Key Points
Disease relapse is a common cause of failure of allogeneic hematopoietic stem cell transplantation in patients with advanced MDS.
High IPSS-R prognostic risk category and monosomal karyotype are independent predictors of relapse after allogeneic transplantation in MDS.
Abstract
Approximately one-third of patients with myelodysplastic syndrome (MDS) receiving allogeneic hematopoietic stem cell transplantation (HSCT) are cured by this treatment. Treatment failure may be due to transplant complications or relapse. To identify predictive factors for transplantation outcome, we studied 519 patients with MDS or oligoblastic acute myeloid leukemia (AML, <30% marrow blasts) who received an allogeneic HSCT and were reported to the Gruppo Italiano Trapianto di Midollo Osseo registry between 2000 and 2011. Univariate and multivariate survival analyses were performed using Cox proportional hazards regression. High-risk category, as defined by the revised International Prognostic Scoring System (IPSS-R), and monosomal karyotype were independently associated with relapse and lower overall survival after transplantation. On the other hand, older recipient age and high hematopoietic cell transplantation-comorbidity index (HCT-CI) were independent predictors of nonrelapse mortality. Accounting for various combinations of patient’s age, IPSS-R category, monosomal karyotype, and HCT-CI, the 5-year probability of survival after allogeneic HSCT ranged from 0% to 94%. This study indicates that IPSS-R risk category and monosomal karyotype are important factors predicting transplantation failure both in MDS and oligoblastic AML. In addition, it reinforces the concept that allogeneic HSCT offers optimal eradication of myelodysplastic hematopoiesis when the procedure is performed before MDS patients progress to advanced disease stages.
Introduction
Myelodysplastic syndromes (MDSs) are myeloid neoplasms that range from conditions with a near-normal life expectancy to forms close to acute myeloid leukemia (AML).1 An increasing body of evidence indicates that their clinical heterogeneity reflects different somatic mutations that cause clonal proliferation and evolution of myelodysplastic cells.2-6 In particular, founding mutations, typically those of RNA splicing machinery or DNA methylation, appear to dictate future trajectories of disease evolution with distinct clinical phenotypes.1,7
The fact that MDSs have a highly variable clinical course makes risk stratification of crucial importance in clinical decision-making.8 To predict the outcome of the individual patient with MDS, in particular to define the risk of progression to AML, several prognostic scoring systems have been developed.9-11 In 2012, an international collaborative group has created the revised International Prognostic Scoring System (IPSS-R) for MDS, which is predominantly based on a new comprehensive cytogenetic scoring system (MDS cytogenetic scoring system)12 and defines 5 risk groups with different clinical outcomes.13
The only potentially curative treatment of MDS patients remains allogeneic hematopoietic stem cell transplantation (HSCT), which is considered a conventional therapeutic option until the age of 65 years in eligible patients.8 The introduction of reduced-intensity conditioning (RIC) regimens has resulted in a significant reduction in transplant-related mortality.14 Despite these recent advances, long-term survival rate is currently about 30% in MDS patients.15-17 A number of studies have shown that advanced risk stage at transplantation is associated with inferior overall survival,16-18 and we have recently demonstrated that allogeneic HSCT offers optimal survival benefit when it is performed early in intermediate risk stages.19 Cytogenetic abnormalities have been found to be highly predictive of outcome after allogeneic HSCT in MDS patients.20,21 In addition, in older patients receiving allogeneic HSCT, age-related factors, such as the presence of extra-hematological comorbidity, significantly affect the risk of nonrelapse mortality.22,23
In the present work, we analyzed disease- and patient-related predictive factors for the outcome of allogeneic HSCT in a cohort of 519 patients with primary MDS or oligoblastic AML.
Methods
Patients and study design
This study was approved by the institutional Ethics Committee (Comitato di Bioetica, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico [IRCCS] Policlinico San Matteo, Pavia, Italy). The procedures followed were in accordance with the Declaration of Helsinki of 1975, as revised in 2000.
We studied 519 patients undergoing allogeneic HSCT for primary MDS or oligoblastic AML between 2000 and 2011 and reported to the Gruppo Italiano Trapianto di Midollo Osseo registry. All clinical variables were analyzed at the time of transplant in patients receiving allogeneic HSCT upfront and at the time of remission-induction chemotherapy in those receiving treatment before transplantation.
Diagnosis of MDS and AML were made or revised according to the World Health Organization criteria,24 and the following MDS categories were considered: refractory cytopenia with unilineage dysplasia (RCUD), refractory anemia with ringed sideroblasts (RARS), refractory cytopenia with multilineage dysplasia (RCMD), refractory anemia with excess blasts-1 (RAEB-1), refractory anemia with excess blasts-2 (RAEB-2), and myelodysplastic syndrome associated with isolated del(5q) (MDS del[5q]). Patients with 20% to 29% bone marrow blasts, previously diagnosed with RAEB in transformation (RAEB-t) according to the French-American-British classification,25 were reclassified as oligoblastic AML (in Ancient Greek, oligos means few). This decision takes into account the fact that in the 2008 World Health Organization classification, a myeloid neoplasm with 20% or more blasts was considered to be AML.24
Cytogenetics was evaluated on bone marrow cells using standard banding techniques. The results of cytogenetic analysis collected from each site using the International System for Cytogenetic Nomenclature were centrally reviewed by an expert geneticist (P.B.) Cytogenetic abnormalities were initially categorized according to the IPSS criteria9 and then reclassified according to the new MDS cytogenetic scoring system.12 Monosomal karyotype (MK) was defined as the presence of ≥2 distinct autosomal chromosome monosomies or 1 single autosomal monosomy in combination with structural abnormalities.26
End points and statistical analysis
The primary end points were overall survival (OS), nonrelapse mortality (NRM), and probability of relapse. OS was defined as the time between transplantation and death (from any cause) or last follow-up (for censored observations). When estimating NRM, any death in the absence of disease relapse was considered an event. The probability of relapse was estimated considering treatment as a failure at the time of hematologic relapse according to standardized criteria.27 Hematological relapse was defined by recurrence of blasts in the peripheral blood or infiltration of the bone marrow by >5% blasts. Molecular relapse was defined by recurrence of cytogenetic abnormalities in hematopoietic cells after achieving complete remission and/or by falling complete donor chimerism (a cutoff of 20% recipient cells was used).
The cumulative probability of OS was estimated using the Kaplan-Meier product limit method. The cumulative incidence of relapse and NRM was estimated with a competing risks approach.28 Univariate and multivariate survival analyses were performed using Cox proportional hazards regression. To compare different statistical models, we used the Akaike information criterion (AIC).29 Among a set of candidate models, a lower AIC value indicates a better trade-off between fit and complexity. A difference between 0 and 2 in AIC values does not indicate a substantial difference of fitness between the models tested. By contrast, a difference ≥3 indicates a substantial difference favoring the model with the lowest AIC value.
Analyses were performed using Stata 11.2 SE (StataCorp, College Station, TX) software.
Results
Posttransplant outcome according to cytogenetic risk
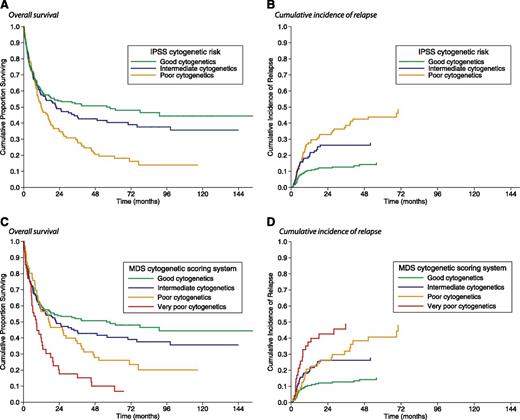
An abnormal karyotype was found in 273 of 519 patients (53%). According to the IPSS categorization,9 karyotype was good in 268 patients (52%), intermediate in 135 (26%), and poor in 116 (22%). In these subgroups, the 5-year OS was 49%, 40%, and 19%, respectively (P = .001; Figure 1A), whereas the 5-year cumulative incidence of relapse was 14%, 27%, and 44%, respectively (P = .001; Figure 1B).
Kaplan-Meier analysis of survival and cumulative incidence of relapse following allogeneic HSCT in MDS patients stratified according to their pre-transplant IPSS cytogenetic risk or the new MDS cytogenetic scoring system used for the IPSS-R. (A) There was a significant difference in posttransplant OS between patients with poor IPSS cytogenetics and those with good (P = .002) or intermediate IPSS cytogenetics (P = .03), whereas no significant difference was found between good and intermediate IPSS cytogenetics (P = .31). (B) Patients with poor IPSS cytogenetics also showed a significantly higher probability of relapse than those with good (P < .001) and intermediate IPSS cytogenetics (P = .02). (C) There was a significant difference in posttransplant OS between patients with good and those with poor (P = .03) or very poor IPSS-R cytogenetics (P = .002), whereas no significant difference was seen between good and intermediate IPSS-R cytogenetics (P = .10). Patients with intermediate IPSS-R cytogenetics had an increased OS compared with those with poor or very poor IPSS-R cytogenetics (P = .02). (D) The cumulative incidence of relapse was lower in patients with good IPSS-R cytogenetics than in those with intermediate, poor, or very poor IPSS-R cytogenetics (P = .006, P = .001, and P < .001, respectively). Patients with intermediate IPSS-R cytogenetics showed a significantly lower incidence of relapse than those with poor or very poor IPSS-R cytogenetics (P = .04).
Kaplan-Meier analysis of survival and cumulative incidence of relapse following allogeneic HSCT in MDS patients stratified according to their pre-transplant IPSS cytogenetic risk or the new MDS cytogenetic scoring system used for the IPSS-R. (A) There was a significant difference in posttransplant OS between patients with poor IPSS cytogenetics and those with good (P = .002) or intermediate IPSS cytogenetics (P = .03), whereas no significant difference was found between good and intermediate IPSS cytogenetics (P = .31). (B) Patients with poor IPSS cytogenetics also showed a significantly higher probability of relapse than those with good (P < .001) and intermediate IPSS cytogenetics (P = .02). (C) There was a significant difference in posttransplant OS between patients with good and those with poor (P = .03) or very poor IPSS-R cytogenetics (P = .002), whereas no significant difference was seen between good and intermediate IPSS-R cytogenetics (P = .10). Patients with intermediate IPSS-R cytogenetics had an increased OS compared with those with poor or very poor IPSS-R cytogenetics (P = .02). (D) The cumulative incidence of relapse was lower in patients with good IPSS-R cytogenetics than in those with intermediate, poor, or very poor IPSS-R cytogenetics (P = .006, P = .001, and P < .001, respectively). Patients with intermediate IPSS-R cytogenetics showed a significantly lower incidence of relapse than those with poor or very poor IPSS-R cytogenetics (P = .04).
Cytogenetic abnormalities were then reclassified according to the new MDS cytogenetic scoring system used for the IPSS-R.12 As shown in supplemental Table 1 on the Blood Web site, 6 (1%) patients had very good, 277 (53%) had good, 127 (25%) had intermediate, 56 (11%) had poor, and 53 (10%) had very poor cytogenetics. Due to their low number, patients with very good cytogenetics were excluded from the analysis. The 5-year OS was 48% in the good, 37% in the intermediate, 28% in the poor, and 15% in the very poor cytogenetic subgroup (P = .003; Figure 1C). By competing risk analysis, the 5-year cumulative incidence of relapse was 16%, 30%, 43%, and 41%, respectively, in the above subgroups (P = .001; Figure 1D), whereas the 5-year cumulative incidence of NRM was 35%, 31%, 27%, and 35%, respectively (P = .65). With respect to the IPSS cytogenetic categorization, by applying the MDS cytogenetic scoring system, the risk group changed in 96 (18%) patients: in 33 (6%) cases into a more favorable and in 63 (12%) into a less favorable prognostic group.
MK was found in 76 (15%) patients. The single most prevalent monosomy was −7, which was observed in 49 of 76 patients (64%). Most patients with MK also had a complex karyotype (68 patients, 89%) according to IPSS criteria (detailed clinical characteristics and transplant procedures in patients with MK are reported in supplemental Results). As shown in Figure 2, the 5-year OS of MK patients was only 10%, which is significantly worse than that of patients without MK (P < .001), whereas the 5-year cumulative incidence of relapse was 49%, which was significantly greater than that of patients without MK (P < .001). MK maintained a significant effect on OS and probability of relapse in patients stratified according to either IPSS cytogenetic categorization or MDS cytogenetic scoring system (OS, P < .001 and P = .001, respectively; probability of relapse, P < .001 and P < .001, respectively). Focusing on patients with complex karyotype, subjects with MK showed a significantly reduced OS (P = .009) and increased probability of disease relapse after allogeneic HSCT (P = .006) than those without MK.
Posttransplant outcome in MDS patients stratisfied according to the absence or presence of monosomal karyotype. (A) OS; (B) cumulative incidence of relapse. The presence of monosomal karyotype significantly affected both OS (P < .001) and probablility of relapse (P < .001).
Posttransplant outcome in MDS patients stratisfied according to the absence or presence of monosomal karyotype. (A) OS; (B) cumulative incidence of relapse. The presence of monosomal karyotype significantly affected both OS (P < .001) and probablility of relapse (P < .001).
We studied the prognostic effect of the MDS cytogenetic scoring system on the posttransplant outcome in a Cox multivariate analysis, considering as covariates age and gender of recipient, absolute neutrophil count (ANC), hemoglobin level, platelet count, percentage of bone marrow blasts, disease stage at transplantation (complete remission vs active/progressive disease), comorbidity (defined according to the HCT-CI), source of hematopoietic stem cells (peripheral blood vs bone marrow), type of donor (HLA-identical sibling vs matched unrelated donor [MUD]), and type of conditioning (standard conditioning vs RIC).
We first focused on MDS patients classified according to World Health Organization criteria. According to this model, the MDS cytogenetic scoring system significantly affected OS (hazard ratio [HR], 1.29; P = .001) and probability of relapse (HR, 1.40; P < .001). The MDS cytogenetic scoring system maintained a significant effect on both OS and probability of relapse when including patients with oligoblastic AML and when stratifying patients according to type of conditioning (P value ranging from .011 to <.001).
We then fitted multivariable models to investigate the prognostic value of MK on posttransplant outcome of MDS patients. MK significantly affected OS (HR, 2.37; P < .001) and probability of relapse (HR, 4.36; P < .001). The prognostic effect of MK on post-transplant outcome was maintained even when including oligoblastic AML patients and when stratifying patients according to type of conditioning (P value ranging from .008 to <.001).
Next we compared different multivariate Cox survival analyses to determine which definition of unfavorable cytogenetics (IPSS poor karyotype vs IPSS-R very poor cytogenetic subgroup vs MK) was the most efficient on in capturing patients with poor prognosis after transplantation. The AIC for the models tested was 970, 971, and 966, respectively, indicating the importance of accounting for MK in the prognostic model.
Posttransplant outcome according to the degree of peripheral blood cytopenia and the percentage of marrow blasts
As a first step, we analyzed the prognostic effect of hemoglobin level, ANC, and platelet count categorized according to IPSS-R criteria.13
Ninety-nine patients (19%) had a hemoglobin level >10 g/dL, 197 (38%) had values between 8 and 10 g/dL, and 223 (43%) had levels <8 g/dL. Hemoglobin level and transfusion dependency were significantly associated (Kendall τ-b rank correlation coefficient = 0.79). The 5-year OS was 51% in patients with a level >10 g/dL, 40% in those with values between 8 and 10 g/dL, and 33% in patients with a hemoglobin level <8 g/dL (P = .001). The 5-year cumulative incidence of NRM was 29%, 37%, and 44%, respectively (P = .003). We then evaluated the impact of secondary iron overload—as assessed by serum ferritin levels—on the outcome of transfusion-dependent MDS patients undergoing allogeneic HSCT. Data on pretransplantation serum ferritin were available in 285 cases. In patients receiving standard conditioning regimen and stratified according to hemoglobin level, a serum ferritin level >1000 ng/mL showed a significant effect on survival (HR, 1.89; P = .001) and risk of NRM (HR, 2.01; P < .001).
With respect to platelet count, the 5-year OS was 47% in patients with a platelet count >100 × 109/L, 38% in those with values between 50 and 100 × 109/L, and 33% in those with a platelet count <50 × 109/L (P = .001). Patients with a platelet count <50 × 109/L showed a higher incidence of relapse and NRM compared with those with a platelet count >50 × 109/L (P = .012 and .03, respectively). No significant difference on posttransplant outcome was found between patients with ANC < 0.8 × 109/L and those with ANC ≥ 0.8 × 109/L.
We then analyzed the effect on posttransplant outcome of bone marrow blasts. Patients with >10% marrow blasts had lower OS and higher probability of relapse with respect to patients with <5% and those with between 5% and 10% marrow blasts (OS, P < .001 and P = .036, respectively; cumulative incidence of relapse, P < .001 and P = .021, respectively).
As a final step, we fitted a Cox multivariate model to analyze the prognostic effect of peripheral blood cytopenia and percentage of bone marrow blasts categorized according to IPSS-R criteria, considering as covariates age and gender of recipient, cytogenetics, disease stage at transplantation, comorbidity, source of hematopoietic stem cells, type of donor, and type of conditioning. Focusing on MDS patients diagnosed according to World Health Organization criteria, hemoglobin level showed a significant effect on both OS (HR, 1.37; P = .002) and NRM (HR, 1.41; P = .009), whereas ANC and platelet count did not show an independent effect on posttransplant outcome. The percentage of marrow blasts (≤10% vs >10%) showed a significant effect on OS (HR, 1.42; P = .023) and probability of relapse (HR, 1.66; P = .014).
Posttransplant outcome of patients stratified according to IPSS-R
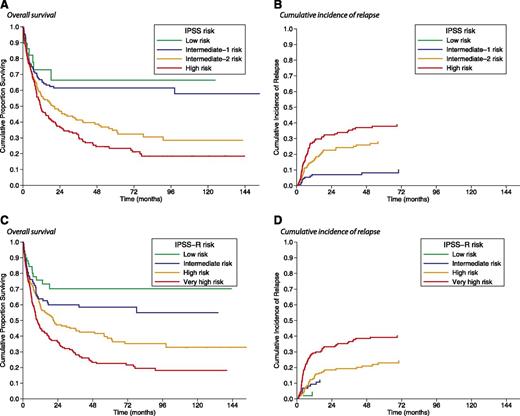
We first classified patients according to the IPSS score9 : 29 (6%) patients had low, 134 (26%) had intermediate-1, 177 (34%) had intermediate-2, and 179 (34%) had high risk. In these risk groups, the 5-year OS was 66%, 61%, 33%, and 24%, respectively (P < .001; Figure 3A). By competing risk analysis, the 5-year cumulative incidence of relapse was not evaluable, 9%, 28%, and 38%, respectively (P < .001; Figure 3B).
Kaplan-Meier analysis of survival and cumulative incidence of relapse following allogeneic HSCT in MDS patients stratified according to their pretransplant IPSS or IPSS-R risk. (A) No significant difference in posttransplant OS was observed between low and intermediate-1 IPSS risk (P = .61) and between intermediate-2 and high IPSS risk (P = .16). By contrast, there was a significant difference in posttransplant OS between low or intermediate-1 and intermediate-2 or high IPSS risk (P < .001). (B) Patients with low or intermediate-1 IPSS risk also showed a lower probability of relapse than those with intermediate-2 or high IPSS risk (P < . 001). (C) No significant difference in posttransplantation OS was observed between low and intermediate IPSS-R risk (P = . 17). There was a significant difference in post-transplant OS between low or intermediate IPSS-R risk and high or very high IPSS-R risk (P = .01 and P < .001, respectively) and also between high and very high IPSS-R risk (P < .001). (D) Patients with low or intermediate IPSS-R risk also showed a lower probability of relapse than those with high or very high IPSS-R risk (P = .01 and P < .001, respectively), whereas patients with very high risk showed a higher probability of relapse than those with high risk (P < .001).
Kaplan-Meier analysis of survival and cumulative incidence of relapse following allogeneic HSCT in MDS patients stratified according to their pretransplant IPSS or IPSS-R risk. (A) No significant difference in posttransplant OS was observed between low and intermediate-1 IPSS risk (P = .61) and between intermediate-2 and high IPSS risk (P = .16). By contrast, there was a significant difference in posttransplant OS between low or intermediate-1 and intermediate-2 or high IPSS risk (P < .001). (B) Patients with low or intermediate-1 IPSS risk also showed a lower probability of relapse than those with intermediate-2 or high IPSS risk (P < . 001). (C) No significant difference in posttransplantation OS was observed between low and intermediate IPSS-R risk (P = . 17). There was a significant difference in post-transplant OS between low or intermediate IPSS-R risk and high or very high IPSS-R risk (P = .01 and P < .001, respectively) and also between high and very high IPSS-R risk (P < .001). (D) Patients with low or intermediate IPSS-R risk also showed a lower probability of relapse than those with high or very high IPSS-R risk (P = .01 and P < .001, respectively), whereas patients with very high risk showed a higher probability of relapse than those with high risk (P < .001).
Patients were then reclassified according to IPSS-R criteria13 : 59 (11%) patients had low, 89 (17%) patients had intermediate, 207 (40%) had high, and 164 (32%) had very high risk (supplemental Table 2). As illustrated in Figure 3C, the 5-year OS was 71% in low, 58% in intermediate, 39% in high, and 23% in very high risk patients (P < .001). In these risk groups, by competing risk analysis, the 5-year cumulative incidence of relapse was 4%, 12%, 23%, and 39%, respectively (P < .001; Figure 3D). IPSS-R significantly stratified posttransplantation OS in patients with low/intermediate-1 IPSS (P = . 03), as well as in patients with intermediate-2 or high IPSS (P = .005; supplemental Figure 1). With respect to the IPSS-based stratification, the IPSS-R risk group changed in 338 (65%) cases: 33 (6%) patients were assigned to a more favorable prognostic group and 305 (59%) to a less favorable prognostic group.
We also analyzed the prognostic effect of the IPSS-R score by a multivariate Cox regression model (Table 3). We first focused on MDS patients classified according to World Health Organization criteria. The IPSS-R score significantly affected OS (HR, 1.41; P < .001) and probability of relapse (HR, 1.81; P < .001). The prognostic effect of IPSS-R on posttransplant outcome was also maintained when including oligoblastic AML patients and when stratifying patients according to type of conditioning (P from .006 to <.001).
Recipient age (≥50 years) and high HCT-CI were significant risk factors for NRM (P = .02 and P = .017, respectively), whereas MK and lack of complete remission after induction chemotherapy showed an independent effect on relapse (P = .001 and P = .001, respectively; Table 3). Probability of survival and cumulative incidence of relapse and NRM are reported in Table 4 and supplemental Table 3, respectively. Accounting for various combinations of patient’s age, IPSS-R category, monosomal karyotype, refractoriness to chemotherapy, and HCT-CI, the 5-year probability of survival after allogeneic HSCT ranged from 0% to 94%.
Year of transplant (2006-2011 vs 2000-2005) showed a significant effect on TRM (HR, 0.50; P = .01), whereas the probability of relapse was not significantly affected. With respect to donor-recipient HLA match, patients receiving transplant from identical sibling or from HLA-MUD showed a significantly better OS (P < .001) and a significantly decreased TRM (P < .001) than those transplanted from a HLA-mismatched donor. Patients receiving transplant from HLA-matched sibling or unrelated donor presented comparable OS.
Finally, to verify whether IPSS-R could improve the IPSS prognostic stratification of MDS who underwent allogeneic HSCT, we fitted 2 separate multivariable Cox analyses including IPSS and IPSS-R as covariates, respectively, and compared them. The AIC for the models tested were 3254 and 3249, respectively, indicating that IPSS-R is more likely to capture prognostic information in this patient setting.
Patient-based and disease status–based risk stratification of posttransplant outcome
Finally, we explored the possibility of developing a predictive model of posttransplant outcome in MDS patients. We included patient-related and disease-related factors that were previously found to have a significant effect on survival after transplantation (Table 3). Each factor was assigned a score proportional to the regression coefficient obtained from the multivariable Cox proportional hazards model. Accordingly, a score value of 1 was assigned for each of the following risk factors: recipient age ≥50 years, monosomal karyotype, high HCT-CI, and refractoriness to induction chemotherapy. In addition, a score value of 1 was assigned to patients with intermediate IPSS-R, a score of 2 to those with high IPSS-R risk, and a score of 3 to those with very high IPSS-R risk.
A transplantation risk index (TRI) was calculated as the sum of these weighted scores, and TRI was then categorized into 4 risk groups: low (score equal to 0 or 1), intermediate (score equal to 2 or 3), high (score equal to 4), and very high (score > 4). The 5-year OS was 76% in patients with low, 48% in patients with intermediate, 18% in patients with high, and 5% in patients with very high transplantation index (P < .001; Figure 4). This realization of a predictive model should be considered just as a proof of concept of its feasibility.
Patient-based and disease status–based risk stratification of outcome among MDS patients receiving allogeneic HSCT. (A) Calculation of the MDS TRI. (B) Posttransplant survival in patients stratified according to their TRI.
Patient-based and disease status–based risk stratification of outcome among MDS patients receiving allogeneic HSCT. (A) Calculation of the MDS TRI. (B) Posttransplant survival in patients stratified according to their TRI.
Discussion
In this study, we analyzed predictive factors for the outcome of allogeneic HSCT in patients with MDS or oligoblastic AML and found that IPSS-R and MK were independent predictors in these patients. IPSS-R is based on the same highly reproducible variables included in IPSS (ie, peripheral blood cytopenias, marrow blast percentage, and cytogenetics) split into different categories according to clinically relevant criteria.13 The prognostic impact of IPSS-R has already been shown in untreated MDS patients, where it identifies 5 risk groups compared with the 4 major prognostic categories of the IPSS.13 We found that IPSS-R significantly stratified overall survival in patients with MDS or oligoblastic AML receiving an allogeneic HSCT and that it provided a better prediction of posttransplant outcome compared with IPSS. Age of recipient, comorbidity, MK, and response to induction chemotherapy were significant additive prognostic factors in MDS patients stratified into IPSS-R categories.
At present, the clinical basis for selection of patients who are candidates for allogeneic HSCT is provided by IPSS.8,30 Several studies showed that transplantation early after the diagnosis is associated with the most favorable outcome.15-17 However, it remains unclear whether such a strategy may lead to the maximal life expectancy for all patient categories. In fact, patients diagnosed in an early stage (typically those with low and intermediate-1 IPSS risk) may experience a long period with stable disease after diagnosis, and the risks of immediate morbidity and mortality related to transplantation may be unacceptably high. In this context, a large clinical heterogeneity in low and intermediate-1 IPSS categories has been observed,10 and IPSS does not efficiently stratify posttransplant outcome in early disease stages.16
In MDS patients treated with allogeneic HSCT, IPSS-R significantly improved the prediction of patient prognosis with respect to IPSS. Compared with the IPSS-based stratification, the IPSS-R risk group changed in ∼65% of patients, and the great majority of these individuals were classified into a less favorable prognostic category. Thus, the clinical implementation of IPSS-R might result in a more effective selection of candidates to allogeneic HSCT among patients with low-risk disease.19,31
A major contribution to the prediction of posttransplant outcome was provided by cytogenetics, ie, the only biological parameter that is currently included in the diagnostic and prognostic assessment of MDS patients.13,24 Cytogenetics was previously found to be an independent predictor of the probability of relapse after transplantation.20 The recently developed MDS cytogenetic scoring system showed a significant effect on overall survival and on probability of relapse of patients with MDS or oligoblastic AML receiving an allogeneic HSCT.21,32
The presence of MK, which was not considered as a separate category by the MDS cytogenetic scoring system,33 has emerged as a further risk factor for worse prognosis in myeloid neoplasms and was associated with a poor outcome after transplantation.26,32,34 MK frequently involves chromosome 5 and 7 abnormalities and also TP53 gene mutations that are significant risk factors for disease relapse after allogeneic HSCT.35 In our study, MK identified a group of patients with dismal outcome after allogeneic HSCT, with an ∼10% 5-year overall survival. MK was found to be more efficient in capturing patients with poorer posttransplant prognosis with respect to the unfavorable karyotypes as defined according to either IPSS or IPSS-R criteria. Overall, these data indicate the importance of accounting for MK in predicting survival after transplantation in MDS.
Among the other components of IPSS-R, we found that the definition of severe anemia as a hemoglobin level <8 g/dL was significantly associated with the presence of transfusion dependency and was more useful in capturing the posttransplant prognosis in MDS patients with respect to anemia as defined according to IPSS criteria (ie, hemoglobin <10 g/dL). As previously reported,36 the negative effect of anemia on posttransplant outcome appeared to be related at least in part to transfusional iron overload as assessed by serum ferritin level. It should be underlined, however, that serum ferritin alone may be inadequate to properly assess body iron status in these patient.36,37
In the last few years, the introduction of RIC regimens has resulted in a significant reduction of transplant-related toxicity and mortality, leading to a rapidly growing number of transplantations in elderly patients with hematologic diseases.30,38 Interestingly, IPSS-R maintained its prognostic effects in patients receiving RIC. In this population, we observed that patient-related factors, such as age itself and the presence of extra-hematological comorbidity, significantly affect the risk of NRM and should therefore be part of transplantation decision-making.8 The analyses reported in Figure 4 illustrate how different patient-related and disease-related factors can be used to predict the posttransplantation outcome. Although we realize that the predictive impact of the TRI we developed needs to be validated, the difference in overall survival shown in Figure 4B is impressive and might at least help in discussing the potential outcome of transplantation with the individual patients.
There are potential sources of bias in our analysis, which are inherent to the retrospective nature of a study based on a national transplant registry. Factors to consider include patient selection, missing data in a proportion of patients, long period of recruitment, and different types of transplant and pretransplant treatment. Although we are aware that a wide validation of these results is needed, we are confident that our findings can be useful to the clinical management of MDS patients. Recent advances in our understanding of the genetic basis of MDS will hopefully improve our ability of predicting the outcome of transplantation in MDS patients.1 However, the findings of our study reinforce the concept that allogeneic HSCT offers optimal eradication of myelodysplastic hematopoiesis when the procedure is performed before MDS patients progress to advanced disease stages.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Fondazione Berlucchi, Brescia; Fondazione Veronesi, Milan; Fondazione Cariplo, Milan; and Regione Lombardia, Milan, Italy (M.G.D.P.); by Fondazione IRCCS Policlinico S. Matteo (E.P.A.); and by Associazione Italiana per la Ricerca sul Cancro, Milan, and Fondazione Cariplo (M.C.).
The members and institutions of the Gruppo Italiano Trapianto di Midollo Osseo who contributed to the trial appear in the online data supplement.
Authorship
Contribution: M.G.D.P., E.P.A., M.C., and A.R. conceived this study; A.B., M.T.v.L., L.M., M.F., M.B., F.O., S.G., A.P.I., R.C., P.M., P.P., E.A., R.O., F.R., P.B., and A.B. collected data; C.P., M.G.D.P., E.P.A., and M.C. analyzed the data; and M.G.D.P. and M.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matteo G. Della Porta, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: matteogiovanni.dellaporta@unipv.it; or Mario Cazzola, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: mario.cazzola@unipv.it.
References
Author notes
M.G.D.P. and E.P.A. contributed equally to this work.
M.C. and A.R. contributed equally to this work.