In this issue of Blood, de Laval et al report on a novel mechanism by which hematopoietic stem cells (HSCs) harboring DNA damage are rescued by thrombopoietin (TPO)-mediated DNA repair.1 It has been recently demonstrated that HSCs use the error-prone nonhomologous end-joining (NHEJ) pathway of DNA repair to fix DNA breaks. Maintenance of genomic integrity is crucial for HSC function. Finding the players involved in HSC DNA repair will provide a better understanding of hematopoietic homeostasis, HSC aging, and leukemogenesis.
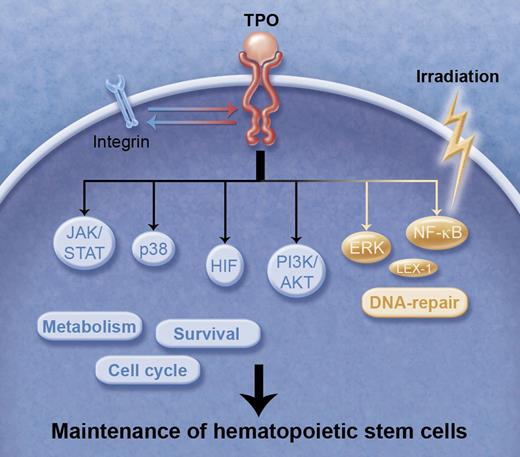
TPO signals in HSCs. TPO is essential for the maintenance of HSCs mediated by regulation of a variety of signaling molecules that are involved in cell cycle, survival, cellular metabolism, and integrin-mediated signal transduction. ERK and NF-κB collaborate to repair IR-induced DNA damage under TPO stimulation, preventing aging and tumorigenesis. These TPO signals working in harmony support the maintenance of HSCs. Professional illustration by Alice Y. Chen.
TPO signals in HSCs. TPO is essential for the maintenance of HSCs mediated by regulation of a variety of signaling molecules that are involved in cell cycle, survival, cellular metabolism, and integrin-mediated signal transduction. ERK and NF-κB collaborate to repair IR-induced DNA damage under TPO stimulation, preventing aging and tumorigenesis. These TPO signals working in harmony support the maintenance of HSCs. Professional illustration by Alice Y. Chen.
TPO is a master regulator of HSC maintenance, as well as megakaryopoiesis.2-4 Congenital amegakaryocytic thrombocytopenia patients, who lack a functioning TPO receptor (MPL), are born with almost absent megakaryocytes and multilineage bone marrow failure eventually develops. TPO-deficient mice show reduced HSC number in age, associated with a multilineage hematopoietic defect, and defective HSC expansion post transplantation.2 The signaling cascade of TPO-MPL has been well studied, mainly by using megakaryocytic cells. For example, TPO activates several major signaling pathways: p44/42 mitogen-activated protein kinase (MAPK, ERK1/2), p38 MAPK, phosphoinositide 3-kinase (PI3K)-AKT, and Janus kinase-signal transducer, and activator of transcription (STAT) 3 and STAT5 (see figure). TPO also activates hypoxia-inducible factor-1α, which functions as a feedback mechanism to block the overproduction of oxidative stress,5 suggesting that glycolytic metabolism is controlled by the TPO signal. In addition, a recent study reported that TPO is required for activation of αvβ3-integrin “inside-out” signaling and that subsequent “outside-in” signaling is indispensable for TPO-dependent HSC functions,6 suggesting that TPO supports HSC function by medicating adhesion signals.
Laval et al have recently published findings that TPO stimulates DNA-PK–dependent NHEJ DNA repair in response to irradiation (IR) in HSCs, contributing to maintenance of chromosomal integrity and prevention of injury from damage.7 Because the maintenance of genomic integrity is crucial for the preservation of DNA damage accumulation in HSC aging,8 the TPO may be indispensable for the prevention of premature aging of HSCs and tumorigenesis. In their study, Laval et al report that TPO-induced double-strand DNA break repair in response to IR is dependent on ERK activity (see figure). Induction of LEX-1, an ERK substrate, is essential for the double-strand DNA break repair. The LEX-1 mRNA upregulation is dependent on NF-κB. IR induces enhancement of physical interaction between pERK and DNA-PK, which is mediated by LEX-1, leading to increased phosphorylation of DNA-PK. The conclusion based on their findings is that NHEJ DNA repair induced by TPO is mediated by an IEX-1/DNA-PK/ERK complex in HSCs. These findings provide novel insights into how TPO protects HSCs from DNA damage. Furthermore, manipulation of these molecules may help protect HSCs and megakaryocytes from genotoxic stress when cancer patients are treated with IR or chemotherapy.
How are these signaling molecules in DNA repair related to other signals under TPO stimulation? As previously mentioned, because TPO activates many signaling pathways, other downstream targets may affect DNA repair. ERK and NF-κB may be involved in other biological phenomena (eg, cell cycle or survival) in HSCs. One of the most intriguing issues is whether DNA repair response contributes to HSC self-renewal under the TPO signal. HSCs lacking TPO-MPL signaling exhibit a failure of dormancy of HSCs in vivo.2,3 Although TPO is a potent stimulator of cell proliferation in vitro, HSCs that lose the TPO signal are actively cycling, associated with decreased negative cell cycle regulators, p57KIP2 and p19INK4D, which seems contradictory. One possible explanation is that there might be distinct types of HSC proliferation—“self-renewal” and “non–self-renewal”—and that TPO may stimulate the self-renewal type of cell proliferation, whereas it may suppress the non–self-renewal type of proliferation. Because TPO administration is reported to expand HSC numbers in vivo, the self-renewal type of HSC proliferation may be dominant in vivo. Alternatively, loss of the TPO signal reduces the self-renewal type of HSC proliferation but may stimulate the non–self-renewal type of proliferation, leading to failure of quiescence of HSCs. Is genomic integrity dependent on this differential signaling? Another question is whether NF-κB or ERK is involved in TPO-mediated HSC survival. Further dissection of the regulation of the TPO signal on maintenance of genomic integrity and elucidation of collaborative function with other TPO downstream molecules are required to understand the mechanism of HSC maintenance. A whole picture of the harmony of the TPO orchestra would lead to an understanding of the nature of HSCs, including self-renewal, expansion, aging, and tumorigenesis.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal