To the editor:
The genetic determinants that govern the phenotype associated with chronic myelomonocytic leukemia (CMML) and myelodysplastic syndromes (MDS) are unknown. Many recurrent mutations occur in both diseases, suggesting that the presence or absence of a solitary mutation is insufficient to drive specific phenotypes.1-3 Early expansion of clones harboring mutations associated with both diseases has been postulated as 1 driver of CMML.4 To dissect the genetic drivers of phenotype, we clinically and molecularly characterized a rare cohort of patients who presented as de novo MDS and transformed to secondary CMML.5
Cases that were identified in the MDS and CMML databases were manually reviewed to ensure that (1) all patients were diagnosed with World Health Organization-defined CMML; (2) all patients had MDS >6 weeks from the diagnosis of CMML; and (3) all patients had a monocyte count of <1000/dL throughout the duration of MDS. Eighteen patients met criteria for secondary CMML, which accounted for 6.6% of the entire CMML cohort (n = 270), with antecedent myeloid malignancies including MDS (n = 13), MDS/myeloproliferative neoplasms–unclassified (U) (n = 4), and refractory anemia with ring sideroblasts and thrombocytosis (n = 1). The median time to CMML transformation was 873 days (range, 230-1523), suggesting that the antecedent malignancy was stable and not an evolving CMML.
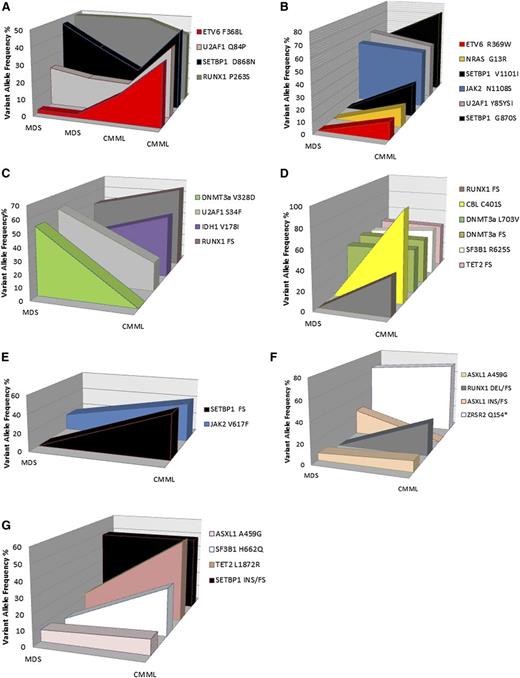
Of the 18 secondary CMML cases, 4 had DNA at the time of CMML and 7 had DNA at the time of MDS and CMML from the same sample. We sequenced our index case at 2 time points for which the patient had clinically stable MDS and 2 time points after CMML transformation. A 26-gene amplicon-based targeted next-generation sequencing panel consisting of all known exons of genes previously identified to be recurrently mutated in CMML achieved an average read depth of 652× on a Mi-Seq personal sequencer. After first pass analysis, sequencing data from sequential MDS time points 1 and 2 identified mutations in SETBP1, U2AF1, and RUNX1 at similar variant allele frequencies (VAFs), consistent with their clinical stability. Analysis of sequential CMML time points 3 and 4 identified the aforementioned mutations but also identified the acquisition of an ETV6 mutation at an increasing VAF that directly correlated with the emergence of monocytosis. Manual review of variant reads identified the ETV6 variant in the initial MDS sequential samples at frequencies of 3% and 4%, suggesting that this variant was present within a subclone at the time of MDS (Figure 1). Sequential samples in 6 other cases identified the acquisition of mutations in NRAS, CBL, and RUNX1 consistent with their role in CMML pathogenesis,6-8 as well as another case with ETV6 during first pass analysis. However, manual review of variant reads with sufficient depth identified that several variants were present at frequencies of ≤5%, suggesting that CMML transformation was associated with expansion of a preexisting MDS subclone.
Genetic variants and frequencies of secondary CMML cases with sequential samples. Cases 1 to 7 (A-G, respectively) were sequentially sequenced cases at ≥1 time point of MDS and 1 time point of CMML. Although previously frozen or fresh bone marrow mononuclear cells (BMNCs) and peripheral mononuclear cells (PBMCs) were included in this analysis, sequential samples were only analyzed if they were isolated from the same source. Genes are color coded and VAFs are annotated for each case. The gene sequenced with our targeted gene panel were as follows: ASXL, CBL, CEBPA, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MLL, NPM1, NRAS, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, WT1, ZRSR2, MPL, and ABL1. To be a true mutation, variants must have resulted in a frame shift or nonsense mutation. Missense mutations were only included if they had been previously reported in hematologic malignancies and confirmed to be somatic in the literature.
Genetic variants and frequencies of secondary CMML cases with sequential samples. Cases 1 to 7 (A-G, respectively) were sequentially sequenced cases at ≥1 time point of MDS and 1 time point of CMML. Although previously frozen or fresh bone marrow mononuclear cells (BMNCs) and peripheral mononuclear cells (PBMCs) were included in this analysis, sequential samples were only analyzed if they were isolated from the same source. Genes are color coded and VAFs are annotated for each case. The gene sequenced with our targeted gene panel were as follows: ASXL, CBL, CEBPA, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MLL, NPM1, NRAS, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, WT1, ZRSR2, MPL, and ABL1. To be a true mutation, variants must have resulted in a frame shift or nonsense mutation. Missense mutations were only included if they had been previously reported in hematologic malignancies and confirmed to be somatic in the literature.
To our knowledge, this is the first report to molecularly characterize a secondary CMML cohort. Although genome-wide sequencing would annotate all genomic changes, our deep sequencing data demonstrate that longitudinal data analyses can generate important insights into molecular architecture during malignant transformation and provide insight into the genotype/phenotype of 2 related diseases.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This Total Cancer Care study was enabled, in part, by the generous support of the DeBartolo Family, and the authors thank the many patients who so graciously provided data and tissue for this study.
Our study also received valuable assistance from the Molecular Genomics and Tissue Core Facilities at the H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute (NCI)-designated Comprehensive Cancer Center, supported under National Institutes of Health, NCI grant P30-CA76292. This work was also funded by the American Cancer Society Internal Review Grant and the Susan and John Sykes Endowed Chair in Hematologic Malignancies.
Contribution: E.P. designed research, analyzed and interpreted data, and wrote the manuscript; S.Y., S.K., T.M., and J.S.P. performed research and analyzed data; J.K.T. analyzed data; N.A.A. and R.S.K. collected clinical data; L.Z., J.L., P.K.E.-B., and E.S. assisted in research design and manuscript preparation; and J.P.M., M.A.S., and A.F.L. assisted in research design, sample acquisition, and manuscript preparation.
Correspondence: Eric Padron, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: eric.padron@moffitt.org.