX 1300Y camera (Princeton Instruments, Trenton, NJ). Pictures were analyzed using ImageJ software (developed at the National Institutes of Health, Bethesda, MD).
Biochemical assays
For western blots, 3 × 104 HSPCs were cultured for 1 hour at 37°C in Iscove modified Dulbecco medium containing 0.2% fetal calf serum and TPO (50 ng/mL), SCF (100 ng/mL), or FL (100 ng/mL). Proteins were extracted in 10% trichloroacetic acid and solubilized as described elsewhere.36 Samples were loaded on 10% Bis-Tris NuPage gels (Invitrogen). For pErk1/2 quantification using capillary isoelectric focusing immunoassays,37 3 × 104 cells were lysed for 15 minutes on ice in Bicine 20 mM, 0.6% CHAPS (pH 7.5) containing phosphatase and protease inhibitors (Sigma-Aldrich). Analysis was performed on a NanoPro 1000 (ProteinSimple, Santa Clara, CA). Electrophoretic mobility shift assays (EMSAs) for analysis of NF-κB DNA-binding activity were performed in 5 × 104 HSPC total lysates using the HIV-LTR tandem κB oligonucleotide as a κB probe.38 Equal amounts of proteins (at least 1.5 µg) for each sample were used. For supershift assays, protein extracts were incubated with antibodies for 30 minutes on ice.
Statistical analysis
A 1-way analysis of variance and Tukey comparison test or unpaired Student t test was applied using GraphPad Prism version 5.0 software (GraphPad Software, San Diego, CA). The value of *P < .05 was determined as significant and **P < .01 or ***P < .001 as highly significant.
For further details, see supplemental Methods (available on the Blood Web site).
Results
The Erk pathway is required for TPO-mediated DSB repair in HSCs
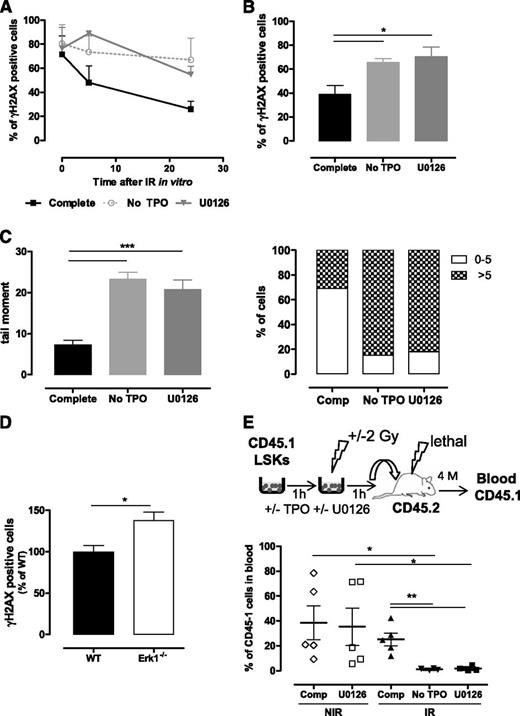
To determine which TPO-dependent signaling pathways promoted DSB repair in HSPCs following IR, cells were cultured in media containing, IL-3, FL, SCF, IL-6, and TPO (referred to as complete medium) and kinase inhibitors of TPO/cytokine-induced signaling before IR.10 Analysis of γH2AX foci was used as a DSB marker. As previously described, cells cultured in TPO-free medium were greatly impaired in their capacity to resolve IR-induced γH2AX foci. The MEK inhibitor U0126 prevented a TPO effect in LSK and HSC-enriched LSK-CD34− cells (Figure 1A-B). By contrast, no significant effect was observed using p38 and JNK MAPK inhibitors (supplemental Figure 1A-B). Single-cell comet assays confirmed that MEK inhibition abolished TPO-promoted DNA DSB rejoining (Figure 1C; supplemental Figure 1C). This effect is specific for TPO, because it could not be detected in Mpl−/− cells (supplemental Figure 1C). Supporting a role for the Erk pathway in HSC DNA repair, Erk1−/− LSK-CD34− HSCs displayed increased γH2AX foci numbers 16 hours after TBI (Figure 1D). The basal levels of DNA damage in Erk1−/− HSCs (14% + 2.5%), although higher than that of WT 7.3% + 2.5%), were not significantly different. As Mpl−/− HSPCs, Erk1−/− cells showed decreased kinetics of γH2AX foci decay after IR in vitro that could not be improved by TPO stimulation (supplemental Figure 1D-E).
Erk activation is necessary for TPO-mediated DSB repair in HSPCs. (A-B) γH2AX foci resolution analysis at the indicated times after IR (2 Gy) of (A) LSK and (B) LSK-CD34− cells cultured in complete medium in the presence or absence of the MEK inhibitor U0126 (10 µM), or in medium without TPO. Data are means + standard error of the mean (SEM) (n = 3). (C) DSB analysis using a neutral comet assay of LSK cells cultured as in panel A. Mean of tail moments (left) and percent of cells with tail moments >5 or <5 (right) are shown. Representative experiment out of 3 similar performed with cells pooled from 3 to 4 mice. Data are means + SEM of tail moment values analyzed in at least 100 cells. (D) γH2AX staining in LSK-CD34− cells isolated 16 hours after TBI of wild type (WT) and Erk1−/− mice. Data are means + SEM normalized to the mean of γH2AX positive cells from WT mice (n = 5). (E) Experimental design to test the effect of Erk inhibition on LSK reconstitution ability (top). LSK cells were cultured as in panel A for 1 hour before injection in CD45-2 lethally irradiated congenic mice. CD45.1-chimerism in peripheral blood 4 months posttransplantation (bottom). Each dot represents an individual mouse. Mice were injected with LSK cells from a pool of 9 mice. Comp, complete medium; NIR, no IR.
Erk activation is necessary for TPO-mediated DSB repair in HSPCs. (A-B) γH2AX foci resolution analysis at the indicated times after IR (2 Gy) of (A) LSK and (B) LSK-CD34− cells cultured in complete medium in the presence or absence of the MEK inhibitor U0126 (10 µM), or in medium without TPO. Data are means + standard error of the mean (SEM) (n = 3). (C) DSB analysis using a neutral comet assay of LSK cells cultured as in panel A. Mean of tail moments (left) and percent of cells with tail moments >5 or <5 (right) are shown. Representative experiment out of 3 similar performed with cells pooled from 3 to 4 mice. Data are means + SEM of tail moment values analyzed in at least 100 cells. (D) γH2AX staining in LSK-CD34− cells isolated 16 hours after TBI of wild type (WT) and Erk1−/− mice. Data are means + SEM normalized to the mean of γH2AX positive cells from WT mice (n = 5). (E) Experimental design to test the effect of Erk inhibition on LSK reconstitution ability (top). LSK cells were cultured as in panel A for 1 hour before injection in CD45-2 lethally irradiated congenic mice. CD45.1-chimerism in peripheral blood 4 months posttransplantation (bottom). Each dot represents an individual mouse. Mice were injected with LSK cells from a pool of 9 mice. Comp, complete medium; NIR, no IR.
Exposure of LSK cells to low doses of IR in vitro induced a striking decrease in their long-term reconstituting activity that was completely prevented by a short treatment with TPO before and after IR1,10 (Figure 1E). This protective effect of TPO was abolished in the presence of U0126, decreasing engraftment of irradiated cells down to levels of cells treated in TPO-free media. This loss of HSC function was not a result of increased apoptosis, which remained low 24 hours after IR even when the inhibitor was not removed from the cultures (data not shown). In addition, U0126 did not alter the reconstitution ability of nonirradiated LSK cells (Figure 1E), showing that Erk pathway inhibition is generally not toxic to HSCs. Taken together, these results indicate that Erk activity is required for TPO-induced HSC DNA repair and functional protection after IR.
TPO is the main activator of Erks in HSPCs
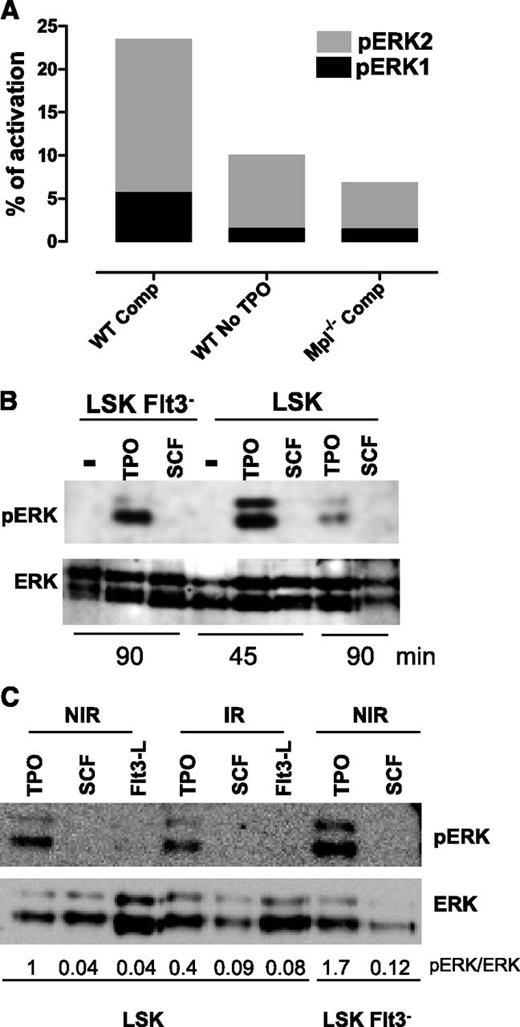
Removal of SCF from the medium had no effect on DSB repair when TPO was present (supplemental Figure 1C). This confirms our previous results demonstrating that the effect of TPO is specific.10 All of the cytokines present in the complete medium are known as Erk activators in HSPCs. However, no comparative quantitative assessment of their ability to trigger Erk activation in HSCs has been performed. To understand why only the TPO/Erk signal was able to regulate DNA damage repair, we measured Erk1/2 activation in HSPCs cultured in complete or TPO-free medium at time where TPO is activating repair using a capillary isoelectric-focusing immunoassay.37 Removal of TPO from the medium led to a striking decrease in total pErk1/2 levels (Figure 2A and supplemental Figure 2A). Likewise, about 2/3 of Erk activation was lost upon incubation of Mpl−/− cells in medium containing TPO. Western blot analysis showed that TPO but not SCF or FL stimulation alone could induce a detectable pErk signal in LSK, LSK-Flt3−, and LSK-CD34− cells (Figure 2B-C; supplemental Figure 2B). IR did not increase but rather slightly decreased Erk activation induced by TPO. In agreement with increased Mpl expression with cell stemness phenotype,11 TPO-triggered Erk phosphorylation was higher in LSK-Flt3− and LSK-CD34− cells than in LSK cells. Thus, TPO is the major Erk activator in HSPCs.
TPO is the major Erk activator in HSPCs. (A) One representative experiment, out of 3 similar experiments, of Erk activity obtained by capillary isoelectric-focusing immunoassay in WT and Mpl−/− LSK cells cultured for 1 hour in complete medium (Comp) or without TPO. Results show the area peak value of phosphorylated Erk1 or Erk2, normalized to the area value of total Erk. Experiment performed with cells pooled from 3 to 5 mice. (B-C) Western blot analysis of Erk activation in LSK or LSK-Flt3− cells cultured for 45 or 90 minutes as indicated with SCF, FL, or TPO or in the absence of cytokine (−) and then irradiated (2 Gy) or not. Analysis was performed 30 minutes after IR. NIR, no IR.
TPO is the major Erk activator in HSPCs. (A) One representative experiment, out of 3 similar experiments, of Erk activity obtained by capillary isoelectric-focusing immunoassay in WT and Mpl−/− LSK cells cultured for 1 hour in complete medium (Comp) or without TPO. Results show the area peak value of phosphorylated Erk1 or Erk2, normalized to the area value of total Erk. Experiment performed with cells pooled from 3 to 5 mice. (B-C) Western blot analysis of Erk activation in LSK or LSK-Flt3− cells cultured for 45 or 90 minutes as indicated with SCF, FL, or TPO or in the absence of cytokine (−) and then irradiated (2 Gy) or not. Analysis was performed 30 minutes after IR. NIR, no IR.
Iex-1 is synergistically and specifically induced by TPO and IR in HSPCs
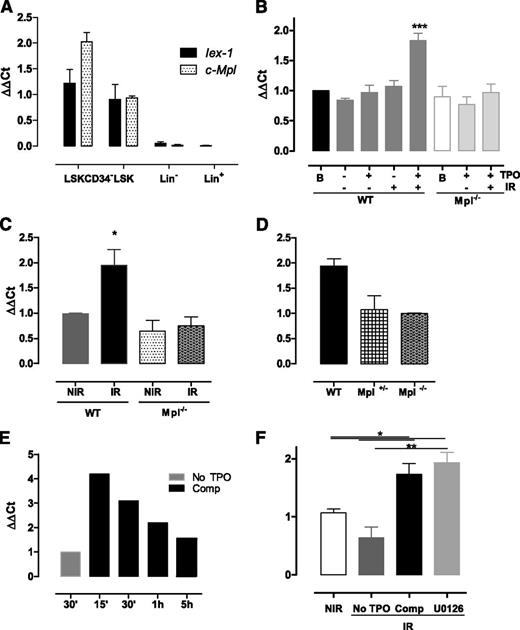
The early gene IEX-1/IER3 was previously found to regulate DNA damage responses upon IR.32 In addition, we have identified the IEX-1 protein as an Erk substrate involved in TPO-mediated function in megakaryocytes,24,25 suggesting that it could play a role in TPO/Erk signaling-mediated DNA repair in HSPCs. Compatible with this possibility, the Iex-1 messenger RNA (mRNA) expression pattern follows that of Mpl, increasing greatly in HSC-enriched populations (Figure 3A). Upon IR in vitro, LSK cells cultured in complete medium showed a significant increase in Iex-1 mRNA levels (Figure 3B). No such increase was observed in the absence of IR or when IR was applied in TPO-free medium or in Mpl−/− cells. Likewise, Iex-1 mRNA levels were significantly increased in vivo in LSK cells isolated from WT but not from Mpl−/− mice 5 hours after TBI (Figure 3C). Supporting a role for Iex-1 induction in the TPO-mediated HSC DNA damage response, Iex-1 mRNA expression remained unchanged in irradiated heterozygous Mpl+/− cells impaired for this function10 (Figure 3D). Kinetics studies showed that Iex-1 mRNA induction upon TPO and IR is rapid (Figure 3E), a timing that is compatible with that of an early-response gene and the effect of TPO on DSB repair. Altogether, these results indicate that Iex-1 induction in HSPCs requires TPO and IR signals, both in vitro and in vivo. However, this effect is independent of TPO-mediated Erk activation because it remained unchanged in the presence of MEK inhibitor (Figure 3F).
Iex-1 is induced in HSPCs in response to TPO and IR. (A) Quantitative polymerase chain reaction analysis of Mpl and Iex-1 mRNA levels in different hematopoietic cell populations. Results are means + SEM (n = 3). (B-F) Quantitative polymerase chain reaction analysis of Iex-1 expression in LSK cells from WT, Mpl−/−, and Mpl+/− mice cultured in vitro in complete medium (Comp) or without TPO and irradiated or not (2 Gy). Analysis was performed at 5 hours (B,D) or at various times after IR (E). (F) Cells were incubated with the MEK inhibitor U0126 (10 µM) 30 minutes before being treated as in panel B. Data are means + SEM (n = 4). (C) Iex-1 mRNA expression in LSK cells isolated 5 hours after TBI of WT and Mpl−/− mice. Data are means + SEM (n = 3). All the results are normalized on gapdh expression.
Iex-1 is induced in HSPCs in response to TPO and IR. (A) Quantitative polymerase chain reaction analysis of Mpl and Iex-1 mRNA levels in different hematopoietic cell populations. Results are means + SEM (n = 3). (B-F) Quantitative polymerase chain reaction analysis of Iex-1 expression in LSK cells from WT, Mpl−/−, and Mpl+/− mice cultured in vitro in complete medium (Comp) or without TPO and irradiated or not (2 Gy). Analysis was performed at 5 hours (B,D) or at various times after IR (E). (F) Cells were incubated with the MEK inhibitor U0126 (10 µM) 30 minutes before being treated as in panel B. Data are means + SEM (n = 4). (C) Iex-1 mRNA expression in LSK cells isolated 5 hours after TBI of WT and Mpl−/− mice. Data are means + SEM (n = 3). All the results are normalized on gapdh expression.
Iex-1 expression is required for TPO-induced DNA repair upon IR
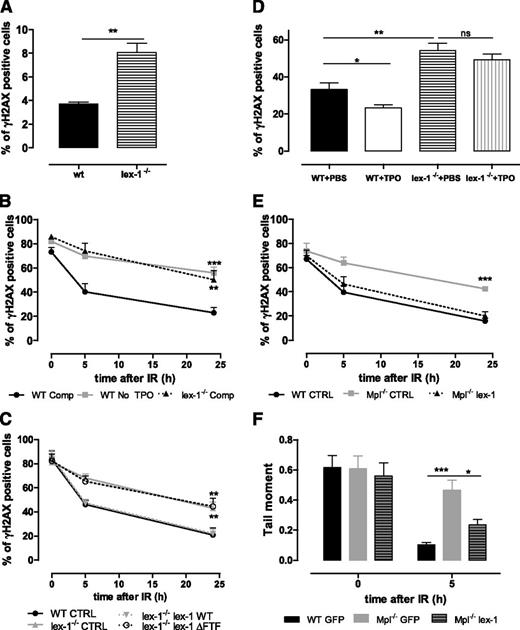
As Mpl−/− HSPCs,10 Iex-1−/− HSPCs showed spontaneous increased DNA damage (Figure 4A). Upon IR in vitro, the presence of TPO in the medium did not accelerate the resolution of γH2AX foci in Iex-1−/− cells (Figure 4B). The defect was similar in extent to that observed in WT cells treated without TPO, showing that Iex-1−/− HSPCs do not respond to TPO. Infection of Iex-1−/− HSPCs with an Iex-1–encoding vector rescued completely the TPO-induced increase in γH2AX foci removal (Figure 4C), indicating that the defect in TPO response is due to Iex-1 deficiency. TBI-induced DNA damage was stronger in LSK-CD34−Iex-1−/− cells than in their WT counterparts (Figure 4D). Moreover, TPO injection to WT but not to Iex-1−/− mice just before TBI significantly reduced the number of LSK-CD34− harboring γH2AX foci at 16 hours compared with mice treated with phosphate-buffered saline alone. This shows that Iex-1−/− HSCs are unable to respond to TPO in vivo. Further confirming the importance of Iex-1 expression in TPO-improved DSB repair, Mpl−/− cells regained γH2AX foci disappearance and DSB rejoining in the presence of TPO upon infection with a vector expressing Iex-1 and green fluorescent protein (GFP), but not with a vector expressing GFP alone (Figure 4E-F).
Iex-1 is necessary and sufficient for TPO-mediated DSB repair in HSPCs. (A) Percentage of γH2AX-positive LSK cells isolated from nontreated WT and Iex-1−/− mice. Data are means + SEM (n = 3). (B) Kinetics of γH2AX foci resolution after IR (2 Gy) of WT and Iex-1−/− LSK cells cultured in complete medium (Comp) or without TPO. (C) WT LSK cells were infected with lentiviruses expressing GFP only or GFP with Iex-1-WT or Iex-1-ΔFTF and treated as in (B). For (B) and (C), the results represent means + SEM of 3 independent experiments performed with pools of 5 to 10 mice. (D) WT and Iex-1−/− mice were treated with TPO (8 µg/kg) or phosphate-buffered saline 30 minutes before TBI (2 Gy). γH2AX staining in LSK-CD34− cells isolated 16 hours after TBI. (E-F) γH2AX foci (E) and comet tail resolution (F) after IR of WT and Mpl−/− LSK cells infected as in (C) and cultured in the presence of TPO complete medium. Means + SEM are shown. (E) n = 4. (F) Data are means + SEM of tail moment values analyzed in at least 35 cells.
Iex-1 is necessary and sufficient for TPO-mediated DSB repair in HSPCs. (A) Percentage of γH2AX-positive LSK cells isolated from nontreated WT and Iex-1−/− mice. Data are means + SEM (n = 3). (B) Kinetics of γH2AX foci resolution after IR (2 Gy) of WT and Iex-1−/− LSK cells cultured in complete medium (Comp) or without TPO. (C) WT LSK cells were infected with lentiviruses expressing GFP only or GFP with Iex-1-WT or Iex-1-ΔFTF and treated as in (B). For (B) and (C), the results represent means + SEM of 3 independent experiments performed with pools of 5 to 10 mice. (D) WT and Iex-1−/− mice were treated with TPO (8 µg/kg) or phosphate-buffered saline 30 minutes before TBI (2 Gy). γH2AX staining in LSK-CD34− cells isolated 16 hours after TBI. (E-F) γH2AX foci (E) and comet tail resolution (F) after IR of WT and Mpl−/− LSK cells infected as in (C) and cultured in the presence of TPO complete medium. Means + SEM are shown. (E) n = 4. (F) Data are means + SEM of tail moment values analyzed in at least 35 cells.
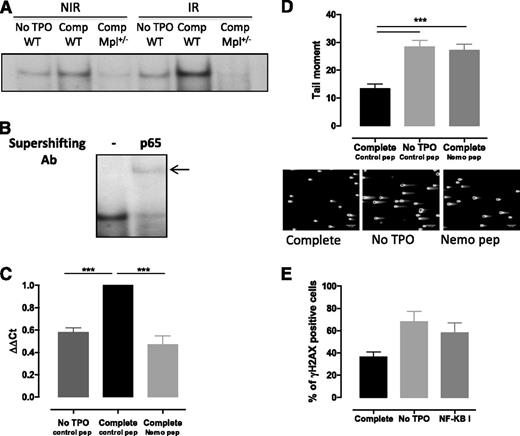
TPO-increased NF-κB activation is required for Iex-1 expression upon IR
Although Erk and Iex-1 are both required for TPO-promoted DSB repair, our results indicate that another pathway is involved in the synergistic induction of Iex-1 mRNA by TPO and IR in HSPCs. Iex-1 has been described as a NF-κB target gene in various types of cells,26 a pathway that can be activated in response to IR.39 This prompted us to evaluate the status of NF-κB activation in HSPCs. EMSA showed that NF-κB DNA binding was induced when TPO was present in the medium (Figure 5A). A weak activity was also detected upon IR in the absence of TPO. Correlating with the effect of TPO on Iex-1 expression, NF-κB activity was greatly enhanced when both stimuli were applied together and was abolished in Mpl+/− cells. Supershift analysis indicated that the complexes induced by TPO and IR contained the NF-κB subunit RelA (Figure 5B). Iex-1 mRNA induction by TPO + IR treatment was inhibited upon incubation with NEMO peptide, a specific IKK complex inhibitor40 (Figure 5C), indicating that TPO-mediated NF-κB activation is required for Iex-1 induction in HSPCs. Moreover, NEMO peptide and CAS 545380-34-5, a second NF-κB inhibitor blocking NF-κB transcription, inhibited TPO-accelerated DNA repair (Figure 5D-E). This further supports the requirement of a NF-κB–mediated increase in Iex-1 expression to ensure a TPO effect on DNA repair.
NF-κB is required for TPO-induced Iex-1 expression and DSB repair in HSPCs. (A) EMSA for NF-κB DNA-binding activity in WT and Mpl+/− LSK cells cultured in complete medium (Comp) or without TPO for 45 minutes and then irradiated (2 Gy) or not. Analysis was performed 30 minutes after IR. Representative experiment out of 4 similar performed. (B) Supershift analysis after addition of anti-p65RelA antibody or no antibody (−) in WT LSK cells treated as in (B) 30 minutes post-IR in the presence of TPO. (C) Iex-1 mRNA expression 30 min after IR in LSK cells precultured in medium without TPO or in complete medium containing either the Nemo peptide or a control peptide (10 µM). Data are means + SEM from 4 independent experiments performed with cells from 3 to 5 mice. (D) Comet tail moment of WT LSK cultured as in panel C and 2 hours after IR. Representative pictures of comets labeled with SYTOXGreen and means + SEM of the tail moment values measured in at least 60 cells are shown. The scale bars on the pictures represent a 107 µm × 10 objective. One representative experiment out of 3 experiments is shown. (E) γH2AX staining in LSK cells cultured like in panel D in the presence or absence or of the NF-κB inhibitor (NF-κB I, 1 µM) 24 hours after IR. Data are means + SEM of 3 independent experiments with at least 100 cells counted per condition.
NF-κB is required for TPO-induced Iex-1 expression and DSB repair in HSPCs. (A) EMSA for NF-κB DNA-binding activity in WT and Mpl+/− LSK cells cultured in complete medium (Comp) or without TPO for 45 minutes and then irradiated (2 Gy) or not. Analysis was performed 30 minutes after IR. Representative experiment out of 4 similar performed. (B) Supershift analysis after addition of anti-p65RelA antibody or no antibody (−) in WT LSK cells treated as in (B) 30 minutes post-IR in the presence of TPO. (C) Iex-1 mRNA expression 30 min after IR in LSK cells precultured in medium without TPO or in complete medium containing either the Nemo peptide or a control peptide (10 µM). Data are means + SEM from 4 independent experiments performed with cells from 3 to 5 mice. (D) Comet tail moment of WT LSK cultured as in panel C and 2 hours after IR. Representative pictures of comets labeled with SYTOXGreen and means + SEM of the tail moment values measured in at least 60 cells are shown. The scale bars on the pictures represent a 107 µm × 10 objective. One representative experiment out of 3 experiments is shown. (E) γH2AX staining in LSK cells cultured like in panel D in the presence or absence or of the NF-κB inhibitor (NF-κB I, 1 µM) 24 hours after IR. Data are means + SEM of 3 independent experiments with at least 100 cells counted per condition.
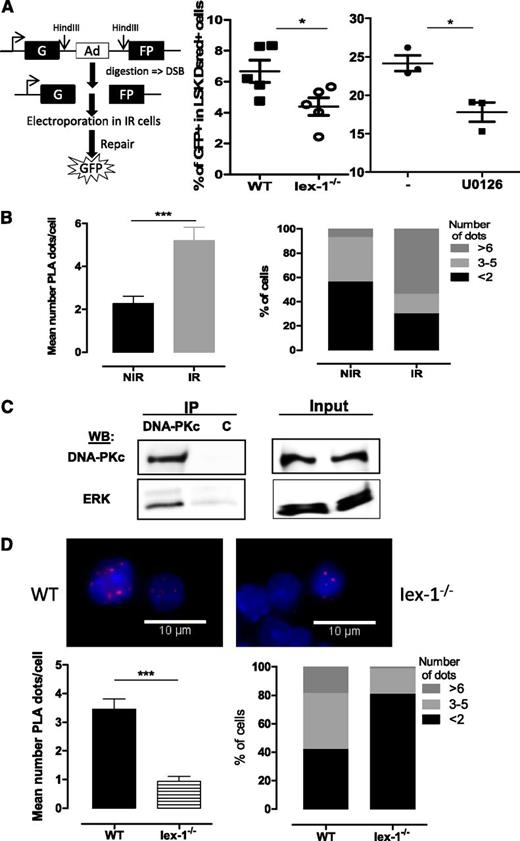
Iex-1 and Erk form a complex with DNA-PKc that is required for DNA-PK activation by TPO
We then asked how the Erk pathway and Iex-1 regulate TPO-induced DNA repair. TPO increases IR-induced DNA-PK activation and classical NHEJ signaling.10 Direct assessment of NHEJ activity in response to IR with a plasmid ligation assay showed that this activity was significantly impaired both in Iex-1−/− LSK cells and in the presence of U0126 (Figure 6A), suggesting that Iex-1, Erk, and TPO act on the same pathway. Iex-1 forms a complex with pErk1/2 through an FXF-specific docking site.24,41 Expression of Iex-1-ΔFTF mutated at this site could not rescue the Iex-1−/− HSPC DNA-damage defect (Figure 4C), showing that Iex-1/pErk interaction is required for this function. To examine if Iex-1–bound pErk1/2 affects DNA-PK activation in HSPCs, we examined interactions between these proteins using PLA, which allows detection in situ of physical protein-protein interactions by immunofluorescence in small cell numbers. Both pErk and pDNA-PK levels are increased upon TPO stimulation. To minimize these changes, we analyzed the interaction between the DNA-PK catalytic subunit, DNA-PKc, and pErk in cells cultured in complete medium, ie, in the presence of TPO. We have previously shown that neither the total level of DNA-PKc nor its localization is altered by TPO and IR treatments.10 PLA between pErk and DNA-PKc could be detected at a low level in LSK cells cultured in complete medium and was significantly increased upon IR (Figure 6B). Confirming the DNA-PK/Erk interaction, anti–DNA-PK antibodies could coimmunoprecipitate ERK1/2 in UT7-Mpl cells, a cell line in which TPO enhances DNA-PK–dependent NHEJ repair (Figure 6C).
pErk and DNA-PKc form a complex that requires the presence IEX-1. (A) NHEJ activity in WT and Iex-1−/− LSK cells cultured in complete medium in the presence or absence of U0126 (10 μM). The experimental procedure (left) and means + SEM percent of GFP+ cells in LSK DsRed+ cells (right) are shown. Each dot represents an individual mouse. (B,D) PLA between pErk and DNA-PKc in WT and Iex-1−/− LSK cells. Cells were cultured in complete medium for 30 minutes and analyzed 30 minutes after IR (2 Gy) or not. Representative pictures (×100) and means + SEM of PLA dot numbers per cell (left) and percent of cells with the indicated amounts of dots (right) are shown. (C) UT7-Mpl cells were treated with TPO and then irradiated (2 Gy). Thirty minutes after IR, nuclear extracts were immunoprecipitated with anti-DNA-PKc or control (c) mouse immunoglobulin G. NIR, no IR.
pErk and DNA-PKc form a complex that requires the presence IEX-1. (A) NHEJ activity in WT and Iex-1−/− LSK cells cultured in complete medium in the presence or absence of U0126 (10 μM). The experimental procedure (left) and means + SEM percent of GFP+ cells in LSK DsRed+ cells (right) are shown. Each dot represents an individual mouse. (B,D) PLA between pErk and DNA-PKc in WT and Iex-1−/− LSK cells. Cells were cultured in complete medium for 30 minutes and analyzed 30 minutes after IR (2 Gy) or not. Representative pictures (×100) and means + SEM of PLA dot numbers per cell (left) and percent of cells with the indicated amounts of dots (right) are shown. (C) UT7-Mpl cells were treated with TPO and then irradiated (2 Gy). Thirty minutes after IR, nuclear extracts were immunoprecipitated with anti-DNA-PKc or control (c) mouse immunoglobulin G. NIR, no IR.
We were not able to perform PLA with the available anti–Iex-1 antibodies that do not detect Iex-1 protein in primary cells. However, the PLA signal between DNA-PKc and pErk was greatly decreased in Iex-1−/− cells as compared with WT cells (Figure 6D), indicating that the IR-induced in situ pErk/DNA-PKc interaction is dependent on the presence of Iex-1.
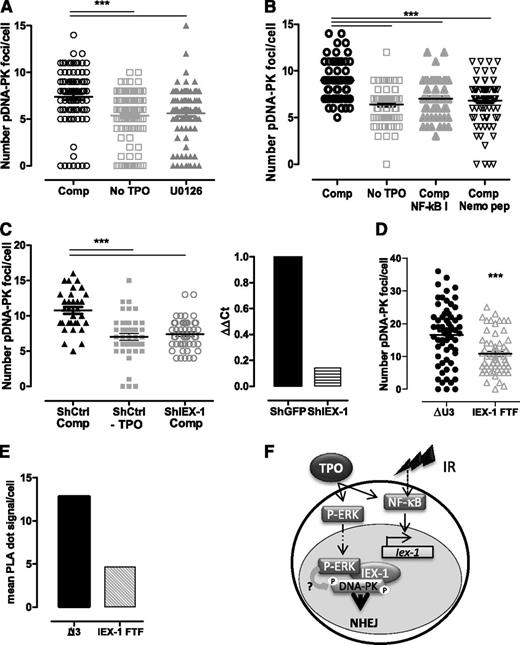
We next assessed whether the pErk/DNA-PKc complex is required for TPO-increased DNA-PK activation upon IR. Ser2056- and Thr2609-phospho–DNA-PK foci formation is a functional marker of IR-induced DNA-PK activation and is required for NHEJ.42,43 Because anti–pDNA-PK antibodies do not react with mouse cells, we used cord-blood–derived human CD34+ HSPCs. Both MEK and NF-κB inhibitors abolished TPO ability to increase IR-induced Ser2506-pDNA-PK foci formation in these cells (Figure 7A-B). Similar results were found in UT7-Mpl cells (supplemental Figure 3A).10,25
Erk and IEX-1 are necessary for DNA-PK activation in human and mouse HSPCs. (A-B) Ser2056-pDNA-PK foci number 30 minutes after IR of CD34+ human progenitor cells cultured without TPO or in complete medium (Comp) in the presence or absence of 10 µM U0126 (A) or 1 µM NF-κB I or 10 µM Nemo inhibitory peptide (B). Each dot represents an individual cell. Data are means + SEM from cells counted in 3 independent experiments. (C) Number of pSer2056-pDNA-PK 30 minutes after IR of CD34+ human cells infected with lentiviruses encoding control GFP-shRNA or IEX-1-shRNA and cultured and analyzed as in panel A (left). Data are means + SEM foci per cell counted from 3 independent experiments. IEX-1 mRNA expression in the shRNA-infected cells analyzed on the left (right). (D) Number of Thr2609-pDNA-PK and (E) pErk/DNA-PKc interaction using PLA in UT7-Mpl infected with control (ΔU3) or with IEX-1-ΔFTF vectors and treated with TPO and IR as in panel A. Representative experiments. (F) Representative model illustrating the cooperation between NF-κB and Erk activation upon TPO and IR, leading to Iex-1 induction and Iex-1 activation, respectively. The complex formed between pErk/Iex-1/DNA-PKc is required for DNA-PK phosphorylation and DNA-repair promotion.
Erk and IEX-1 are necessary for DNA-PK activation in human and mouse HSPCs. (A-B) Ser2056-pDNA-PK foci number 30 minutes after IR of CD34+ human progenitor cells cultured without TPO or in complete medium (Comp) in the presence or absence of 10 µM U0126 (A) or 1 µM NF-κB I or 10 µM Nemo inhibitory peptide (B). Each dot represents an individual cell. Data are means + SEM from cells counted in 3 independent experiments. (C) Number of pSer2056-pDNA-PK 30 minutes after IR of CD34+ human cells infected with lentiviruses encoding control GFP-shRNA or IEX-1-shRNA and cultured and analyzed as in panel A (left). Data are means + SEM foci per cell counted from 3 independent experiments. IEX-1 mRNA expression in the shRNA-infected cells analyzed on the left (right). (D) Number of Thr2609-pDNA-PK and (E) pErk/DNA-PKc interaction using PLA in UT7-Mpl infected with control (ΔU3) or with IEX-1-ΔFTF vectors and treated with TPO and IR as in panel A. Representative experiments. (F) Representative model illustrating the cooperation between NF-κB and Erk activation upon TPO and IR, leading to Iex-1 induction and Iex-1 activation, respectively. The complex formed between pErk/Iex-1/DNA-PKc is required for DNA-PK phosphorylation and DNA-repair promotion.
TPO and IR act additively to induce IEX-1 mRNA and protein expression in UT7-Mpl cells and in CD34+ HSPCs (supplemental Figure 3B-D). Small hairpin RNA (shRNA)-mediated IEX-1 knockdown abolished TPO-promoted DNA-PK phosphorylation in CD34+ and UT7-Mpl cells (Figure 7C; supplemental Figure 3E). Interestingly, in UT7-Mpl cells, the IR + TPO–induced pDNA-PK foci formation and pErk/DNA-PKc in situ interaction (Figure 7D-E) were inhibited upon expression of IEX-1-ΔFTF as compared with vector expressing GFP alone, suggesting that this mutant behaves as a dominant negative on DNA-PK activation and pErk/DNA-PKc interaction and that TPO induces the formation of a tripartite complex (pErk/IEX-1/DNA-PKc) upon IR. Preventing the interaction between DNA-PKc and pErk through mutation of IEX-1’s Erk docking site, inhibition of Erk signaling, or Iex-1 deficiency alters the ability of TPO to increase DNA-PK activation upon IR.
Altogether, these data show that TPO-triggered NF-κB and Erk pathways leading to IEX-1 expression and activation, respectively, upon DNA damage constitute a unique pathway allowing the selective activation of DNA-PK–dependent NHEJ in HSPCs in response to TPO and IR (Figure 7F).
Discussion
Studies from mice deficient in various kinases or signaling intermediates have increased our knowledge of the pathways controlling HSC self-renewal and quiescence. Although signaling pathways are shared by growth factors, their activation may vary with the cellular context. In addition, within a unique cellular context, they can be used differently by different growth factors, leading to different final functional outcome. Little is known concerning the unique signals triggered by a given cytokine in HSCs and those regulating HSC response following IR. Here we identify a new pathway specifically bridging Mpl signaling to DNA-PK–dependent DSB repair in HSCs upon IR. This involves activation both Erk and NF-κB, which cooperate to induce and activate the early gene Iex-1 in response to DNA damage.
Phosphoflow assays have shown that SCF and FL trigger Erk1/2 activation in mouse HSPCs.16 However, our results indicate that TPO is the main activator of this pathway in both LSK and HSC-enriched cells. Indeed, removal of TPO from WT cell cultures, or using Mpl−/− cells, led to a 65% to 70% decrease in the Erk1/2 phosphorylation signal despite the presence of IL-3, IL-6, SCF, and FL in the medium. Moreover, Erk1/2 activation could be detected by western blots after single stimulation with TPO, but not with SCF. This might be due to the fact that our studies were performed after 45 to 90 minutes of treatment, a time required before and after IR to stimulate TPO-mediated repair,10 while the maximum activation of Erks by SCF has been found to be around 10 minutes. On the other hand, SCF-mediated Erk activation was found to be higher in Lin−Kit+ myeloid progenitors than in HSCs,16 whereas TPO-induced Erk activation was found to augment in HSC-enriched populations.
TPO-induced Erk activation is required for HSC DSB repair and to prevent HSC loss of function following IR. By contrast, U0126 has no effect on the reconstitution ability of nonirradiated cells. A similar observation was found for Erk1−/−34 and Iex-1−/− HSCs (data not shown). This suggests that the Erk/Iex-1 pathway does not play a critical role in TPO-controlled HSC function in the absence of DNA damage. This assumption is supported by the fact that HSCs expressing a truncated Mpl receptor defective in its ability to activate Erk have unaltered self-renewal.44 pErk1 represents only about 30% of the total pErk1/2 signal (Figure 2A). The increased DNA damage upon IR and the loss of TPO-promoted DNA repair in Erk1−/− HSCs suggest that threshold levels of Erk activity need to be reached to regulate HSC response to genotoxic stress but not for the maintenance of HSC self-renewal or numbers. A recent study has shown that HSCs deficient for both Erk1 and Erk2 display decreased proliferation and contribute poorly to hematopoiesis.45 The difference between this study and ours might be due the fact that we induced partial or transient Erk inhibition, contrasting with a total removal of Erk signal induced by Erk1/2 ablation. On the other hand, it is possible that total Erk1/2 deficiency significantly alters HSPC proliferation and survival in response to another growth factor in vivo.
Erk inhibition abolished a TPO-mediated increased in DNA-PK autophosphorylation in human HSPCs and UT7-Mpl cells. Using PLA, we found that pErk and DNA-PKc form a complex that is greatly increased upon IR. Several studies have shown that γ-IR can trigger Erk activation in different cell lines and have implied this pathway in the proper execution of apoptosis or G2/M checkpoint.46 However, few data have been described concerning the involvement of Erks in DSB repair mechanisms. MEK inhibitors, as well as AKT inhibitors, were found to block epidermal growth factor–stimulated DSB repair through both HR and NHEJ, but the mechanism by which this inhibition occurs was not described.47 A recent report has demonstrated DNA-PKc can interact with AKT upon IR and epidermal growth factor stimulation, enhancing its autophosphorylation that is needed for efficient DSB repair.48 However, we were unable to detect AKT activation in HSPCs in response to TPO and/or IR. This is the first evidence of an interaction between DNA-PKc and activated Erks and of a direct role of this pathway in DNA-PK and NHEJ activation. Although further studies are required to determine how this occurs, our results indicate that induction of the Erk substrate IEX-1 is specifically required for this effect in both human and mouse HSPCs. We have previously shown that IEX-1 acts as a scaffold protein for activated Erks, bringing them in complex with other substrates and enabling their phosphorylation.41 Our results with the IEX-1-ΔFTF mutant show that Iex-1 is required to bring pErks to DNA-PKc and for TPO-enhanced DNA-PK autophosphorylation. Thus, IEX-1 might increase DNA-PK activation by promoting its phosphorylation by Erks upon IR. However, the numerous DNA-PKc phosphorylation sites and the paucity of HSPCs preclude us to test this hypothesis.
Nuclear translocation of NF-κB-RelA has been observed in HSCs of aged mice.49 It was recently demonstrated that the self-renewal potential and the expression of key HSC maintenance genes are severely impaired in RelA-deficient HSCs.50 This indicates that NF-κB signaling is activated in vivo in HSCs but the stimulus responsible for this activation under homeostasis is unknown. Our results show for the first time that a noninflammatory cytokine regulating HSCs induces NF-κB activation in these cells. This activation required full Mpl expression and is specific because no NF-κB DNA binding was detected in Mpl+/− HSPCs. In addition, although IR has only a slight effect by itself, TPO and IR act in synergy to stimulate NF-κB in WT but not in Mpl+/− cells. The majority of the NF-κB DNA-binding activity contains the RelA/p65 subunit, suggesting that loss of TPO signaling may participate to the RelA−/− HSC phenotype in addition to its effect in the HSC DNA-damage response.
NF-κB–induced expression of antiapoptotic Bcl-2 family members plays an important role in cell survival following DNA damage.51 Our results show that NF-κB–triggered Iex-1 induction is sufficient to promote the TPO-mediated effect on DNA repair. This is in agreement with our previous data showing that the TPO-mediated effect in HSPCs is unrelated to changes in apoptosis and results specifically from an alteration in the DNA repair process. Megakaryocytes resist marrow radioablation and facilitate niche osteoblast expansion.52 TPO administration before TBI enhances megakaryocyte-induced promotion of HSC engraftment.53 One study showed that TPO can activate NF-κB in megakaryocytes.54 It is intriguing that the same signaling pathway involving Iex-1, NF-κB, and Erk activation could be triggered by Mpl/TPO to protect both HSCs and megakaryocytes from TBI. Manipulation of one of these downstream actors may limit thrombocytopenia and HSC injury and allow niche reconstruction.
Interestingly, IEX-1 is one of the most downregulated genes in CD34+ cells from low-risk MDS patients, and low expression is associated with short survival and adverse prognosis.55-57 IEX-1 downregulation has been suggested to be linked to the high level of apoptosis observed in these patients. Our results showing that Iex-1−/− HSPCs display increased DNA damage even in the absence of IR suggest that decreased IEX-1 expression in low-risk MDS patients could lead to impaired DNA repair through the classical DNA-PK–dependent NHEJ. This could accelerate MDS disease progression and predispose to radiotherapy-induced secondary MDS development. This is in agreement with increased DNA instability in low-risk MDS patients58,59 and decreased DNA-PK–dependent classical NHEJ repair in a mouse model of MDS.60
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Richard Dyunga for technical assistance, the Cochin Institute animal facility and cellular imaging and immunobiology platforms, and the Paris Descartes University Proteomic Platform (3P5). This work was supported by Institut National de la Santé et de la Recherche Médicale and by grants from Ligue Contre le Cancer, Comité d’Ile de France (RS12/75-67); INCa Plan Cancer (A12162KS) (F.P.); and from Agence Nationale de la Recherche (V.B.). B.L. and D.B. are recipients of fellowships from the ARC Foundation and Ligue Nationale Contre le Cancer, respectively.
Authorship
Contribution: B.d.L. and P.P. designed experiments, performed most research, and analyzed data; D.B. and A.C. performed comet assays and analyzed data; C.B.-G. and V.B. designed and performed NF-κB analysis experiments; M.G. and R.K. provided Erk1−/− and Iex-1−/− mice, respectively, and helped write the manuscript; F.P. conceived the project, planned research, and wrote the manuscript; and all authors reviewed and agreed with the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for P.P. is Gustave Roussy Cancer Campus, Unité Mixte de Recherche 8200, Villejuif, France.
The current affiliation for D.B., M.G., and F.P. is Gustave Roussy Cancer Campus, Institut National de la Santé et de la Recherche Médicale U1009, Villejuif, France.
Correspondence: Françoise Porteu, Gustave Roussy, Cancer Campus, 114 rue Édouard-Vaillant, 94805 Villejuif Cedex, France; e-mail: francoise.porteu@gustaveroussy.fr.
References
Author notes
B.d.L. and P.P. contributed equally to this study.