Key Points
Ex vivo addition of OKT3 is an economical and easy method to prevent xenogeneic GVHD and rescue patient leukemia sample xenografts.
OKT3-treated whole UCB produces robust, durable hematopoietic xenografts that are indistinguishable from CD34+ grafts.
Abstract
Immunodeficient mice are now readily engrafted with human hematopoietic cells. However, these mice are susceptible to graft-versus-host disease (GVHD) induced by the engraftment and rapid expansion of coinjected human T cells. Therefore, highly purified sample populations must be used, adding significant time, expense, and effort. Here, we have explored in vivo and in vitro methods utilizing anti-T–cell antibodies to circumvent this problem. Intraperitoneal injection of the antibody within 48 hours prevented GVHD. Alternatively, short-term in vitro incubation of cells with antibody immediately before transplant was equally effective. Although in vitro antithymocyte globulin treatment resulted in a dramatic loss of SCID-repopulating cells (SRCs), treatment with OKT3 or UCHT1 abrogated GVHD risk and preserved engraftment potential. Leukemia samples that presented with substantial human T-cell contamination were effectively rescued from GVHD. In addition, OKT3 treatment of unfractionated cord blood resulted in robust engraftment of primary and secondary mice that was indistinguishable from grafts obtained using purified CD34+ cells. Limiting dilution analysis of unfractionated blood demonstrated a SRC frequency of 1 in 300 to 500 CD34+ cells, similar to that of purified hematopoietic stem and progenitor cells. This protocol streamlines xenograft studies while significantly reducing the cost and time of the procedure.
Introduction
Since their introduction, the nonobese diabetic (NOD) and NOD SCID γ strains of immunodeficient mice have quickly become the gold standard for xenotransplantation of human cells.1,2 They have proven to be much more hospitable to engraftment of a wide range of normal and malignant human cells.3-5 One such useful trait is the reliability with which one can induce a fatal graft-versus-host disease (GVHD) using normal human peripheral blood (PB) mononuclear cells (MNCs).6-8 This model has been successfully exploited for numerous studies of immune function and treatment of GVHD. Unfortunately, it is exactly this trait that hampers studies in these mice focused on the engraftment of primary human hematopoietic tissues. Diagnostic hematologic malignancy specimens frequently contain large numbers of mature T cells, the population responsible for xenogeneic GVHD. Without expensive and time-consuming procedures that remove T cells by positive or negative selection, many samples invariably induce GVHD, effectively rendering the engrafted mice useless and often resulting in loss of the patient sample.9,10 Additionally, either lin− or CD34+ cells must be purified from umbilical cord blood (UCB), bone marrow (BM), or PB progenitor cell products before initiation of hematopoietic xenografts. A better model would allow for modulation of the CD34 negative fraction to enable the study of various facilitating cells. Although the existence of rare, poorly engrafting CD34−CD38−lin− SCID-repopulating cells (SRCs) has been previously postulated,11,12 recent reports have convincingly identified CD34− cells that are readily engrafted and give rise to CD34+ cells in vivo, raising the possibility that the use of CD34+ cells in xenotransplant studies could be omitting some critical cells that are included in clinical stem cell transplants in humans.13,14
In this study, we set out to prevent GVHD from ruining precious primary leukemia samples. We found that anti T-cell antibodies were effective therapies in xenografts, with OKT3 and UCHT1 showing superior results to ATG due to off-target effects of ATG at the dose used. Additionally, we found that in vitro incubation with antibody was just as effective as later in vivo treatment. This in vitro protocol is preferred because of its simplicity and significant savings in antibody expense.
We applied this protocol to unfractionated UCB and found that injection of as few as 1 million OKT3-treated white blood cells (WBCs) resulted in stable, long-term, multi-lineage engraftment without GVHD, similar to results observed with CD34+ purified samples. Importantly, BM from primary mice readily transferred the human graft to secondary hosts, indicating engraftment and retention of HSPCs/SRCs. Furthermore, limiting dilution analysis revealed SRC frequency to be ∼1 in 7 × 104 WBCs, which corresponded to ∼1 in 400 CD34+ cells.
Materials and Methods
Mice
NSG, NOD/RAG/interleukin-2 (IL-2)RG (NRG) (both from The Jackson Laboratory), NOD/LtSz-SCID IL-2RG−/− SGM3 (NSGS),15 and NRG-SGM3 (NRGS) mice were maintained according to standard procedures and in accordance with an institutional review board-approved protocol. The NRGS strain was generated by cross breeding female NSGS with male NRG. The litters contained males hemizygous for the IL2RG knockout and heterozygous for the human stem cell factor/granulocyte-macrophage-colony stimulating factor/interleukin-3 cytokine cassette. These were used in a backcross to NRG females to eliminate the SCID mutation. The NRGS mouse line will be available from The Jackson Laboratory as stock no. 024099.
Cells
PB MNCs.
PB was obtained from normal adults following proper consent. Blood was passed through a ficoll gradient (Ficoll-Paque PLUS; GE Healthcare) to obtain MNCs. Then 20 M cells were intravenously (IV) injected into sublethally irradiated mice to induce GVHD.
UCB.
UCB was obtained from the Translational Trials Development Support Laboratories of Cincinnati Children’s Hospital Medical Center after informed consent. Whole UCB was subjected to red blood cell depletion by incubation with one-seventh volume of a 6% hetastarch solution (Hespan, B. Braun Medical Inc.) for 90 minutes at room temperature. Recovered WBCs were enumerated by complete blood count analysis using a Hemavet 9500 (Drew Scientific). For induction of GVHD, 1 to 15 M recovered cells were injected into the tail vein of each preconditioned mouse. Alternatively, for the CD34+ engraftment experiments, recovered cells were subjected to CD34+ selection using the direct CD34 microbead conjugate kit from Miltenyi Biotec. The manufacturer’s protocol was modified in that total WBCs recovered after hetastarch depletion were suspended in 600 μL phosphate buffered saline (PBS)/2% fetal bovine serum (FBS)/1 mM EDTA buffer along with 200 μL antibody and 200 μL FcyR blocker. Incubation time was extended to 30 minutes at 4°C. An additional wash was performed on LS selection columns. For humanization, we used samples containing CD34+ ≥90% CD34+ as determined by fluorescence-activated cell sorter (FACS) analysis.
PB-natural killer (NK) cells.
Normal adult donor apheresis product was obtained from the Children’s Hospital Medical Center Translational Trials Development Support Laboratories after proper informed consent. NK cells were isolated with the CD56+ microbead kit (Miltenyi Biotec) according to the manufacturer’s protocol. Then 6 M cells were injected into the tail vein of NSGS mice preconditioned with 250 cGy total body irradiation from a 137Cs source at a rate of 50 Rad/minute 4 hours prior. Mice received 500 ng rhIL-15 (Invitrogen) 3 times/week by intraperitoneal (IP) injection for the duration of the experiment. PB was analyzed for NK, T, and NKT frequency by FACS at 2 weeks following engraftment.
AML and B-ALL patient samples and cell lines.
Diagnostic BM and PB samples were obtained from patients at Cincinnati Children’s Hospital Medical Center. After informed consent and under the auspices of an institutional review board-approved protocol, we obtained residual specimens for use in the research presented here. Additional samples were obtained from the Stem Cell and Xenograft Core at the University of Pennsylvania's Perelman School of Medicine. The AML1001 patient sample was provided by Dr Yogen Saunthararajah (Cleveland Clinic). MA9.3RAS is an in vitro-generated human UCB-derived AML as previously described.15 This study was conducted in accordance with the Declaration of Helsinki.
Xenografts
Mice were placed on doxycycline chow prior to and immediately following conditioning or BM aspiration. Conditioning consisted of IP injection of busulfan (BU) 24 hours prior to IV injection of cells by modified methods similar to those previously published by others.16 BU powder (Sigma-Aldrich) was freshly dissolved in dimethylsulfoxide to a stock concentration of 30 mg/mL and temporarily kept at room temperature. Immediately before injection, this stock solution was diluted 10-fold in PBS. Conditioning (30 mg/kg SCID mice and 40 mg/kg RAG mice, IP) occurred 24 hours prior to IV injection of cells. Alternatively, mice received 250 cGy of total body irradiation 4 hours prior to cell injection. For secondary transplants, bones from primary xenografted mice were crushed and filtered. Then 10 to 15 million total cells were infused into BU-conditioned recipients.
Cytotoxic antibodies
OKT3, UCHT1 (BioXCell), and ATG (Thymoglobulin, Genzyme) were diluted to 1-mg/mL solutions in sterile PBS containing 2% FBS. These dilutions were stored at 4°C until use. Antibodies were injected IP (1-10 mg/kg) or incubated with cells in suspension on ice for ∼30 minutes at a concentration of 1 μL/1 million WBC (1 μg/million) just prior to IV injection.
Flow cytometry
Samples were stained in PBS/3%FBS/1 mM EDTA with Pen-Strep antibiotics (flow buffer) for 30 minutes at 4°C, followed by washing to remove unbound antibody. For OP9-DL1 co-culture experiments, a panel of CD8-FITC, CD4-PE, CD45-PE-Cy7, CD3-APC, and mCD45-APC-Cy7 was used. The general antibody panel for xenografts was hCD45-FITC, CD19-VioBlue (Miltenyi Biotec), CD13-PE, CD33-PE, CD3-PE-Cy7, and CD56-APC. CD34-APC was used to determine progenitor cells separately. Anti mCD45-APC-Cy7 (clone 30-F11) was added to allow exclusion of murine cells. For the PB-NK experiment, the antibodies were mCD45-APC-Cy7, CD45-FITC, CD56-APC, and CD3-PECy7. All antibodies were from BD, unless otherwise noted. 7-Aminoactinomycin D was added to eliminate nonviable events from analysis. Unlabeled antibodies against mouse and human FcγR (Miltenyi Biotec) were also used to prevent nonspecific labeling.
OP9-DL1 stroma co-culture assays
OP9-DL117 stroma was maintained in α minimum essential medium supplemented with 20% FBS and antibiotics. For UCB/macrophage/stroma coculture, monolayers were formed on gelatin-coated, 35-mm dishes, and media were switched to Iscove modified Dulbecco medium with 10% FBS, 1000 U/mL human IL-2, and 10 ng/mL each of human IL-7, human macrophage colony-stimulating factor (crosses to mouse cells), murine granulocyte macrophage colony-stimulating factor, and murine IL-3. Then 2.5 × 105 UCB T cells prelabeled at 4°C for 60 minutes with 1 μg of antibody/million total cells were added to each 35-mm dish followed by 1 × 106 cells isolated from pooled peritoneal cavity flushes from NSG mice. T cell yields were obtained from viable counts and FACS analysis after 4 days.
SRC frequency determination by limiting dilution
Conditioned mice received 5-fold dilutions between 5 × 106 and 8 × 103 total UBC-WBCs by IV delivery. Ten weeks after engraftment, BM aspirates were evaluated for multi-lineage engraftment. The number of CD34+ cells injected was calculated by staining an aliquot of the cell suspension with CD34-APC at the time of injection. SRC frequency was determined using the Extreme Limiting Dilution Analysis application.18
Results
OKT3 and ATG eliminate human T cells in humanized mice
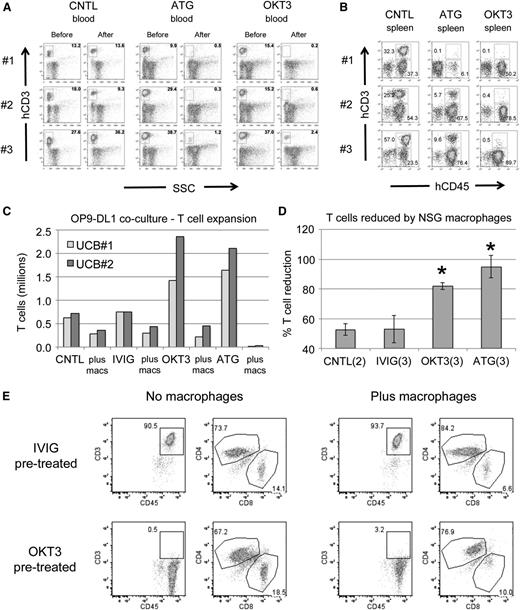
We first examined the activity of anti T-cell antibodies in the xenograft setting. Humanized mice with significant T cells present in PB were injected with either ATG or OKT3 at 10 mg/kg or with PBS. Mice that received either of the antibodies exhibited nearly complete elimination of CD45+CD3+ cells in PB (Figure 1A). The spleens of treated animals had far fewer T cells than the spleens of control mice, indicating efficient targeting of human T cells in vivo (Figure 1B). Importantly, human CD3 negative cells were readily detectable in the mice, indicating some degree of targeted specificity for T cells.
Human T cells are effectively targeted in vivo and in vitro. (A) Flow plots showing levels of human T cells in the PB of UCB-CD34+ humanized NSGS mice before and 3 days after injection of control (CNTL), ATG, or OKT3 antibodies. Three separate mice are shown for each group. SSC, side scatter. (B) The spleens of each of the mice from A were analyzed 7 days after antibody injection. Both CD45+CD3+ T cells and CD45+CD3− non-T cells are gated. (C) Antibody-labeled UCB was co-cultured for 4 days on OP9-DL1 stroma to promote T-cell expansion with or without peritoneal macrophages from NSG mice. IVIG, intravenous immunoglobulin. (D) The decrease in T cells in the presence of macrophages relative to the same antibody labeled culture without macrophages is shown. *P < .05. (E) Representative flow plots after 4 days of coculture to highlight OKT3 blockage of CD3 and the decreased CD8+ T-cell percentages after macrophage exposure.
Human T cells are effectively targeted in vivo and in vitro. (A) Flow plots showing levels of human T cells in the PB of UCB-CD34+ humanized NSGS mice before and 3 days after injection of control (CNTL), ATG, or OKT3 antibodies. Three separate mice are shown for each group. SSC, side scatter. (B) The spleens of each of the mice from A were analyzed 7 days after antibody injection. Both CD45+CD3+ T cells and CD45+CD3− non-T cells are gated. (C) Antibody-labeled UCB was co-cultured for 4 days on OP9-DL1 stroma to promote T-cell expansion with or without peritoneal macrophages from NSG mice. IVIG, intravenous immunoglobulin. (D) The decrease in T cells in the presence of macrophages relative to the same antibody labeled culture without macrophages is shown. *P < .05. (E) Representative flow plots after 4 days of coculture to highlight OKT3 blockage of CD3 and the decreased CD8+ T-cell percentages after macrophage exposure.
NSG macrophages suppress antibody-labeled T cells in vitro
NSG mice retain limited innate immune activity, primarily comprised of monocytes and neutrophils, and lack adaptive immunity and complement activity.19 Macrophages and dendritic cells are present, but efficient antigen presentation may be impaired because of defects found in the parental NOD strain.20,21 However, NSG and NOD/SCID macrophage phagocytosis has been observed to eliminate human erythrocytes22 and AML cells,23 implying some level of functionality.
To gain insight into the mechanisms of antibody-mediated depletion in immunodeficient mice, we tested effects of peritoneal macrophages obtained from NSG mice on UCB T cells growing on OP9-DL1 stroma. Prelabeling T cells with either OKT3 or ATG resulted in a robust expansion of T cells relative to control (no antibody) or intravenous immunoglobulin (irrelevant antibody) cultures (Figure 1C). The addition of NSG peritoneal macrophages greatly diminished the number of human T cells within 4 days (Figure 1C-D). Whereas ATG prelabeled cells were completely eliminated, some T cells remained in the OKT3 prelabeled cultures. However, CD3 was either blocked or internalized on these T cells (Figure 1E). Interestingly, we noted a consistent drop in the fraction of CD8+ T cells present in all macrophage cocultures. It is worth noting that CD8+ T cells are primarily responsible for xenogeneic GVHD.24 Together, these studies were proof of principle that monoclonal antibodies could effectively target human cells and display cytotoxic activity in NSG mice despite numerous immune deficiencies and gave us a strong rationale for testing of these antibodies to control or prevent GVHD in primary human sample xenografts.
In vitro preincubation with antibody is an effective means to prevent xenogeneic GVHD
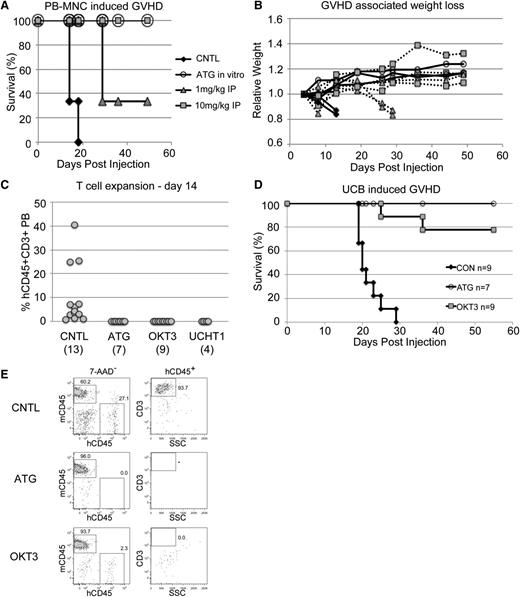
Human PB MNCs effectively induce xenogeneic GVHD when injected into NSG mice, and this model has been used in numerous studies.6-8 We took advantage of this model to test the effectiveness of early prophylactic anti T-cell therapy. We tested multiple methods of antibody exposure. For one cohort, normal donor adult PB MNCs were incubated in the syringe with 1uL of a 1-mg/mL ATG solution per million cells. Alternatively, transplanted mice were treated with a single dose of either 10 mg/kg or 1 mg/kg antibody within the first 48 hours. As expected, control mice developed a rapid GVHD characterized by significant weight loss and died within 30 days (Figure 2A-B). In contrast, mice that received the 10-mg/kg dose of ATG were fully protected from GVHD, whereas the lower 1-mg/kg dose gave partial protection. These findings fit well with clinical data that indicate the effective ATG dose in humans is between 7.5 and 15 mg/kg.25 Notably, direct in vitro incubation of cells with antibody prior to injection was completely protective. In addition to being simpler, this method requires significantly less antibody than in vivo treatment. Therefore, going forward, we limited most of our analyses to in vitro antibody treatment prior to transplant (pretreatment).
Anti-T–cell antibodies prevent GVHD. (A) Survival of sublethally irradiated NSG mice was monitored after tail vein injection of 20 million normal donor human PB MNCs/mouse. (B) Weights of the mice from A showed a characteristic decline over time. (C) PB from BU-conditioned NSGS and NRGS mice injected with 13 to 17 million antibody pretreated UCB cells showed significant human T cells only in control mouse PB 2 weeks after injection. (D) Survival of mice in C was monitored. (E) Analysis of the week 2 PB samples analyzed in C highlight the potential non-T–cell specific effects of ATG.
Anti-T–cell antibodies prevent GVHD. (A) Survival of sublethally irradiated NSG mice was monitored after tail vein injection of 20 million normal donor human PB MNCs/mouse. (B) Weights of the mice from A showed a characteristic decline over time. (C) PB from BU-conditioned NSGS and NRGS mice injected with 13 to 17 million antibody pretreated UCB cells showed significant human T cells only in control mouse PB 2 weeks after injection. (D) Survival of mice in C was monitored. (E) Analysis of the week 2 PB samples analyzed in C highlight the potential non-T–cell specific effects of ATG.
Preincubation with either ATG or OKT3 prevents UCB-induced GVHD
We subsequently performed experiments using fresh, unselected, UCB cells incubated with either ATG or OKT3. Within 2 weeks of injection, human T cells were detectable in the PB of control mice, whereas mice that received cells treated with ATG, OKT3, or UCHT1 antibody were devoid of these cells (Figure 2C). Importantly, the T cells expanded and led to a rapid GVHD in the control mice, whereas most mice that received antibody-treated samples survived (Figure 2D).
Notably, the PB of mice that received OKT3 or UCHT1 pretreated cells contained some non-T human cells, most of which stained for myeloid markers (Figure 2E and not shown). In contrast, mice that received ATG pretreated cells did not show evidence of any human cells at this time point, implying that ATG may have effects beyond the T-cell compartment using this approach.
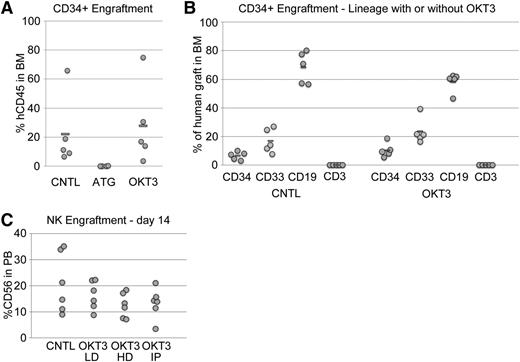
ATG but not OKT3 pretreatment interferes with SCID-repopulating ability of human CD34+ cells
ATG consists of a rabbit polyclonal antibody against human thymus extract. Therefore, it may contain activities against a wide range of antigens, many of which may be nonspecific to T cells. In contrast, OKT3 is a murine monoclonal antibody directed against human CD3, which is strictly limited to T and NKT cells. Given the data presented above suggesting some nonspecific activity of ATG, we evaluated the effects of ATG and OKT3 on the engraftment of SRCs. To this end, we transplanted a cohort of conditioned mice with purified UCB CD34+ cells. The recipients of OKT3 pretreated CD34+ cells had BM grafts very similar to mice that received control untreated CD34− cells by both quantitative and qualitative measures (Figure 3A-B). In contrast, mice that received ATG pretreated CD34+ cells had nearly undetectable human grafts, implying that ATG pretreatment interferes with human SRC engraftment. However, ATG pretreatment did not have any direct effects on the growth of CD34+ cells in liquid culture or methylcellulose assays (not shown), suggesting “off target” effects of ATG rather than a direct cytotoxic effect.
ATG negatively affects SRC activity whereas OKT3 does not. (A) Human CD45+ cell engraftment in the BM of NSG mice 8 weeks after injection of purified UCB CD34+ cells was not affected by pretreatment with OKT3 but was completely eliminated by ATG. (B) The lineage distribution of these BM grafts was also not affected by OKT3 relative to controls. (C) Normal donor human PB-NK cells engrafted NSGS mice and were present at high levels in the PB with or without OKT3 pretreatment. Cell preparations were incubated with a low (LD) or high dose (HD) of OKT3 in vitro or mice were injected (IP) with a single 10-mg/kg dose of antibody 24 hours after cell transfer.
ATG negatively affects SRC activity whereas OKT3 does not. (A) Human CD45+ cell engraftment in the BM of NSG mice 8 weeks after injection of purified UCB CD34+ cells was not affected by pretreatment with OKT3 but was completely eliminated by ATG. (B) The lineage distribution of these BM grafts was also not affected by OKT3 relative to controls. (C) Normal donor human PB-NK cells engrafted NSGS mice and were present at high levels in the PB with or without OKT3 pretreatment. Cell preparations were incubated with a low (LD) or high dose (HD) of OKT3 in vitro or mice were injected (IP) with a single 10-mg/kg dose of antibody 24 hours after cell transfer.
We also monitored engraftment of human PB NK cells, which can transiently engraft NSG mice and persist when additional cytokine support is provided.26 No significant decrease in NK engraftment was observed upon OKT3 pretreatment in vitro or in vivo (Figure 3C). Thus, in addition to myeloid, B, and CD34+ populations, NK subsets are not significantly affected by OKT3 exposure.
OKT3 pretreatment rescues leukemic SRC-engrafted mice from GVHD
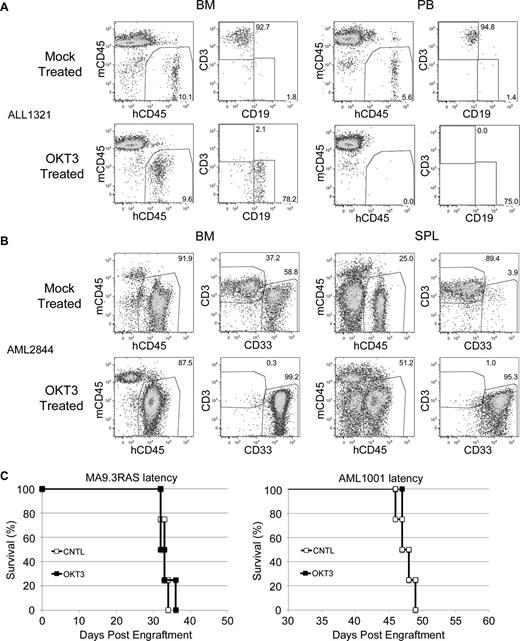
We have shown the efficacy of OKT3 pretreatment in the prevention of GVHD induced by normal donor PB or UCB. Although this treatment has no apparent negative effects on human CD34+ or NK cell engraftment, this finding may not extend to leukemia SRCs. Therefore, we obtained patient leukemia specimens and subjected these to OKT3 pretreatment. No significant engraftment differences were realized between cohorts with or without antibody exposure, demonstrating that OKT3 pretreatment did not damage the leukemic initiating cell activity (Table 1). Importantly, we did identify some leukemia patient samples with significant GVHD-inducing potential due to contaminating human T cells. OKT3 pretreatment proved to be a very effective means to eliminate T-cell engraftment from these samples (Table 2). Notably, some of these samples would have been lost due to expansion of the T cells and resultant GVHD. Instead, OKT3 pretreatment effectively rescued these samples (Figure 4A-B).
OKT3 rescues leukemia engraftment. (A) Representative FACS plots showing predominant T-cell engraftment in BM and PB of control mice and predominant leukemia engraftment in the NSG mice that received OKT3 pretreated human B-ALL patient cells. (B) A similar analysis and result is shown for the NSGS BM and spleen (SPL) of mice that received human patient AML cells. (C) Latency of AML induced in NSGS mice by the MA9.3RAS cell line (left) or the AML1001 patient sample (right) was not altered by pretreatment of cells with OKT3.
OKT3 rescues leukemia engraftment. (A) Representative FACS plots showing predominant T-cell engraftment in BM and PB of control mice and predominant leukemia engraftment in the NSG mice that received OKT3 pretreated human B-ALL patient cells. (B) A similar analysis and result is shown for the NSGS BM and spleen (SPL) of mice that received human patient AML cells. (C) Latency of AML induced in NSGS mice by the MA9.3RAS cell line (left) or the AML1001 patient sample (right) was not altered by pretreatment of cells with OKT3.
Additionally, we performed experiments to determine whether OKT3 pretreatment affected the latency of 2 independent xenograft AML models. We observed no delay in the kinetics of lethality when using either the MA9.3RAS human leukemia cell line or a primary AML patient sample (Figure 4C), again demonstrating that OKT3 pretreatment did not damage leukemic initiating cell activity. It should be noted that a subset of AML samples does aberrantly express T-cell antigens, including CD3.27 Although we did not obtain any such samples, OKT3 would likely disrupt engraftment of these samples.
OKT3 pretreatment allows long-term engraftment of unfractionated UCB
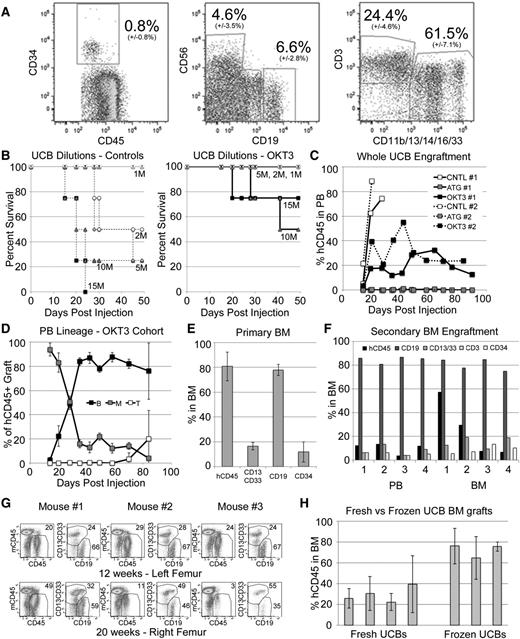
We next sought to determine if our protocol could be extended to the engraftment of UCB, which contains ∼25% T cells in addition to the 0.8% of cells expressing CD34 (Figure 5A). Limiting dilution analysis showed that untreated UCB efficiently induced GVHD while OKT3 pretreatment led to complete protection at doses <10 M total WBCs/mouse (Figure 5B). Mice receiving 5 M OKT3-treated cells were followed over time with serial bleeds and found to contain durable PB engraftment characterized by an early myeloid dominance, which was gradually replaced by a B-cell majority and an eventual appearance of T cells (Figure 5C-D). Again, ATG pretreatment resulted in loss of SRC activity and no detectable human cells in recipient mice (Figure 5C). For mice receiving OKT3-pretreated WBCs, multi-lineage BM engraftment was also observed at high levels and included a population of CD34+ cells (Figure 5E). BM from primary NSG mice engrafted for 12 to 16 weeks could be readily transplanted to secondary recipients, with robust grafts detectable after an additional 16 weeks in secondary mice, indicating excellent retention of primitive HSPC/SRCs (Figure 5F). Furthermore, primary engrafted NSG mice retained hCD45 multipotent BM grafts at 20 weeks, indicating successful long-term repopulation with this approach (Figure 5G). Importantly, we were able to generate grafts from both fresh and frozen UCB samples, with frozen samples generally leading to higher levels of human cell engraftment (Figure 5H).
OKT3 pretreatment allows establishment of long-term hematopoietic xenografts from unfractionated UCB. (A) A representative hetastarch-depleted UCB, showing average and standard deviation (14 unique UCBs) stem and progenitor (CD34+), NK (CD56+), B (CD19+), T (CD3+), and myeloid (CD11b/13/14/16/33+) cells. Units contained an average of 850 M WBCs with average recovery of 84% (±11%). (B) One to 15 million total UCB-WBCs were infused, and NSGS and NRGS mice were followed for GVHD. Samples were pretreated with OKT3 (solid lines, right) or control (dashed lines, left). (C) Mice injected with UCB cells were followed for engraftment of hCD45+ cells in the PB. Plots show average levels from 2 unique UCBs in 2 experiments (1 and 2) utilizing NSGS (1) and NRGS (2) mice. (D) Average PB lineage distribution of human grafts observed in the OKT3 cohorts. (E) The 12- to 16-week BM engraftment in NSG mice receiving OKT3 pretreated UCB. Data from 3 separate UCB samples engrafted into 2 or 3 mice each (n = 7) is shown. Populations are shown as percentages of the hCD45+ graft. (F) Secondary NSG engraftment was measured at 16 weeks in the PB and BM. Results from 4 secondary mice are shown. (G) OKT3 pretreated WUCB engraftment of 3 NSG mice was measured at 12 weeks and again at 20 weeks to determine the potential for long-term engraftment. (H) BM engraftment in mice that received 1 to 5 million WBCs from unique fresh or frozen UCB units. Each column represents the average from 3 to 9 mice at 10 to 12 weeks.
OKT3 pretreatment allows establishment of long-term hematopoietic xenografts from unfractionated UCB. (A) A representative hetastarch-depleted UCB, showing average and standard deviation (14 unique UCBs) stem and progenitor (CD34+), NK (CD56+), B (CD19+), T (CD3+), and myeloid (CD11b/13/14/16/33+) cells. Units contained an average of 850 M WBCs with average recovery of 84% (±11%). (B) One to 15 million total UCB-WBCs were infused, and NSGS and NRGS mice were followed for GVHD. Samples were pretreated with OKT3 (solid lines, right) or control (dashed lines, left). (C) Mice injected with UCB cells were followed for engraftment of hCD45+ cells in the PB. Plots show average levels from 2 unique UCBs in 2 experiments (1 and 2) utilizing NSGS (1) and NRGS (2) mice. (D) Average PB lineage distribution of human grafts observed in the OKT3 cohorts. (E) The 12- to 16-week BM engraftment in NSG mice receiving OKT3 pretreated UCB. Data from 3 separate UCB samples engrafted into 2 or 3 mice each (n = 7) is shown. Populations are shown as percentages of the hCD45+ graft. (F) Secondary NSG engraftment was measured at 16 weeks in the PB and BM. Results from 4 secondary mice are shown. (G) OKT3 pretreated WUCB engraftment of 3 NSG mice was measured at 12 weeks and again at 20 weeks to determine the potential for long-term engraftment. (H) BM engraftment in mice that received 1 to 5 million WBCs from unique fresh or frozen UCB units. Each column represents the average from 3 to 9 mice at 10 to 12 weeks.
OKT3 treated whole UCB engrafts as well as purified CD34+ cells
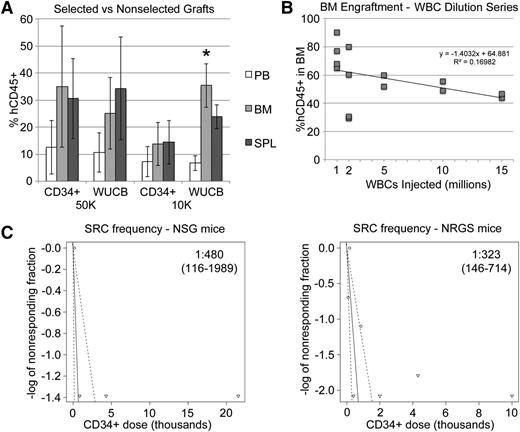
To compare our protocol with established xenograft approaches, we tested engraftment of equal numbers of CD34+ cells from the same UCB units, injected either as a whole, unfractionated OKT3-pretreated sample (WUCB) or as a magnetically purified sample (CD34+) and found nearly identical engraftment in PB, BM, and SPL of recipient animals (Figure 6A). Interestingly, at 10 000 CD34+ cells/mouse, we noticed slightly better engraftment in the WUCB cohort, particularly in the BM. A dilution series with decreasing numbers of WUCB-WBCs revealed efficient engraftment, even at very low cells doses (Figure 6B). We repeated these experiments with limiting numbers of cells to estimate the SRC frequency of OKT3-pretreated WUCB and found it to be ∼1 in 70 000 WBCs, which was in the range of 1 in 300 to 500 CD34+ cells (Figure 6C).
Unfractionated UCB engrafts to levels similar to those achieved with CD34+ cells. (A) Fifteen-week hCD45+ engraftment in BM, SPL, and PB from mice that received either UCB WBCs or UCB CD34+ cells from the same UCB unit. Flow cytometry was performed prior to engraftment to ensure that each mouse received either 10 000 or 50 000 total CD34+ cells for each approach. (B) Mice received various numbers of OKT3-pretreated UCB WBCs. The 12-week BM graft is plotted against original cell dose. (C) Limiting dilution analysis was performed to estimate the SRC frequency as measured by unfractionated UCB engraftment at 10 weeks in the BM. SRC frequency and 95% confidence intervals are shown on the plots. Plots and calculations were drawn and determined by extreme limiting dilution analysis.18
Unfractionated UCB engrafts to levels similar to those achieved with CD34+ cells. (A) Fifteen-week hCD45+ engraftment in BM, SPL, and PB from mice that received either UCB WBCs or UCB CD34+ cells from the same UCB unit. Flow cytometry was performed prior to engraftment to ensure that each mouse received either 10 000 or 50 000 total CD34+ cells for each approach. (B) Mice received various numbers of OKT3-pretreated UCB WBCs. The 12-week BM graft is plotted against original cell dose. (C) Limiting dilution analysis was performed to estimate the SRC frequency as measured by unfractionated UCB engraftment at 10 weeks in the BM. SRC frequency and 95% confidence intervals are shown on the plots. Plots and calculations were drawn and determined by extreme limiting dilution analysis.18
Discussion
Primary leukemia patient samples are a precious resource in biomedical research but are often available in only limited quantities. The utility of working with primary human samples is obvious, and the recent deluge of information obtained from proteomic and nucleic acid analyses of these samples is significant.28-33 The advances made in our understanding of the molecular mechanisms driving human disease cannot be overstated. However, the ability to use primary patient samples in functional and translational studies is frequently limited by the inability to propagate these samples in vitro and in vivo. The advent of superior strains of immunodeficient mice, primarily those based on deletion of the common γ cytokine receptor, has opened new avenues for research using human samples.1,2,34-36 These strains are highly permissive for engraftment of both solid and liquid tumors from humans as well as for engraftment of increasingly functional human immune systems. The use of a xenograft approach for translational studies relating to hematologic malignancy, solid tumor grafting, infectious diseases, and immunological studies of human hematopoiesis and cellular therapy have increased exponentially over the past few years.37,38 Nevertheless, the propensity for these highly immunodeficient strains to permit the expansion of mature differentiated T cells has led to significant difficulties with GVHD when hematologic malignancy samples are engrafted.9,10 This problem results in lost time and effort and often leads to loss of irreplaceable patient samples. As a simple method to overcome this problem, we have now shown that a short in vitro incubation with the anti-T–cell monoclonal antibody OKT3 results in complete abrogation of GVHD with no effect on SRC activity, normal human NK cell engraftment, or the ability of leukemia patient samples to expand in vivo and induce malignancy.
Traditionally, UCB xenotransplant has been performed with highly purified CD34+ cells. Not only does this add extra effort and expense, it also does not accurately reflect the clinical protocol of hematopoietic stem cell transplant. Other studies have examined the engraftment of MNC preparations of UCB cells. Although MNCs engrafted, GVHD developed unless the preparations were T-cell depleted by magnetically removing CD2 positive cells first.39 The protocol described in the current paper addresses the GVHD issue in a simple, efficient, and inexpensive way.
There is some clinical experience with regard to our in vitro opsonization protocol. A very similar approach utilizing 30-minute pretreatment with OKT3 at concentrations slightly higher than used in the present study was found to not inhibit engraftment and reconstitution in BM transplant recipients.40,41 Importantly, the percentage of OKT3-labeled cells in these preparations correlated well with the number of T cells in the sample, demonstrating the specific nature of the OKT3 monoclonal antibody.40 One of these small-scale trials additionally found grade II or worse GVHD to be reduced from 79% to 18% with OKT3 labeling.41
The engraftment we observed at more limiting cell numbers is rather striking (Figure 6A-B) and clearly demonstrates the greatly expanded possibilities using unfractionated cord blood compared with CD34-selected samples. Because only 1 million WBCs are required to generate efficient grafts, one can easily produce very large cohorts of mice at a greatly reduced cost, considering the average cord blood sample yields 850 million WBCs. The ability to work with frozen aliquots of the same WBCs in numerous experiments has the added benefit of controlling for the large variability that is often introduced when using independent cord blood samples. The efficient engraftment at limiting cell numbers is an important finding considering that GVHD was not completely avoided when higher cell doses of 10 to 15 million cells were infused (Figure 5B). These data indicate the ideal cell dose is between 1 and 5 million WBCs/mouse (∼8-40 × 103 CD34+ cells). Interestingly, this optimal cell dose is very similar to the minimum total nucleated cell dose of 40 million/kg required in a recent report of a UCB transplant clinical trial.42 In addition, our estimate of ∼1 SRC/500 CD34+ cells is similar to a recent report that also made use of the NSG strain, demonstrating the in vitro opsonization approach is not dramatically affecting the SRC frequency.43
At least one study has found that T cells play an active role in promoting the engraftment of SRCs in mice and that their removal can lead to lower engraftment rates.39 Adding back CD3/CD28 activated cells to the T-cell–depleted MNC samples restored the higher engraftment rates while lowering the risk of GVHD. These findings may indicate that the exceptional engraftment we observed could be related to the high T-cell doses that accompany these transplants. It is possible that the T cells act transiently to provide support for the engraftment of SRCs in this approach even while destined for destruction soon after engraftment.
In general, mouse strains were interchangeable for these experiments. We used cytokine mice (NSGS/NRGS) for AML experiments and NSG/NRG for B-ALL experiments. As our focus was initially on preventing GVHD in AML xenografts, many of the GVHD experiments were done using cytokine mice. However, we observed the same antibody protection in NSG mice as well (Figure 2A). We first noted normal UCB engraftment in surviving cytokine mice from these initial GVHD experiments. We therefore used cytokine mice for many of the engraftment experiments presented in Figures 5 and 6. We did repeat engraftment in several smaller NSG/NRG experiments and found similar results. These NSG mice were used for the secondary transplants, because this is the strain that most investigators would use to assay for serial transplantation.
In summary, we show that OKT3 pretreatment of both normal and malignant hematopoietic samples efficiently controls the GVHD problem often associated with these samples, with no deleterious effect on the repopulating ability of the treated cells. We believe this simple protocol will eliminate the GVHD problems associated with human xenografts in immunodeficient mice lacking the common γ receptor and will allow for more accurate modeling of stem cell transplants in xenograft recipients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christina Sexton for technical assistance, the Comprehensive Mouse and Cancer Core of the Cincinnati Children’s Hospital Research Foundation for help with irradiation of mice, the Cincinnati Children’s Hospital Medical Center Research Flow Cytometry Core for flow cytometry help, the Translational Trials and Development Support Laboratories of Cincinnati Children’s Hospital for acquisition of normal donor blood products, and the Stem Cell and Xenograft Core at the University of Pennsylvania's Perelman School of Medicine for primary patient samples.
This work was supported by an Institutional Clinical and Translational Science Award, National Institutes of Health/National Center for Research Resources grant 1UL1RR026314-01, a Center of Excellence in Molecular Hematology P30 award (DK090969 to G.D.-D.), a Center of Excellence in Molecular Hematology P30 award (DK090971 to J.C.M.), and National Cancer Institute grant CA168369 (J.C.M.). J.C.M. is a Leukemia and Lymphoma Scholar.
Authorship
M.W. designed the study, performed experiments, analyzed data, interpreted data, and wrote the manuscript; R.A.B., R.P., and G.W.R. performed experiments and analyzed data; G.D.-D. supplied essential reagents; and J.C.M. designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark Wunderlich, 3333 Burnet Ave, ML 7013, Cincinnati, OH, 45229; e-mail: mark.wunderlich@cchmc.org; and James C. Mulloy, 3333 Burnet Ave, ML 7013, Cincinnati, OH, 45229; e-mail: james.mulloy@cchmc.org.