Key Points
There is no difference in survival after BMT among children of different BMI.
Abstract
The rising incidence of pediatric obesity may significantly affect bone marrow transplantation (BMT) outcomes. We analyzed outcomes in 3687 children worldwide who received cyclophosphamide-based BMT regimens for leukemias between 1990 and 2007. Recipients were classified according to age-adjusted body mass index (BMI) percentiles as underweight (UW), at risk of UW (RUW), normal, overweight (OW), or obese (OB). Median age and race were similar in all groups. Sixty-one percent of OB children were from the United States/Canada. Three-year relapse-free and overall survival ranged from 48% to 52% (P = .54) and 55% to 58% (P = .81) across BMI groups. Three-year leukemia relapses were 33%, 33%, 29%, 25%, and 21% in the UW, RUW, normal, OW, and OB groups, respectively (P < .001). Corresponding cumulative incidences for transplant-related mortality (TRM) were 18%, 19%, 21%, 22%, and 28% (P < .01). Multivariate analysis demonstrated a decreased risk of relapse compared with normal BMI (relative risk [RR] = 0.73; P < .01) and a trend toward higher TRM (RR = 1.28; P = .014). BMI in children is not significantly associated with different survival after BMT for hematologic malignancies. Obese children experience less relapse posttransplant compared with children with normal BMI; however, this benefit is offset by excess in TRM.
Introduction
The growing incidence of overweight and obesity worldwide is a major health concern not only in developed countries but also in the developing world.1-3 The prevalence of a body mass index (BMI) >85 percentile has tripled among children and adolescents since 1980,2 and >30% of children are at or above the 85th percentile.4 Little is known about the pharmacokinetics of high-dose chemotherapy in overweight patients; several reviews have raised the concern about increased toxicity without dose adjustment or increased relapse risk with dose adjustments for ideal body weight (IBW).5,6 Overweight patients may have altered chemotherapy distribution, which may lead to differences in kidney and liver blood flow, with diminished clearance.5 In the setting of hematopoietic stem cell transplantation (HSCT), complications more common in overweight patients, such as hyperglycemia, have been associated with an increased risk of acute graft-versus-host disease (GVHD) and infection and a subsequent increase in transplant-related mortality (TRM).7,8
Furthermore, relatively few data are available in pediatric-specific populations. To date, there has not been a comprehensive analysis of the risks of TRM and relapse in over- and underweight pediatric patients receiving HSCT. The extrapolation of adult data to pediatric populations is further complicated by limited data on the age- and development-associated alterations in the clearance of busulfan and other chemotherapy conditioning regimens.
Several studies have evaluated the impact of weight extremes, under- or overweight, in adults receiving HSCT.6,9,10 However, few studies have addressed the impact of BMI extremes in children receiving HSCT. The aim of this study was to assess the effect of BMI on transplant outcomes in pediatric recipients of bone marrow transplants for hematologic malignancies.
Patients and methods
Data sources
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive HSCTs to a Statistical Center located at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) Coordinating Center in Minneapolis. Participating centers are required to report all transplantations consecutively; compliance is monitored by onsite audits. The CIMBTR maintains an extensive database of detailed patient-, transplant-, and disease-related information and prospectively collects data longitudinally with yearly follow-ups.11 Observational studies conducted by the CIBMTR are performed in compliance with HIPAA regulations as a public health authority and also in compliance with all applicable federal regulations pertaining to the protection of human research participants, as determined by a continuous review by the Institutional Review Boards of NMDP and the Medical College of Wisconsin. This study was conducted in accordance with the Declaration of Helsinki.
Patients
The study included patients ages 2 to 19 years who underwent allogeneic bone marrow transplant (BMT) for hematologic malignancies between 1990 and 2007 reported to the CIBMTR. Diagnoses included acute lymphoblastic leukemia (ALL) in first or second complete remission, acute myeloid leukemia (AML) in first or second complete remission, chronic myeloid leukemia, and myelodysplastic syndromes. To minimize confounding factors, the study was restricted to patients who received myeloablative conditioning with cyclophosphamide (CY) plus either total body irradiation (TBI) or busulfan-based regimens and bone marrow as the hematopoietic stem cell source. Patients undergoing second transplant, or those with DNA fragility syndromes, cord blood, or peripheral stem cell grafts, and myeloproliferative disorders were excluded. The median follow-up of the study cohort was 86 months, and the completeness index (the observed/the expected follow-up) for a 5-year analysis was >87%.Patients were divided into 5 BMI categories based on their age-adjusted BMI. BMI as a measure of obesity has been validated in children and adolescents.12,13 Age-adjusted BMIs were calculated using the 2000 Centers for Disease Control and Prevention BMI for age growth charts to obtain percentile rankings.14 The weight categories were defined as: underweight (UW): <5th percentile; at risk for UW (RUW): 5th to <25th percentile; normal: 25th to 85th percentile; overweight (OW): 86th to 95th percentile; and obese (OB): >95th percentile according to the revised childhood obesity terminology by Ogden and Flegal.15 The sample size of this cohort allowed inclusion of patients in the threshold percentiles by adding groups at risk for underweight or overweight, which could demonstrate a possible dose-effect relationship of BMI and outcome.
Study end points and variables
Primary end points were TRM, relapse, relapse-free survival (RFS), and overall survival (OS). Variables analyzed included recipients’ weight according to age-adjusted BMI as the main effect. Other variables included age, gender, performance score, race, disease risk, time from diagnosis to transplant, year of transplant, HLA match and donor type (according to Weisdorf et al16 ), marrow cell dose, cytomegalovirus serologic status, GVHD prophylaxis, and conditioning regimen.
Statistical analysis
OS and RFS were calculated using Kaplan-Meier estimates. Cumulative incidence analysis was used to describe TRM and relapse. Cox proportional hazards model was used to analyze the effect of BMI on the outcomes of interest after controlling for clinically relevant covariates. The proportional hazards assumption was assessed for each variable using a time-dependent or graphical approach. Time-dependent covariates were used when nonproportional hazards were detected. Forward stepwise regression with α = 0.05 was used to build models, with the BMI group variable forced into the model. Two-way interactions were checked between the main effect (BMI groups) and all other variables in the model.
To determine whether the effect of obesity on survival differed depending on chemotherapy dose intensity, the interaction between chemotherapy dose delivered and overweight status was examined in the Cox proportional hazards models. Conditioning regimen chemotherapy dose adjustment estimates were performed for patients with an age-adjusted BMI >85%. Based on the most common published protocols, standard dose CY was considered 120 mg/kg if TBI or other chemotherapy agents were given and 200 mg/kg if busulfan and no other agent was given. The standard dose of busulfan considered was 16 mg/kg. Total doses of CY and busulfan were divided by actual and IBW; the final doses per weight were compared, and determination of dose adjustment was performed for each case according to the regimen, chemotherapy agents used, and commonly prescribed doses. Cases were considered as not adjusted if the chemotherapy dose by actual weight fell within 10% of commonly prescribed doses for Cy or busulfan. The impact of dose adjustment on outcomes was analyzed in this subset of patients with BMI >85%.
Results
Patient characteristics
There were 3687 children who met the eligibility criteria. The clinical features of this study population are shown in Table 1. Median age ranged from 10 to 13 years. Of these, 282 (7.2%) were UW, 509 (13.8%) were at RUW, 1880 (56.8%) were normal weight, 573 (15.5%) were at OW, and 443 (12%) were OB. The distribution of age groups was similar in patients in the overweight groups. Among OW and OB patients, 56.8% were transplanted for ALL, but again, there were no significant differences in BMI distributions among the diagnoses. However, the OB group had a higher number of patients with ALL (63%), unrelated donor recipients (56%), and from transplant centers in the United States (61%). There were no significant differences in BMI distributions across donor types, conditioning, cell doses, or GVHD prophylaxis (Table 2). There were 1194 patients who received busulfan Cy-containing regimens and 2493 who received CyTBI regimens.
TRM
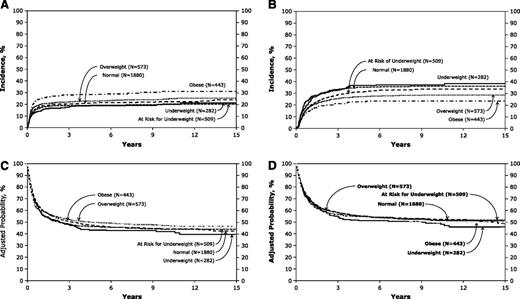
Cumulative incidences of 3-year TRM ranged from 18% (95% confidence interval [CI] 13-22) to 28% (95% CI = 24-32; P < .001) from the lower to the higher BMI groups (Figure 1A). Multivariate analysis of TRM adjusted for significant variables revealed relative risk (RR) of 1.28 (95% CI = 1.05-1.56; P = .014) in OB patients compared with children with normal weight (Table 2). However, the overall P value in the TRM model was 0.12. Other variables that predicted higher RR of TRM included age 10 to 18 years vs 2 to 9 years (P < .001), non-white race (< 0.001), time from diagnosis to transplant >12 months (P = .013), and year of BMT 1990 to 1998 (P < .001).
Posttransplant outcomes according to BMI groups. (A) Cumulative incidence of TRM by BMI groups. (B) Cumulative incidence of disease relapse by BMI groups. (C) Probability of leukemia free survival by BMI groups. (D) Probability of OS by BMI groups.
Posttransplant outcomes according to BMI groups. (A) Cumulative incidence of TRM by BMI groups. (B) Cumulative incidence of disease relapse by BMI groups. (C) Probability of leukemia free survival by BMI groups. (D) Probability of OS by BMI groups.
There were no statistically significant differences among BMI groups in 100-day mortality. Of patients in the OB group, 16% died within day 100 of nonrelapse causes, and 10% to 13% in the other BMI groups. Organ failure (interstitial pneumonia syndrome and other organ failure) accounted for 57% and 40% of early deaths (<100 days) in patients in the OW and OB groups, respectively (supplemental Table 1 available on the Blood Web site).
Relapse
Cumulative incidences of 3-year disease relapse ranged from 33% (95% CI = 28-39) to 21% (95% CI = 17-25; P < .001) from the lower to the higher BMI groups (Figure 1B). Multivariate analysis revealed lower RR of relapse for both the OW and OB groups (Table 2). Decreased relapse was also observed in patients who had female donors or sex-mismatched donors (P = .0016), time to transplant <12 months (P = .017), and well-matched unrelated donor (P < .0001).
Relapse-free and OS
There were no significant differences in either RFS or OS among the weight groups (Table 3; Figure 1C-D). Multivariate analysis for treatment failure (1-RFS) and overall mortality showed superior survival for younger age, performance score >90, white race, time to BMT <12 months, and matched sibling donor recipients.
Patients in the US and Canada
Because the largest proportion of OB children (61%) who received a BMT were from the United States and Canada, a subset analysis in this population was pursued. Table 3 outlines these results and demonstrates a significantly higher TRM (RR = 1.44, 95% CI = 1.14-1.83; P < .001) among OB children compared with children with normal BMI. Other results in this subset were comparable with the larger group.
Chemotherapy dosage adjustments
Dose information was available for 1016 OW and OB patients (supplemental Table 2). Only 13.1% of patients appeared to have dose adjustments; most patients were >10 years. Of patients with dose adjustments, 80% were >10 kg over IBW. There were no statistically significant differences between the groups with and without dose adjustments in TRM, relapse, RFS, or OS.
Discussion
The effects of obesity on allogeneic transplant outcomes in children have not been well elucidated. In this large registry-based analysis of 3687 pediatric patients, we observed that overweight children experienced decreased incidence of relapse, a trend toward higher TRM, and no significant difference in RFS and OS compared with other BMI groups. There was also no effect of underweight BMI on outcomes, and all analyses were adjusted for other covariates, such as year of transplant and HLA matching that were previously shown to be significantly associated with the risks of TRM and relapse. This finding is in contrast to studies of weight extremes in children with leukemias in which weight extremes are associated with an increased relapse risk and increased mortality.17,18
There are a few HSCT studies in children with findings that contrast ours. A single institutional retrospective study by Bulley et al19 demonstrated inferior OS in OB patients. In this study of 324 children, 17% were OW, but the study population comprised a diverse group of diagnoses, stem cell sources, and conditioning regimens. Almost one-half of the patients in the OB group had nonmalignant diseases. The OB group had inferior OS, event-free survival, and higher early TRM compared with patients with normal weight. Dose adjustments were performed in 41% of the 54 OB children. Two recent studies assessed the impact of weight extremes with allogeneic HSCT in more homogenous populations. A study of children transplanted for severe aplastic anemia by Barker et al20 found that obese patients had a higher probability of grades III-IV acute GVHD (RR = 1.55) compared with normal-weight patients. In addition, TRM at day 100 was higher in OB patients: 29% compared with 12% for normal-weight patients, and OB patients had worse OS, with an increase in TRM. This study included various intensities of multiple conditioning regimens and donor sources, and dose adjustments for OB were not analyzed. The prevalence of children receiving unrelated cord blood transplant who had age-adjusted BMI >85% was 37% in one series.19 There were no differences in any of the outcomes measured, including time to engraftment, TRM, DFS, and OS when the OW or OB patients were compared with normal-weight patients. The conditioning regimens were uniform and included either busulfan or TBI, and chemotherapy dose adjustments were consistently done for patients >125% IBW.
Obesity is now considered an independent prognostic factor for nonrelapse mortality in an adult HSCT-specific comorbidity index, with a hazard ratio of 1.9.21 In adults, obesity is associated with other important comorbidity, including heart disease and diabetes. We investigated the number of reported comorbidities in this study, and 13% of cases had reported at least one comorbidity, which were most commonly asthma, pulmonary conditions other than asthma, and seizures. The presence of the reported comorbidities in this pediatric population was not correlated with any BMI group. The American Society of Clinical Oncology recently published guidelines for chemotherapy dosing for obese adult patients.22 These recommendations note that there is no evidence that short- or long-term toxicities are increased with nonadjusted dosing and recommend full weight-based chemotherapy dosing. However, studies of the effects of obesity on outcomes in adults have been limited by diverse conditioning regimens, diseases, and stem cell sources; the majority of studies comprise autologous transplants.10,23-25 Another CIBMTR study by Navarro et al26 compared outcomes by BMI for adult patients with AML who received various stem cell sources, including autologous, matched related donor, and unrelated donor bone marrow or peripheral blood. OW and OB patients did have a lower risk of relapse in multivariate analysis compared with the UW group, but similar to our study, this did not result in improved OS. RFS was worse in UW patients who received related donor transplants. There are fewer adult studies of the effect of BMI upon outcomes of allogeneic HSCT. Deeg et al27 noted that adults >95% IBW had TRM and day 150 survival comparable with normal-weight patients. Survival among patients 85% to 95% and <85% IBW was significantly worse. Considering the degree of variability across studies, there is an impact of obesity on posttransplant outcomes in adults and children with malignant and nonmalignant diseases.
The effect of obesity on pharmacokinetics of drug metabolism has not been well studied in children. In adults, drug absorption is rarely affected by obesity, but drug distribution may vary with differences in kidney and liver blood flow with diminished clearance.5 Busulfan is the drug most studied in the BMT setting, which is not surprising given the routine use of pharmacokinetics for dosing. A retrospective study of oral busulfan in children by Dupuis et al28 noted no significant effect of obesity on busulfan dose requirements. Browning et al29 recently reported a retrospective study of pharmacokinetics in children who received an IV busulfan test dose in a reduced-intensity conditioning regimen using actual weight to determine dose. Pharmacokinetic data from both the test dose and regular dose were available for 19 patients with BMI >85%. Children with high BMI had higher area under the curves when dosed on actual body weight compared with children with normal or low BMI and needed less drug to reach goal area under the curve. Although data on the clinical use of busulfan pharmacokinetics in patients in this study are not available, the absence of these data likely have a modest effect on the study conclusions, because multivariable modeling demonstrated no association between study end points and conditioning regimen. For this reason, the analyses were not restricted to TBI-containing regimens.
Dose adjustments and possible effects on TRM and relapse have not been evaluated in most studies. The decision to adjust chemotherapy dosing is inconsistent, even within single institutions,30 and the CIBMTR forms that comprise our data do not include this information. More recent pediatric transplant protocols are consistent in the approach to dose adjustment. Children’s Oncology Group protocols for leukemia transplants now mandate chemotherapy adjustments for children with ABW >125% IBW, and the multi-institutional COBLT study used chemotherapy dosing based on 40% adjusted IBW.20 In addition to chemotherapy dosing, cell dose may be an important factor in outcome. Surprisingly, there was no significant difference in cell doses among the BMI groups. Low cell doses may increase TRM,31 and marrow cell doses >3.6 × 108/kg may accelerate engraftment, decrease severe acute GVHD, and improve leukemia-free survival.32
Our data demonstrate that age is significantly associated with risk of TRM and relapse. These data are consistent with the increased risk of TRM and relapse in older adolescent patients with acute leukemia. In addition, the risks of TRM and relapse in the older adolescent population appear similar to those reported in a recent comparison between adolescents and young adults and older adults transplanted for AML. However, given the heterogeneity of diagnoses and conditioning regimens in our cohort, additional analyses will be required to fully evaluate the impact of increasing age on the risk of TRM and relapse.
Subset analysis on children receiving transplant in the United States or Canada was performed to further analyze the impact of obesity, as most OB children were reported from centers in these countries. When enriching the population with a greater number of OB children, the relationship between BMI and TRM became clear. The multivariate analysis of TRM including all patients was not significant, even though the RR was higher for the OB group. Among children in the United States and Canada, TRM was significantly associated with BMI, mainly related to significantly higher TRM in OB children compared to children in the normal weight range. This subset analysis is important, because the approach to calculate age-adjusted BMI is derived from U.S. population norms and determination of weight categories can vary according to norms in a particular country or region.
This study is the largest to date of the effect of BMI on transplant outcomes in children. The large sample size, uniform stem cell source, and restriction of subjects to hematologic malignancies and myeloablative conditioning lend credence to the results. Our study demonstrates that children who are overweight who receive allogeneic BMT for hematologic malignancies do not have worse RFS or OS than normal- or underweight children; the decrease in disease relapsed is offset by an increase in TRM. This may comprise a dose-response effect requiring further study. There are obvious limitations to the study, with heterogenous approaches to GVHD prophylaxis and other supportive care as well as insufficient data regarding intended chemotherapy doses and actual administered doses. Additional information regarding the effects of dose adjustment will prove useful for designing conditioning regimens for these patients and ensuring better outcomes. Prospective multi-institutional studies with uniform approaches to dose adjustment will be necessary to fully evaluate the impact of BMI on transplant outcomes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Institutes of Health National Cancer Institute, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases; Grant/Cooperative Agreement 5U10HL069294 from National Heart, Lung and Blood Institute and National Cancer Institute; a contract HHSH250201200016C with Health Resources and Services Administration; 2 grants (N00014-12-1-0142 and N00014-13-1-0039) from the Office of Naval Research; and grants from Actinium Pharmaceuticals, Allos Therapeutics, Inc., Amgen, Inc., nnonymous donation to the Medical College of Wisconsin, Ariad, Be the Match Foundation, Blue Cross and Blue Shield Association, Celgene Corporation, Fred Hutchinson Cancer Research Center, Fresenius-Biotech North America, Inc., Gamida Cell Teva Joint Venture Ltd., Genentech, Inc.,Gentium SpA, Genzyme Corporation, GlaxoSmithKline, Health Research, Inc. Roswell Park Cancer Institute, HistoGenetics, Inc., Incyte Corporation, Jeff Gordon Children’s Foundation, Kiadis Pharma, The Leukemia & Lymphoma Society, Medac GmbH, The Medical College of Wisconsin, Merck & Co, Inc., Millennium: The Takeda Oncology Co., Milliman USA, Inc., Miltenyi Biotec, Inc., NMDP, Onyx Pharmaceuticals, Optum Healthcare Solutions, Inc., Osiris Therapeutics, Inc., Otsuka America Pharmaceutical, Inc., Perkin Elmer, Inc., Remedy Informatics, Sanofi US, Seattle Genetics, Sigma-Tau Pharmaceuticals, Soligenix, Inc., St. Baldrick’s Foundation, StemCyte, A Global Cord Blood Therapeutics Co., Stemsoft Software, Inc., Swedish Orphan Biovitrum, Tarix Pharmaceuticals, TerumoBCT, Teva Neuroscience, Inc., THERAKOS, Inc., University of Minnesota, University of Utah, and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the U.S. Government.
Authorship
Contribution: R.A., L.S., M.C.P., and N.B. designed the study and interpreted the results; M-J.Z., X.Z., and M.C.P. analyzed data and performed statistical analysis; R.A., N.B. and M.C.P. wrote the paper; M.P., V.T.H., K.C., C.D., G.H., L.M.I., H.M.L., P.L.M., R.O., and M.C.P. reviewed and critiqued the study design and manuscript and contributed revisions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcelo C. Pasquini, 9200 West Wisconsin Ave, CCC5500, Milwaukee, WI 53226; e-mail: mpasquini@mcw.edu.