Abstract
The International Prognostic Scoring Sytem (IPSS) is an important standard for ssessing prognosis of primary untreated adult patients with myelodysplastic syndromes (MDS). To refine the IPSS, MDS patient databases from international institutions were coalesced to assemble a much larger combined database (Revised-IPSS [IPSS-R], n = 7012, IPSS, n = 816) for analysis. Multiple statistically weighted clinical features were used to generate a prognostic categorization model. Bone marrow cytogenetics, marrow blast percentage, and cytopenias remained the basis of the new system. Novel components of the current analysis included: 5 rather than 3 cytogenetic prognostic subgroups with specific and new classifications of a number of less common cytogenetic subsets, splitting the low marrow blast percentage value, and depth of cytopenias. This model defined 5 rather than the 4 major prognostic categories that are present in the IPSS. Patient age, performance status, serum ferritin, and lactate dehydrogenase were significant additive features for survival but not for acute myeloid leukemia transformation. This system comprehensively integrated the numerous known clinical features into a method analyzing MDS patient prognosis more precisely than the initial IPSS. As such, this IPSS-R should prove beneficial for predicting the clinical outcomes of untreated MDS patients and aiding design and analysis of clinical trials in this disease.
Introduction
The myelodysplastic syndromes (MDS) consist of a heterogeneous spectrum of myeloid clonal hemopathies. The International Prognostic Scoring System (IPSS) has been an important standard for assessing prognosis of primary untreated adult MDS patients.1 However, since its publication in 1997, modification of existing parameters and additional prognostic systems have been suggested as providing meaningful differences for patients' clinical outcomes,2-5 and the World Health Organization (WHO) added morphologic refinement of the French-American-British (FAB) classification.6,7 In addition, the WHO Prognostic Scoring System (WPSS)2,3 has provided new insights into prognostic variables, adding red blood cell (RBC) transfusion dependence along with IPSS cytogenetic classification and WHO dysplastic categories. Importantly, recent newer cytogenetic groupings are reported to be prognostically valuable and to refine those features used in the IPSS.8 Additional variables suggested as providing prognostic information in MDS included serum lactate dehydrogenase (LDH),9-11 ferritin,12 and β2-microglobulin13,14 as well as marrow fibrosis15-17 and patient comorbidities and performance status.5,18-20
To examine the prognostic impact of these new clinical and cytogenetic variables and attempt to refine the IPSS, coordination of investigators and coalescence of MDS databases from multiple international institutions provided a much larger combined database of patients by the International Working Group for Prognosis in MDS (IWG-PM) project.
The aims of this study were to refine the IPSS by reassessing the prior major predictive features, determining the impact of the newer clinical features for prognostic power, incorporating larger and more differentiated cytogenetic subgroups, and reevaluating their prognostic impact. Statistically weighted clinical features were used to generate a prognostic categorization model. This larger combined database permitted better analyses of the specific impact of marrow blast percentage, depth of cytopenias, and of the less frequent features, particularly further evaluating the relatively rare cytogenetic subgroups. In addition, as some features had only been reported from single centers, this combined database extended such findings.
Methods
Under the aegis of the MDS Foundation, MDS databases of primary untreated MDS patients from multiple international institutions from 11 countries, including data from the Spanish, French, Piemonte (Italy) and Brazilian MDS Registries and that from the International MDS Risk Analysis Workshop (IMRAW), were submitted and evaluated by the IWG-PM project. Databases came from both university- and nonuniversity-based hospitals associated with the country's MDS-focused groups. Institutional review board approval was obtained from the respective institutions. After careful vetting for accuracy, a combined IWG-PM database of 7012 patients, classified morphologically by FAB (n = 7000) and, in most cases, by the WHO criteria (n = 5504),6 was created. Inclusion criteria were: primary MDS patients whose disease had not been treated with disease-altering therapy during their MDS phase (ie, no hypomethylating agents, intensive chemotherapy, or hematopoietic stem cell transplantation). Marrow blasts were required to be ≤ 30%, peripheral blood blasts ≤ 19%, white blood count (WBC) ≤ 12 × 109/L, and absolute neutrophil count (ANC) ≤ 8 × 109/L). The patient's blood counts needed to demonstrate ≥ 2 months of stable disease. Marrow blasts and cytogenetic results, hemoglobin, ANC, and platelet levels at diagnosis were documented, and data regarding patient's survival and development of acute myeloid leukemia (AML) were obtained. The patient ages were ≥ 16 years. Data regarding use of erythropoiesis-stimulating agents or myeloid growth factors were not systematically collected.
The results of cytogenetic analysis of bone marrow were reviewed by the Cytogenetics Committee (D.H., Chair; J.S., C.F., M.M.L.B., F.S., and M.L.S.) using standard ISCN criteria.21 Specific karyotypic abnormalities and their risk categories were used as per Schanz et al, which required ≥ 10 patients for inclusion as a specific abnormality. Parameters evaluated were cytogenetic risk category,8 marrow aspirate blast percent, depth of cytopenias, degree of marrow fibrosis (0-1+ vs 2-3+), Eastern Cooperative Oncology Group performance status, serum LDH (normal values defined by each hospital), ferritin and β2-microglobulin levels, RBC transfusion dependence, and patient age at diagnosis. The database of untreated primary MDS patients from the Medical University of Vienna was used as an external independent validation cohort.
Statistical methods
Modeling of prognostic risk was based on multivariate analysis of survival time and time to AML transformation. Functional relations of bone marrow blasts and cytopenias with prognostic risk were analyzed to define appropriate categories for score calculation.22 Robust Cox models23 for survival, time to transformation, and combination of both were built to derive the relative weights within the score. To compensate for possible heterogeneities, analyses were stratified by data source, year of diagnosis, and age. This led to generation of reference scores for these clinical outcomes.
The score was developed following a hierarchical approach. The main score was built based on the initially elaborated categories for bone marrow blasts and cytopenias together with the cytogenetic risk categories.8 The categorizations of cytopenias were adjusted to clinically relevant cutpoints. To calculate a specific feature's added score, the proportional weight of the score was used. A “combined” score model was effectively used rather than having 2 separate models for survival and AML transformation. Separate specific score variants for survival and AML transformation were considered, but they provided very little gain. Therefore, for ease of communication and implementation, 1 unified model was preferred. This model approximated (statistically) both outcomes adequately, particularly for survival when age was included. Risk-scoring values were rounded to the nearest 0.5 unit with re-estimation of all statistics for the rounded scores. To ease interpretation, boundaries of the 5 risk categories of the final score were chosen to build a scale with approximately equal risk increments between 2 adjacent levels.
The effect of age was modeled as an optional additive feature for overall survival prediction by including age in a model with the already defined main scores. Additional potentially differentiating features were analyzed to estimate their incremental prognostic values, given the already defined main score.
As a measure of prognostic power, the Dxy coefficient for censored data24 was used. Dxy is a concordance coefficient varying between −1 and 1, with 0 representing no predictive power and 1 perfect concordance of ascribed risk and survival and time to transformation, respectively. For a potentially additive feature to be considered clinically significant, both a P < .05 and a gain in prognostic power (Dxy) were required. Dxy's were internally validated by bootstrapping the related Cox models.24 Two-sided P values < .05 were reported as significant. Correlations between ordered categorical variables were measured by Kendall tau. In line with the nature of the project, no adjustment for multiple testing was applied. All analyses were performed using the open source software R Version 2.14.1.25,26 Kaplan-Meier curves were used to demonstrate clinical outcomes.
Results
Patient characteristics
Data from 7012 patients from multiple institutional databases in the combined IWG-PM database were evaluated. Their median age was 71 years, 77% were > 60 years, the male/female ratio 1.5:1, and median follow-up time 3.9 years. The 7012 patients obtained for evaluation were classified by FAB (n = 7000, 99.8%)7 and additionally by WHO (n = 5504, 78.5%)6 and/or WPSS (n = 2325, 33.2%).9 Table 1 shows the individual clinical variables and outcomes (survival, AML evolution) for our patient cohort, with Dxy concordance coefficients (indicating prognostic power) and univariate P values. Bootstrap-validated Dxy values were almost identical with the sample results given in Table 1 (all differences were at most .01, except the value for β2-microglobulin).
Clinical variables of MDS patients
| . | No. of patients . | % of patients . | Survival years, median . | Dxy (95% CI; P*) . | No. of patients . | % of patients . | AML/25% y . | Dxy (95% CI; P*) . |
|---|---|---|---|---|---|---|---|---|
| Cytogenetics | 7012 | 100 | .25 | 6485 | 100 | .27 | ||
| Very good | 255 | 4 | 5.4 | (.23-.26) | 255 | 3 | NR | (.24-.31) |
| Good | 5069 | 72 | 4.8 | 4657 | 72 | 9.4 | ||
| Intermediate | 947 | 13 | 2.7 | 875 | 14 | 2.5 | ||
| Poor | 283 | 4 | 1.5 | 276 | 4 | 1.7 | ||
| Very poor | 458 | 7 | 0.7 | 452 | 7 | 0.7 | ||
| BM blasts | 7012 | 100 | .30 | 6485 | 100 | .47 | ||
| 0-2% | 3279 | 47 | 5.9 | (.28-.32) | 3004 | 46 | NR | (.44-.50) |
| > 2- < 5% | 1266 | 18 | 4.2 | 1172 | 18 | 8.5 | ||
| 5-10% | 1377 | 19 | 2.3 | 1263 | 20 | 2.2 | ||
| > 10%-30% | 1090 | 16 | 1.3 | 1046 | 16 | 1.0 | ||
| > 10%-20% | (901) | (13) | (1.3) | (860) | (13) | (0.93) | ||
| > 20%-30% | (189) | (3) | (1.4) | (186) | (3) | (1.0) | ||
| Hemoglobin, g/dL | 7012 | 100 | .21 | 6485 | 100 | .16 | ||
| ≥ 10 | 3377 | 48 | 5.5 | (.19-.23) | 3109 | 48 | 9.5 | (.12-.19) |
| 8-< 10 | 2464 | 35 | 2.9 | 2286 | 35 | 5.5 | ||
| < 8 | 1171 | 17 | 2.0 | 1090 | 17 | 2.4 | ||
| Platelets† | 7012 | 100 | .23 | 6485 | 100 | .17 | ||
| ≥ 100 | 4195 | 60 | 5.1 | (.21-.25) | 3823 | 59 | 8.7 | (.14-.21) |
| 50- < 100 | 1469 | 21 | 2.8 | 1368 | 21 | 3.1 | ||
| < 50 | 1348 | 19 | 1.6 | 1294 | 20 | 3.1 | ||
| ANC† | 7012 | 100 | .11 | 6485 | 100 | .16 | ||
| ≥ 0.8 | 5758 | 82 | 4.4 | (.10-.13) | 5303 | 82 | 9.2 | (.13-.19) |
| < 0.8 | 1254 | 18 | 1.9 | 1182 | 18 | 1.9 | ||
| IPSS-R | 7012 | 100 | .43 | 6485 | 100 | .52 | ||
| Very low | 1313 | 19 | 8.8 | (.42-.45) | 1212 | 19 | NR | (.49-.55) |
| Low | 2646 | 38 | 5.3 | 2395 | 37 | 10.8 | ||
| Intermediate | 1433 | 20 | 3.0 | 1310 | 20 | 3.2 | ||
| High | 898 | 13 | 1.6 | 857 | 13 | 1.4 | ||
| Very high | 722 | 10 | 0.8 | 711 | 11 | 0.7 | ||
| Sex | 7012 | 100 | .07 | 6485 | 100 | .04 | ||
| Male | 4243 | 61 | 3.3 | (.05-.09) | 3962 | 61 | 5.8 | (.00-.07) |
| Female | 2769 | 39 | 4.8 | 2523 | 39 | 8.0 | (.030) | |
| Age | 7012 | 100 | .05 | 6485 | −.02 | |||
| ≤ 60 y | 1582 | 23 | 5.7 | (.03-.06) | 1489 | 23 | 8.1 | (−.05-.01) |
| > 60 y | 5430 | 77 | 3.5 | 4996 | 77 | 6.1 | (.082) | |
| ECOG Performance Status | 2496 | 36 | .16 | 2489 | 38 | .09 | ||
| 0 | 751 | 30 | 4.3 | (.13-.18) | 748 | 30 | 8.8 | (.04-.15) |
| 1 | 1477 | 59 | 2.2 | 1473 | 59 | 6.3 | (.005) | |
| 2-4 | 268 | 11 | 1.6 | 268 | 11 | 3.5 | ||
| Serum ferritin | 3049 | 43 | .16 | 2747 | 42 | .11 | ||
| ≤ 350 ng/mL | 1602 | 53 | 6.3 | (.13-.20) | 1435 | 52 | NR | (.05-.17) |
| > 350 ng/mL | 1447 | 47 | 4.2 | 1312 | 48 | 14.5 | (.004) | |
| Serum LDH | 4257 | 61 | .12 | 4130 | 64 | .12 | ||
| Normal | 3103 | 73 | 4.1 | (.10-.14) | 3007 | 73 | 9.2 | (.08-.16) |
| High | 1154 | 27 | 2.1 | 1123 | 27 | 3.2 | ||
| Serum β2-microglobulin | 1005 | 14 | .14 (.10-.18) | 1005 | 15 | .02 (−.08-.11) | ||
| ≤ 2 g/mL | 263 | 26 | 3.8 | 263 | 26 | 6.7 | (.498) | |
| > 2 g/mL | 742 | 74 | 1.7 | 742 | 74 | 4.6 | ||
| Marrow fibrosis | 1323 | 19 | .04 | 1183 | 18 | .05 | ||
| No | 1158 | 88 | 5.2 | (.01-.07) | 1055 | 89 | 14.5 | (−.01-.12) |
| Yes | 165 | 12 | 3.2 | (.004) | 128 | 11 | 4.8 | (.069) |
| RBC transfusion dependence | 2933 | 42 | .26 | 2645 | 41 | .27 | ||
| No | 2003 | 68 | 6.9 | (.23-.29) | 1808 | 68 | 14.5 | (.22-.32) |
| Yes | 930 | 32 | 2.3 | 837 | 32 | 2.1 | ||
| IPSS | 7008 | 100 | .37 | 6481 | 100 | .48 | ||
| Low | 2625 | 37 | 7.0 | (.35-.39) | 2394 | 37 | NR | (.45-.51) |
| Intermediate-1 | 2778 | 40 | 3.6 | 2541 | 39 | 6.1 | ||
| Intermediate-2 | 1126 | 16 | 1.5 | 1074 | 17 | 1.2 | ||
| High | 479 | 7 | 0.9 | 472 | 7 | 0.7 |
| . | No. of patients . | % of patients . | Survival years, median . | Dxy (95% CI; P*) . | No. of patients . | % of patients . | AML/25% y . | Dxy (95% CI; P*) . |
|---|---|---|---|---|---|---|---|---|
| Cytogenetics | 7012 | 100 | .25 | 6485 | 100 | .27 | ||
| Very good | 255 | 4 | 5.4 | (.23-.26) | 255 | 3 | NR | (.24-.31) |
| Good | 5069 | 72 | 4.8 | 4657 | 72 | 9.4 | ||
| Intermediate | 947 | 13 | 2.7 | 875 | 14 | 2.5 | ||
| Poor | 283 | 4 | 1.5 | 276 | 4 | 1.7 | ||
| Very poor | 458 | 7 | 0.7 | 452 | 7 | 0.7 | ||
| BM blasts | 7012 | 100 | .30 | 6485 | 100 | .47 | ||
| 0-2% | 3279 | 47 | 5.9 | (.28-.32) | 3004 | 46 | NR | (.44-.50) |
| > 2- < 5% | 1266 | 18 | 4.2 | 1172 | 18 | 8.5 | ||
| 5-10% | 1377 | 19 | 2.3 | 1263 | 20 | 2.2 | ||
| > 10%-30% | 1090 | 16 | 1.3 | 1046 | 16 | 1.0 | ||
| > 10%-20% | (901) | (13) | (1.3) | (860) | (13) | (0.93) | ||
| > 20%-30% | (189) | (3) | (1.4) | (186) | (3) | (1.0) | ||
| Hemoglobin, g/dL | 7012 | 100 | .21 | 6485 | 100 | .16 | ||
| ≥ 10 | 3377 | 48 | 5.5 | (.19-.23) | 3109 | 48 | 9.5 | (.12-.19) |
| 8-< 10 | 2464 | 35 | 2.9 | 2286 | 35 | 5.5 | ||
| < 8 | 1171 | 17 | 2.0 | 1090 | 17 | 2.4 | ||
| Platelets† | 7012 | 100 | .23 | 6485 | 100 | .17 | ||
| ≥ 100 | 4195 | 60 | 5.1 | (.21-.25) | 3823 | 59 | 8.7 | (.14-.21) |
| 50- < 100 | 1469 | 21 | 2.8 | 1368 | 21 | 3.1 | ||
| < 50 | 1348 | 19 | 1.6 | 1294 | 20 | 3.1 | ||
| ANC† | 7012 | 100 | .11 | 6485 | 100 | .16 | ||
| ≥ 0.8 | 5758 | 82 | 4.4 | (.10-.13) | 5303 | 82 | 9.2 | (.13-.19) |
| < 0.8 | 1254 | 18 | 1.9 | 1182 | 18 | 1.9 | ||
| IPSS-R | 7012 | 100 | .43 | 6485 | 100 | .52 | ||
| Very low | 1313 | 19 | 8.8 | (.42-.45) | 1212 | 19 | NR | (.49-.55) |
| Low | 2646 | 38 | 5.3 | 2395 | 37 | 10.8 | ||
| Intermediate | 1433 | 20 | 3.0 | 1310 | 20 | 3.2 | ||
| High | 898 | 13 | 1.6 | 857 | 13 | 1.4 | ||
| Very high | 722 | 10 | 0.8 | 711 | 11 | 0.7 | ||
| Sex | 7012 | 100 | .07 | 6485 | 100 | .04 | ||
| Male | 4243 | 61 | 3.3 | (.05-.09) | 3962 | 61 | 5.8 | (.00-.07) |
| Female | 2769 | 39 | 4.8 | 2523 | 39 | 8.0 | (.030) | |
| Age | 7012 | 100 | .05 | 6485 | −.02 | |||
| ≤ 60 y | 1582 | 23 | 5.7 | (.03-.06) | 1489 | 23 | 8.1 | (−.05-.01) |
| > 60 y | 5430 | 77 | 3.5 | 4996 | 77 | 6.1 | (.082) | |
| ECOG Performance Status | 2496 | 36 | .16 | 2489 | 38 | .09 | ||
| 0 | 751 | 30 | 4.3 | (.13-.18) | 748 | 30 | 8.8 | (.04-.15) |
| 1 | 1477 | 59 | 2.2 | 1473 | 59 | 6.3 | (.005) | |
| 2-4 | 268 | 11 | 1.6 | 268 | 11 | 3.5 | ||
| Serum ferritin | 3049 | 43 | .16 | 2747 | 42 | .11 | ||
| ≤ 350 ng/mL | 1602 | 53 | 6.3 | (.13-.20) | 1435 | 52 | NR | (.05-.17) |
| > 350 ng/mL | 1447 | 47 | 4.2 | 1312 | 48 | 14.5 | (.004) | |
| Serum LDH | 4257 | 61 | .12 | 4130 | 64 | .12 | ||
| Normal | 3103 | 73 | 4.1 | (.10-.14) | 3007 | 73 | 9.2 | (.08-.16) |
| High | 1154 | 27 | 2.1 | 1123 | 27 | 3.2 | ||
| Serum β2-microglobulin | 1005 | 14 | .14 (.10-.18) | 1005 | 15 | .02 (−.08-.11) | ||
| ≤ 2 g/mL | 263 | 26 | 3.8 | 263 | 26 | 6.7 | (.498) | |
| > 2 g/mL | 742 | 74 | 1.7 | 742 | 74 | 4.6 | ||
| Marrow fibrosis | 1323 | 19 | .04 | 1183 | 18 | .05 | ||
| No | 1158 | 88 | 5.2 | (.01-.07) | 1055 | 89 | 14.5 | (−.01-.12) |
| Yes | 165 | 12 | 3.2 | (.004) | 128 | 11 | 4.8 | (.069) |
| RBC transfusion dependence | 2933 | 42 | .26 | 2645 | 41 | .27 | ||
| No | 2003 | 68 | 6.9 | (.23-.29) | 1808 | 68 | 14.5 | (.22-.32) |
| Yes | 930 | 32 | 2.3 | 837 | 32 | 2.1 | ||
| IPSS | 7008 | 100 | .37 | 6481 | 100 | .48 | ||
| Low | 2625 | 37 | 7.0 | (.35-.39) | 2394 | 37 | NR | (.45-.51) |
| Intermediate-1 | 2778 | 40 | 3.6 | 2541 | 39 | 6.1 | ||
| Intermediate-2 | 1126 | 16 | 1.5 | 1074 | 17 | 1.2 | ||
| High | 479 | 7 | 0.9 | 472 | 7 | 0.7 |
AML/25% indicates time for 25% of patients to develop AML.
All univariate P values not explicitly stated are P < .001.
× 109/L.
Identification of significant prognostic variables
As in the IPSS, marrow cytogenetic subset, marrow blast percentage, and cytopenias were considered as the basis of this new prognostic system (the Revised IPSS [IPSS-R]) given their statistical weight compared with the other variables analyzed herein using Cox proportional hazard regression analyses, with overall survival and AML transformation as outcomes. Multivariate analysis of these variables led to their relative statistical weighting, determining their impact on prognostic risk, using Cox proportional hazard regression. In descending order, these 5 major variables for evaluating clinical outcomes were: cytogenetic risk groups, marrow blast percentage, and depth of cytopenias (hemoglobin, platelet, and ANC levels, respectively). The novel components obtained in the current analysis included: 5 rather than 3 cytogenetic prognostic subgroups with specific classification of a number of less common cytogenetic subsets and alteration of others (Table 2)8 ; the < 5% marrow blast category was split between 0%-2% and > 2-< 5%, whereas all patients with > 10% blasts were grouped in the same category; depth of cytopenias at clinically and statistically relevant cutpoints rather than merely the number of these abnormalities; and modification of the ANC cutpoint to 0.8 × 109/L from 1.8 × 109/L in the IPSS.
MDS Cytogenetic Scoring System
| Prognostic subgroups, % of patients . | Cytogenetic abnormalities . | Median survival,* y . | Median AML evolution, 25%,* y . | Hazard ratios OS/AML* . | Hazard ratios OS/AML† . |
|---|---|---|---|---|---|
| Very good (4%*/3%†) | −Y, del(11q) | 5.4 | NR | 0.7/0.4 | 0.5/0.5 |
| Good (72%*/66%†) | Normal, del(5q), del(12p), del(20q), double including del(5q) | 4.8 | 9.4 | 1/1 | 1/1 |
| Intermediate (13%*/19%†) | del(7q), +8, +19, i(17q), any other single or double independent clones | 2.7 | 2.5 | 1.5/1.8 | 1.6/2.2 |
| Poor (4%*/5%†) | −7, inv(3)/t(3q)/del(3q), double including −7/del(7q), complex: 3 abnormalities | 1.5 | 1.7 | 2.3/2.3 | 2.6/3.4 |
| Very poor (7%*/7%†) | Complex: > 3 abnormalities | 0.7 | 0.7 | 3.8/3.6 | 4.2/4.9 |
| Prognostic subgroups, % of patients . | Cytogenetic abnormalities . | Median survival,* y . | Median AML evolution, 25%,* y . | Hazard ratios OS/AML* . | Hazard ratios OS/AML† . |
|---|---|---|---|---|---|
| Very good (4%*/3%†) | −Y, del(11q) | 5.4 | NR | 0.7/0.4 | 0.5/0.5 |
| Good (72%*/66%†) | Normal, del(5q), del(12p), del(20q), double including del(5q) | 4.8 | 9.4 | 1/1 | 1/1 |
| Intermediate (13%*/19%†) | del(7q), +8, +19, i(17q), any other single or double independent clones | 2.7 | 2.5 | 1.5/1.8 | 1.6/2.2 |
| Poor (4%*/5%†) | −7, inv(3)/t(3q)/del(3q), double including −7/del(7q), complex: 3 abnormalities | 1.5 | 1.7 | 2.3/2.3 | 2.6/3.4 |
| Very poor (7%*/7%†) | Complex: > 3 abnormalities | 0.7 | 0.7 | 3.8/3.6 | 4.2/4.9 |
OS indicates overall survival; and NR, not reached.
Data from patients in this IWG-PM database, multivariate analysis (n = 7012).
Data from Schanz et al8 (n = 2754).
Regarding the cytogenetic classification, good correlation was demonstrated regarding the proportional hazard ratios for clinical outcomes (survival and AML evolution) of the subgroups from the recently developed cytogenetic system8 on which we based our analysis and from our data (Table 2). Because of the higher number of patients analyzed in our database, more cytogenetic subtypes were analyzable for prognosis in our study than had been possible for the IPSS (15 vs 6). A double independent review of the cytogenetic data was performed by the IWG-PM Cytogenetic Committee. Differences between this categorization and that of the IPSS included the finding of complex karyotypes with > 3 abnormalities being distinct from those with 3 abnormalities and with poorer prognosis; chromosome 7 abnormalities were similarly prognostically separable from the Very poor category (when observed in karyotypes with ≤ 3 abnormalities; supplemental Figures 1 and 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The specific cytogenetic abnormalities that now were able to be placed into distinct prognostic subgroups included inv(3)/t3q)/del(3q), del(11q), del(12p), i(17q), +19, double anomalies including del(5q), double abnormalities including del(7q) or monosomy 7, and any other double changes [in addition to the previously IPSS-denoted −Y, del(5q), del(20q), all as single abnormalities] (Table 2).
The distributions of the IPSS cytogenetic categories in the present IWG-PM database was similar to those in the IMRAW database, which generated the IPSS (1): IPSS Good/Intermediate/Poor 73%/15%/12% (IMRAW 70/14/16%). This contrasted with the cytogenetic categorization in the IPSS-R: Very good/Good/Intermediate/Poor/Very poor 4%/72%/13%/4/7%. The IPSS clinical subgroups in our patient cohort were: Low 37%, Intermediate-1 40%, Intermediate-2 16%, and High 7%. These groups were also similar to the IMRAW patients: 33%/38%/22%/7% (1). As both FAB and WHO morphologic classifications were used, refractory anemia with excess blasts in transformation 6%, chronic myelomonocytic leukemia 9%, and isolated del(5q) 4% were represented in our patients.
Model development
Analysis of the marrow blast cutpoints indicated that striking differences were evident for both survival and AML evolution for patients with blasts 0- ≤ 2% (lower risk) versus blasts > 2- < 5%: Cox univariate pairwise comparison hazard ratio 1.4 (95% CI, 1.3-1.5, P < .001) for survival and 2.4 (95% CI, 1.9-2.9 P < .001) for AML evolution (Figures 1 and 2). Multivariate results confirmed this finding. Thus, we incorporated these distinct categorical values into the scoring model. Further, the statistical analysis of clinical outcomes for blasts > 10-≤ 20% vs > 20-≤ 30% indicated that these values had similar risk: hazard ratio = 1.0 (95% CI, 0.8-1.2, P = .996) for survival and 0.8 (95% CI, 0.6-1.1 P = .174) for AML evolution (supplemental Figures 3 and 4). Thus, we combined these 2 categories in the scoring model. In addition, this finding of the statistical relevance of the specific blast cutpoints in the combined database was also present in the individual databases, including that of the IMRAW database from which the IPSS1 had been derived. For the IMRAW patients, the hazard ratios for survival and AML evolution were substantially the same as those for the combined database for both the lower and higher blast group analyses.
IWG-PM patients marrow blast subgroups. Impact on survival. Survival related to MDS patients' individual marrow blast percent categories (Kaplan-Meier curves, Dxy 0.3, P < .001). The number of patients in each category and their proportional representation are shown in Table 1.
IWG-PM patients marrow blast subgroups. Impact on survival. Survival related to MDS patients' individual marrow blast percent categories (Kaplan-Meier curves, Dxy 0.3, P < .001). The number of patients in each category and their proportional representation are shown in Table 1.
IWG-PM patients marrow blast subgroups: Impact on AML evolution. Progression to AML related to MDS patients' individual marrow blast percent categories (Kaplan-Meier curves, Dxy 0.47, P < .001). The number of patients in each category and their proportional representation are shown in Table 1.
IWG-PM patients marrow blast subgroups: Impact on AML evolution. Progression to AML related to MDS patients' individual marrow blast percent categories (Kaplan-Meier curves, Dxy 0.47, P < .001). The number of patients in each category and their proportional representation are shown in Table 1.
Review of the data indicated that baseline depths of cytopenias were statistically and clinically important (Table 3). The relevant cutpoints were: hemoglobin values of < 8, 8-< 10, and ≥ 10 g/dL, platelets of < 50, 50-100 and ≥ 100 × 109/L, and ANC of < 0.8 versus ≥ 0.8 × 109/L.
IPSS-R prognostic score values
| Prognostic variable . | 0 . | 0.5 . | 1 . | 1.5 . | 2 . | 3 . | 4 . |
|---|---|---|---|---|---|---|---|
| Cytogenetics | Very good | — | Good | — | Intermediate | Poor | Very poor |
| BM blast, % | ≤ 2 | — | > 2%- < 5% | — | 5%-10% | > 10% | — |
| Hemoglobin | ≥ 10 | — | 8- < 10 | < 8 | — | — | — |
| Platelets | ≥ 100 | 50-< 100 | < 50 | — | — | — | — |
| ANC | ≥ 0.8 | < 0.8 | — | — | — | — | — |
| Prognostic variable . | 0 . | 0.5 . | 1 . | 1.5 . | 2 . | 3 . | 4 . |
|---|---|---|---|---|---|---|---|
| Cytogenetics | Very good | — | Good | — | Intermediate | Poor | Very poor |
| BM blast, % | ≤ 2 | — | > 2%- < 5% | — | 5%-10% | > 10% | — |
| Hemoglobin | ≥ 10 | — | 8- < 10 | < 8 | — | — | — |
| Platelets | ≥ 100 | 50-< 100 | < 50 | — | — | — | — |
| ANC | ≥ 0.8 | < 0.8 | — | — | — | — | — |
— indicates not applicable.
The changes from the cutpoints used in the IPSS-R compared with those from the IPSS include (1) separating marrow blasts < 5% into 0%-2% and > 2-< 5%; and (2) providing differing depths of cytopenias; also, as patients with marrow blasts of 10%-20% had similar outcomes as those with 21%-30%; and (3) the category of marrow blasts > 10%-30% usefully described the statistical impact of this parameter compared with having separated these groups in the IPSS.
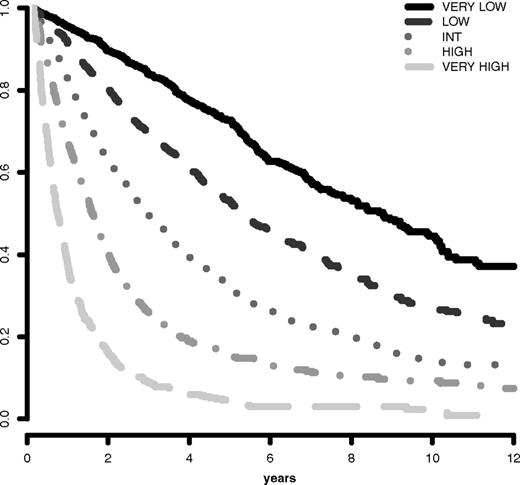
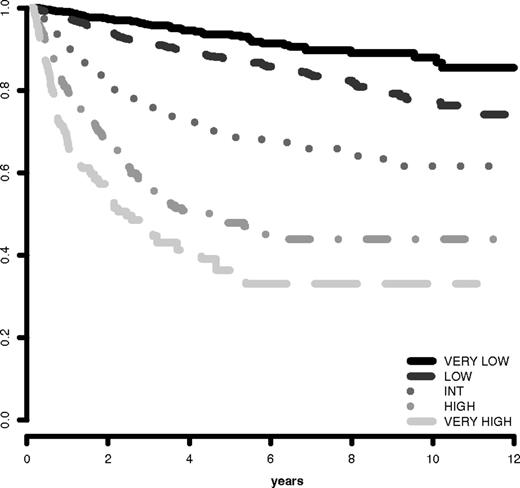
The IPSS-R prognostic risk categories were determined by combining the scores of these main 5 features (Table 4). The model permitted the definition of 5 well-separated prognostic categories for both survival and AML evolution in the IPSS-R (Very low, Low, Intermediate, High, Very high) rather than the 4 categories that are present in the IPSS (Tables 4 and 5; Figures 3 and 4). These risk categories describe scores for 70-year-old patients.
IPSS-R prognostic risk categories/scores
| Risk category . | Risk score . |
|---|---|
| Very low | ≤ 1.5 |
| Low | > 1.5-3 |
| Intermediate | > 3-4.5 |
| High | > 4.5-6 |
| Very high | > 6 |
| Risk category . | Risk score . |
|---|---|
| Very low | ≤ 1.5 |
| Low | > 1.5-3 |
| Intermediate | > 3-4.5 |
| High | > 4.5-6 |
| Very high | > 6 |
IPSS-R prognostic risk category clinical outcomes
| . | No. of patients . | Very low . | Low . | Intermediate . | High . | Very high . |
|---|---|---|---|---|---|---|
| Patients, % | 7012 | 19 | 38 | 20 | 13 | 10 |
| Survival, all* | 8.8 | 5.3 | 3.0 | 1.6 | 0.8 | |
| (7.8-9.9) | (5.1-5.7) | (2.7-3.3) | (1.5-1.7) | (0.7-0.8) | ||
| Hazard ratio | 0.5 | 1.0 | 2.0 | 3.2 | 8.0 | |
| (95% CI) | (0.46-0.59) | (0.93-1.1) | (1.8-2.1) | (2.9-3.5) | (7.2-8.8) | |
| Patients, % | 6485 | 19 | 37 | 20 | 13 | 11 |
| AML/25%*† | NR | 10.8 | 3.2 | 1.4 | 0.73 | |
| (14.5-NR) | (9.2-NR) | (2.8-4.4) | (1.1-1.7) | (0.7-0.9) | ||
| Hazard ratio | 0.5 | 1.0 | 3.0 | 6.2 | 12.7 | |
| (95% CI) | (0.4-0.6) | (0.9-1.2) | (2.7-3.5) | (5.4-7.2) | (10.6-15.2) |
| . | No. of patients . | Very low . | Low . | Intermediate . | High . | Very high . |
|---|---|---|---|---|---|---|
| Patients, % | 7012 | 19 | 38 | 20 | 13 | 10 |
| Survival, all* | 8.8 | 5.3 | 3.0 | 1.6 | 0.8 | |
| (7.8-9.9) | (5.1-5.7) | (2.7-3.3) | (1.5-1.7) | (0.7-0.8) | ||
| Hazard ratio | 0.5 | 1.0 | 2.0 | 3.2 | 8.0 | |
| (95% CI) | (0.46-0.59) | (0.93-1.1) | (1.8-2.1) | (2.9-3.5) | (7.2-8.8) | |
| Patients, % | 6485 | 19 | 37 | 20 | 13 | 11 |
| AML/25%*† | NR | 10.8 | 3.2 | 1.4 | 0.73 | |
| (14.5-NR) | (9.2-NR) | (2.8-4.4) | (1.1-1.7) | (0.7-0.9) | ||
| Hazard ratio | 0.5 | 1.0 | 3.0 | 6.2 | 12.7 | |
| (95% CI) | (0.4-0.6) | (0.9-1.2) | (2.7-3.5) | (5.4-7.2) | (10.6-15.2) |
NR indicates not reached.
Medians, years (95% CI), P < .001.
Median time to 25% AML evolution (95% CIs), P < .001.
Survival based on IPSS-R prognostic risk-based categories. Survival related to MDS patients' prognostic risk categories (Kaplan-Meier curves, n = 7012; Dxy 0.43, P < .001). The number of patients in each category and their proportional representation are shown in Table 1.
Survival based on IPSS-R prognostic risk-based categories. Survival related to MDS patients' prognostic risk categories (Kaplan-Meier curves, n = 7012; Dxy 0.43, P < .001). The number of patients in each category and their proportional representation are shown in Table 1.
AML evolution based on IPSS-R prognostic risk-based categories. Progression to AML related to MDS patients' prognostic risk categories (Kaplan-Meier curves, n = 6485; Dxy 0.52, P < .001). The number of patients in each category and their proportional representation are shown in Table 1.
AML evolution based on IPSS-R prognostic risk-based categories. Progression to AML related to MDS patients' prognostic risk categories (Kaplan-Meier curves, n = 6485; Dxy 0.52, P < .001). The number of patients in each category and their proportional representation are shown in Table 1.
Survival duration and time to AML evolution for patients within these 5 prognostic categories are shown in Table 5 and Figures 3 and 4. As indicated in Table 5, in which hazard ratios are shown, ∼ 56% of the patients were in the lower risk (Very low and Low) and ∼ 23% were in the higher (High and Very high) risk prognostic subgroups for both of these clinical outcomes. For both survival and time to AML evolution, the individual centers' Dxy's were in good agreement with that for the total patient cohort.
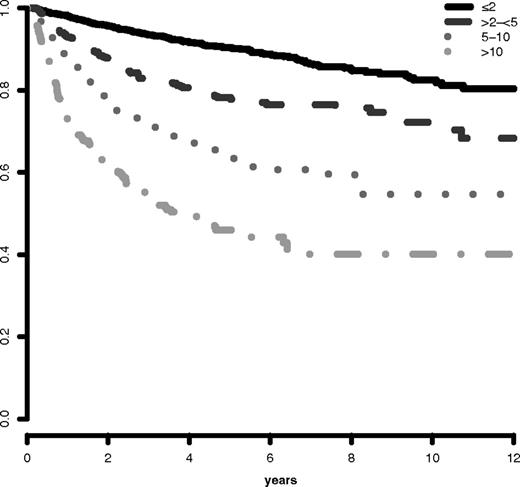
Ready extrapolation is available to adjust the score for patients of any age by use of the following formula: (years − 70) × [0.05 − (IPSS-R risk score × 0.005)], add the result to the sum of the 5 major variables. Patient age clearly had major impact on survival (ie, decreased survival with aging), but not for AML evolution (Figure 5; supplemental Figure 5). Table 6 provides specific survival data within each risk category for patients of differing ages. Figure 6 provides a nomogram, based on the just noted formula, which visually describes the method to determine predicted survival based on patient's age and risk status, generating age-adjusted IPSS-R categorization (IPSS-RA).
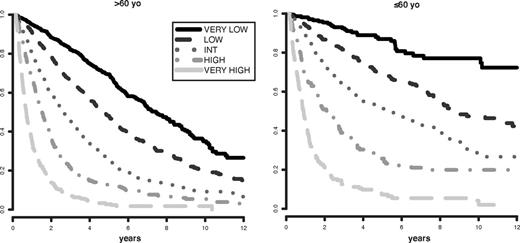
Survival based on patient ages > 60 years vs ≤ 60 years related to their IPSS-R prognostic risk-based categories (Kaplan-Meier curves). Age-related differential survivals are shown for patients in all groups, particularly for those in lower risk categories.
Survival based on patient ages > 60 years vs ≤ 60 years related to their IPSS-R prognostic risk-based categories (Kaplan-Meier curves). Age-related differential survivals are shown for patients in all groups, particularly for those in lower risk categories.
IPSS-R survival related to age
| Ages, y . | IPSS-R prognostic risk categories . | ||||
|---|---|---|---|---|---|
| Very low . | Low . | Intermediate . | High . | Very high . | |
| All | 8.8 | 5.3 | 3.0 | 1.6 | 0.8 |
| ≤ 60 | NR | 8.8 | 5.2 | 2.1 | 0.9 |
| (13.0-NR) | (8.1-12.1) | (4.0-7.7) | (1.7-2.8) | (0.8-1.0) | |
| > 60-70 | 10.2 | 6.1 | 3.3 | 1.6 | 0.8 |
| (9.1-NR) | (5.3-7.4) | (2.5-4.0) | (1.5-2.0) | (0.7-1.0) | |
| > 70-80 | 7.0 | 4.7 | 2.7 | 1.5 | 0.7 |
| (5.9-9.0) | (4.3-5.3) | (2.4-3.1) | (1.3-1.7) | (0.6-0.8) | |
| > 80 | 5.2 | 3.2 | 1.8 | 1.5 | 0.7 |
| (4.2-5.9) | (2.8-3.8) | (1.6-2.6) | (1.2-1.7) | (0.5-0.8) | |
| ≤ 60 (median, 52) | NR | 8.8 | 5.2 | 2.1 | 0.9 |
| > 60 (median, 74) | 7.5 | 4.7 | 2.6 | 1.5 | 0.7 |
| ≤ 70 (median, 62) | 13.3 | 7.7 | 3.9 | 1.7 | 0.9 |
| > 70 (median, 77) | 5.9 | 4.2 | 2.5 | 1.4 | 0.7 |
| Ages, y . | IPSS-R prognostic risk categories . | ||||
|---|---|---|---|---|---|
| Very low . | Low . | Intermediate . | High . | Very high . | |
| All | 8.8 | 5.3 | 3.0 | 1.6 | 0.8 |
| ≤ 60 | NR | 8.8 | 5.2 | 2.1 | 0.9 |
| (13.0-NR) | (8.1-12.1) | (4.0-7.7) | (1.7-2.8) | (0.8-1.0) | |
| > 60-70 | 10.2 | 6.1 | 3.3 | 1.6 | 0.8 |
| (9.1-NR) | (5.3-7.4) | (2.5-4.0) | (1.5-2.0) | (0.7-1.0) | |
| > 70-80 | 7.0 | 4.7 | 2.7 | 1.5 | 0.7 |
| (5.9-9.0) | (4.3-5.3) | (2.4-3.1) | (1.3-1.7) | (0.6-0.8) | |
| > 80 | 5.2 | 3.2 | 1.8 | 1.5 | 0.7 |
| (4.2-5.9) | (2.8-3.8) | (1.6-2.6) | (1.2-1.7) | (0.5-0.8) | |
| ≤ 60 (median, 52) | NR | 8.8 | 5.2 | 2.1 | 0.9 |
| > 60 (median, 74) | 7.5 | 4.7 | 2.6 | 1.5 | 0.7 |
| ≤ 70 (median, 62) | 13.3 | 7.7 | 3.9 | 1.7 | 0.9 |
| > 70 (median, 77) | 5.9 | 4.2 | 2.5 | 1.4 | 0.7 |
Data are median (95% CI).
NR indicates not reached.
Age-adjusted IPSS-R risk categories. The nomogram describes predicted survival based on patient age and IPSS-R risk status (IPSS-RA). To determine an age-adjusted risk categorization, for example, follow the horizontal line, starting at the IPSS-R risk score 3.5 on the vertical axis (Int [Intermediate] risk category per Table 4) to the age of the patient and record the color at that point. If the patient is 45 years, the 3.5′-line and the vertical 45-year line cross in the gray field, placing the patient in the Low risk category, whereas if the patient is 95 years the 3.5′-line and the 95-year line cross in the yellow field, placing the patient in the Intermediate risk category. As indicated, for most patients in the Very high risk category there is no change of risk group, whereas for most patients in the lower risk categories there is greater possibility of category change. Note the “dotted” vertical line at 70 years, which is at the median age of the IWG-PM patient cohort from which the basic risk category scores were calculated (ie, without need for age correction for these patients). The formula to generate the age-adjusted risk score in the figure: (years − 70) × [0.05 − (IPSS-R risk score × 0.005)]. Example: For the 45-year-old patient with an IPSS-R risk score of 3.5 (Intermediate risk): (45-70) × [0.05 − (3.5 × 0.005)] = −0.81. Thus, 3.5-0.8 = 2.7 [age-adjusted IPSS-R score, IPSS-RA: Low risk].
Age-adjusted IPSS-R risk categories. The nomogram describes predicted survival based on patient age and IPSS-R risk status (IPSS-RA). To determine an age-adjusted risk categorization, for example, follow the horizontal line, starting at the IPSS-R risk score 3.5 on the vertical axis (Int [Intermediate] risk category per Table 4) to the age of the patient and record the color at that point. If the patient is 45 years, the 3.5′-line and the vertical 45-year line cross in the gray field, placing the patient in the Low risk category, whereas if the patient is 95 years the 3.5′-line and the 95-year line cross in the yellow field, placing the patient in the Intermediate risk category. As indicated, for most patients in the Very high risk category there is no change of risk group, whereas for most patients in the lower risk categories there is greater possibility of category change. Note the “dotted” vertical line at 70 years, which is at the median age of the IWG-PM patient cohort from which the basic risk category scores were calculated (ie, without need for age correction for these patients). The formula to generate the age-adjusted risk score in the figure: (years − 70) × [0.05 − (IPSS-R risk score × 0.005)]. Example: For the 45-year-old patient with an IPSS-R risk score of 3.5 (Intermediate risk): (45-70) × [0.05 − (3.5 × 0.005)] = −0.81. Thus, 3.5-0.8 = 2.7 [age-adjusted IPSS-R score, IPSS-RA: Low risk].
Additional significant differentiating features for predicting survival were found, although their impact on prognostic score was relatively low compared with the 5 major features and age. These were: performance status, serum ferritin, LDH, and possibly β2-microglobulin (supplemental Table 1). For determining the contribution of each of these features to the patient's risk category, the numerical values (with the categorized values for each variable) are indicated in the table and should be added to the raw scores of the major variables. This table provides multivariate P values as well as indicating the incremental contribution of a feature to the already defined score. Of note, none of these variables was a statistically significant additive feature for predicting AML evolution.
As shown in Table 7, differences were noted in the proportion of our patient cohort who died with or without developing AML in relation to their initial prognostic risk category. Of the patients who died, if observed until death, the proportions dying with leukemia in the groups were 13%-33%, positively related to their higher-risk categories.
Mortality of MDS patients with or without AML evolution
| Risk category . | No. (%) of patients . | Patients who . | ||
|---|---|---|---|---|
| Died, no. (%) . | Died with AML, no. (%) . | Died without AML, no. (%) . | ||
| Very low | 1313 | 350 (27) | 46 (13) | 304 (87) |
| Low | 2646 | 1053 (40) | 174 (17) | 861 (83) |
| Intermediate | 1433 | 782 (55) | 205 (26) | 568 (74) |
| High | 898 | 633 (71) | 207 (33) | 421 (67) |
| Very high | 722 | 619 (86) | 193 (31) | 422 (69) |
| Total | 7012 | 3437 (49) | 825 (24) | 2576 (76) |
| Risk category . | No. (%) of patients . | Patients who . | ||
|---|---|---|---|---|
| Died, no. (%) . | Died with AML, no. (%) . | Died without AML, no. (%) . | ||
| Very low | 1313 | 350 (27) | 46 (13) | 304 (87) |
| Low | 2646 | 1053 (40) | 174 (17) | 861 (83) |
| Intermediate | 1433 | 782 (55) | 205 (26) | 568 (74) |
| High | 898 | 633 (71) | 207 (33) | 421 (67) |
| Very high | 722 | 619 (86) | 193 (31) | 422 (69) |
| Total | 7012 | 3437 (49) | 825 (24) | 2576 (76) |
Distinction between IPSS-R and IPSS
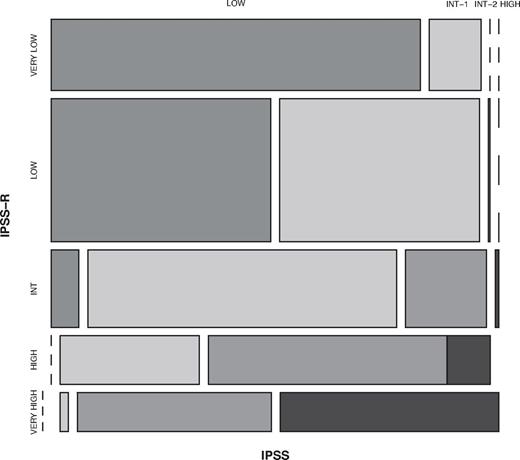
A summary of the refinements of the IPSS-R beyond the IPSS is shown in Table 8. The IPSS-R model showed effective separation of the IPSS patient risk categories and more effectively discriminated prognostic risk for these patients than the IPSS, as indicated by the higher Dxy values (.43 vs .37 for survival, .52 vs .48 for AML evolution; Table 1). Data indicated that 99% of the patients in the IPSS-R Very low and Low risk subgroups encompassed those who had been classified as IPSS Low and Intermediate-1; 81% of those in the IPSS-R High and Very high risk subgroups had been classified as IPSS Intermediate-2 and High (Figure 7, Kendall tau = 0.73). The IPSS-R Intermediate category (20% of the patients) was composed of 73% IPSS Intermediate-1, 19% Intermediate-2, 7% Low, 1% High (Table 9). In the IPSS lower risk group (Low/Intermediate-1), 27% of these patients were shifted into higher risk IPSS-R categories (mainly Intermediate). At the other prognostic extreme, 18% of the IPSS higher-risk (Intermediate-2/High) were downstaged into lower-risk IPSS-R categories (predominantly IPSS-R Intermediate).
Refinements of the IPSS-R beyond the IPSS
| 1. New marrow blast categories |
| ≤ 2%, > 2%-< 5%, 5%-10%, > 10%-30% |
| 2. Refined cytogenetic abnormalities and risk groups |
| 16 (vs 6) specific abnormalities, 5 (vs 3) subgroups |
| 3. Evaluation of depth of cytopenias |
| Clinically and statistically relevant cutpoints used |
| 4. Inclusion of differentiating features* |
| Age, Performance Status, serum ferritin, LDH; β2-microglobulin† |
| 5. Prognostic model with 5 (vs 4) risk categories |
| Improved predictive power |
| 1. New marrow blast categories |
| ≤ 2%, > 2%-< 5%, 5%-10%, > 10%-30% |
| 2. Refined cytogenetic abnormalities and risk groups |
| 16 (vs 6) specific abnormalities, 5 (vs 3) subgroups |
| 3. Evaluation of depth of cytopenias |
| Clinically and statistically relevant cutpoints used |
| 4. Inclusion of differentiating features* |
| Age, Performance Status, serum ferritin, LDH; β2-microglobulin† |
| 5. Prognostic model with 5 (vs 4) risk categories |
| Improved predictive power |
For survival.
Provisional.
Comparison of IPSS-R and IPSS subgroups within the IWG-PM database patient cohort. Vertical axis represents IPSS-R categories' and horizontal axis, IPSS categories. The proportion of patients in each category is shown in Table 9. Kendall τ = 0.73.
Comparison of IPSS-R and IPSS subgroups within the IWG-PM database patient cohort. Vertical axis represents IPSS-R categories' and horizontal axis, IPSS categories. The proportion of patients in each category is shown in Table 9. Kendall τ = 0.73.
Distribution (%) of IWG-PM patients who would previously have been categorized by IPSS now categorized by IPSS-R
| IPSS . | Very low . | Low . | Intermediate . | High . | Very High . |
|---|---|---|---|---|---|
| Low (37) | 44 | 52 | 4 | 0 | 0 |
| Intermediate-1 (40) | 6 | 45 | 38 | 10 | 1 |
| Intermediate-2 (16) | 0 | 1 | 24 | 45 | 30 |
| High (7) | 0 | 0 | 3 | 19 | 78 |
| Total | 19 | 38 | 20 | 13 | 10 |
| IPSS . | Very low . | Low . | Intermediate . | High . | Very High . |
|---|---|---|---|---|---|
| Low (37) | 44 | 52 | 4 | 0 | 0 |
| Intermediate-1 (40) | 6 | 45 | 38 | 10 | 1 |
| Intermediate-2 (16) | 0 | 1 | 24 | 45 | 30 |
| High (7) | 0 | 0 | 3 | 19 | 78 |
| Total | 19 | 38 | 20 | 13 | 10 |
% indicated within rows. Kendall tau = 0.73.
Model validation
After construction and acceptance of the IPSS-R within the IWG-PM, an external validation cohort of 200 MDS patients from the Medical University of Vienna was evaluated and demonstrated good comparability of demographic features with the global IWG-PM database (supplemental Table 2). Their median age was 71 years, 83% were > 60 years, and the male/female ratio was 1.2:1 with a median follow-up time of 4.6 years. A similar proportion of these patients composed the cytogenetic, clinical, and IPSS-R subgroups, as did the global IWG-PM cohort (supplemental Table 2). Our IPSS-R multivariate model fit these data well, exhibiting high prognostic power, as indicated by the high Dxy's and the clearly differing temporal medians and hazard ratios between prognostic risk categories for both survival and AML evolution (Table 10, supplemental Table 2), including age-related survival (supplemental Table 3). Cox model analyses also supported the improved prognostic ability compared with the IPSS.
IPSS-R prognostic risk category: clinical outcomes of Medical University of Vienna patients (n = 200)
| . | Very low . | Low . | Intermediate . | High . | Very high . |
|---|---|---|---|---|---|
| Patients, % | 21 | 38 | 18 | 14 | 8 |
| Survival | |||||
| All* | 9.3 | 6.3 | 3.4 | 1.2 | 0.6 |
| Hazard ratio | 0.8 | 1 | 2.1 | 4.3 | 9.4 |
| 95% CI | 0.4-1.5 | 0.7-1.5 | 1.3-3.5 | 2.4-7.7 | 4.3-20.8 |
| AML transformation | |||||
| AML/25%*† | NR | NR | 2.4 | 0.8 | 0.6 |
| Hazard ratio | 0 | 1 | 8.0 | 18.7 | 52.2 |
| 95% CI | 0-∞ | 0.3-3.9 | 3.1-20.5 | 7.0-49.7 | 13.8-198.2 |
| . | Very low . | Low . | Intermediate . | High . | Very high . |
|---|---|---|---|---|---|
| Patients, % | 21 | 38 | 18 | 14 | 8 |
| Survival | |||||
| All* | 9.3 | 6.3 | 3.4 | 1.2 | 0.6 |
| Hazard ratio | 0.8 | 1 | 2.1 | 4.3 | 9.4 |
| 95% CI | 0.4-1.5 | 0.7-1.5 | 1.3-3.5 | 2.4-7.7 | 4.3-20.8 |
| AML transformation | |||||
| AML/25%*† | NR | NR | 2.4 | 0.8 | 0.6 |
| Hazard ratio | 0 | 1 | 8.0 | 18.7 | 52.2 |
| 95% CI | 0-∞ | 0.3-3.9 | 3.1-20.5 | 7.0-49.7 | 13.8-198.2 |
NR indicates not reached.
Medians, years (95% CI; P < .001).
Median time to 25% AML evolution (95% CI; P < .001).
Discussion
We herein describe the IPSS-R, which provides useful advances and more discriminatory prognostic risk assessment beyond the IPSS for assessing clinical outcomes in MDS (Table 8). Although the IPSS has been an important standard for assessing prognosis of primary untreated adult MDS patients over the past decade, additional refinements27 and prognostic variables have been suggested as providing meaningful differences for patient clinical outcomes.2-5,9-20 In addition, cytogenetic subgroups have recently been defined as providing improved prognostic evaluation of clinical outcomes of primary MDS patients.8 A number of prior prognostic systems in addition to the IPSS have demonstrated merit, although the relative value of each variable was unclear. Thus, in this collaborative IWG-PM project, with the large number of patients evaluated from multiple coalesced databases (n = 7012), we have integrated the various recently independently defined clinical factors for MDS in a comprehensive method and assessed their relative prognostic impact. Multivariate analyses demonstrated that the same major features present in the IPSS (cytogenetic subgroups, marrow blast percentage, and cytopenias) retained major prognostic impact in IPSS-R (in descending order of statistical weight). However, more precise prognostication of these clinical outcomes (survival and AML evolution) in the IPSS-R was demonstrated by effective refinement of these features within the IPSS-R (depth of cytopenias, splitting of marrow blasts < 5%, and more precise cytogenetic subgroups). The IPSS-R also demonstrated improved predictive prognostic power with more precise prognostic categories5 versus 4 groups in the IPSS. In particular, a substantial proportion of those patients previously placed within the IPSS Intermediate-1 and -2 categories were more precisely separated into all 5 IPSS-R categories.
Analogous to the IPSS, based on our clinical outcome data, lower-risk patients are composed within the IPSS-R Very low and Low categories; higher-risk patients are composed within the High and Very high categories. However, as shown in Table 9, the IPSS-R has permitted improved refinement of risk categories for the IPSS Intermediate-1 and Intermediate-2 patients because a substantial portion of the patients who would have been categorized as IPSS Intermediate-1 are now in the IPSS-R Low category; a substantial portion of the patients who would have been categorized as IPSS Intermediate-2 are now in the IPSS-R High category. In other words, the “better risk” IPSS Intermediate-1 patients have been categorized into the lower-risk IPSS-R category; the “poorer risk” IPSS Intermediate-2 patients are now in the higher-risk IPSS-R category. Remaining within the IPSS-R Intermediate category are those who, indeed, have “intermediate” risk (Tables 5 and 7). On review, the clinical outcome data indicate that the IPSS-R Intermediate category appears closer to the initial IMRAW IPSS Intermediate-1 group (survival 3.0 years for IPSS-R Intermediate vs 3.5 years for IPSS Intermediate-1) than it is to IPSS Intermediate-21 ; the proximity of AML evolution for the IPSS-R Intermediate category is also closer to Intermediate-1 than to Intermediate-2 (ie, the “lower risk” patient group). However, per Table 7, the proportion of patients dying with leukemia for the IPSS-R Intermediate category is distinctively worse than for the lower-risk categories. As survival is the major endpoint for most MDS clinical trials, and of predominant concern to patients and caregivers, it seems reasonable to suggest placement of IPSS-R Intermediate patients into the lower-risk group regarding their potential therapeutic management. However, given the distinctiveness of this patient category, assessment of these patients within both lower- and higher-risk treatment protocols appears warranted. Clinical trial evaluation and recommendations by practice guidelines committees will be needed to substantiate this point. Use of the additional differentiating features (eg, age, performance status, ferritin, LDH; see below) could be of particular value for categorization of these patients.
A major component of this schema was the provision of 5 cytogenetic subgroups (vs 3 in the IPSS) based on an increased number of specific prognostic chromosomal categories15 versus 6 in the IPSS. This increase in defined cytogenetic categories, with their increased prognostic weight, was the result of a larger number of patients available for analysis of some of the relatively rare cytogenetic categories. This increased number of cases permitted specific characterization of many of the cytogenetic subgroups that had previously been labeled in the IPSS as “other” and also separated the prior Good and Poor groups into Very good and Good and Poor and Very poor, respectively, thus improving their prognostic accuracy.
Splitting patients with marrow blasts < 5% into those with 0%-2% and > 2-< 5% provided groups with very low risk versus low risk features. The issue of splitting this “low blast group” into 2 separate subgroups may present a challenge for reporting these values in some routine clinical laboratories. However, these differences in blast enumeration were reproducible within the various databases from the different institutions in our study. The discriminatory lower blast percentages should be of particular importance in helping to ensure balanced representation of patients in clinical trials.
The presence of 10%-20% marrow blasts had similar impact on clinical outcomes as did 21%-30% blasts. Thus, these 2 categories were combined in the prognostic model. The underlying reason(s) for this finding is unclear but could relate to the stringent entry criteria for our patient cohort (eg, excluding treated patients and those with high circulating blasts or patients' innate biologic similarity). Of interest, this similarity of clinical outcomes for patients within both the low marrow blast group and the 10%-30% blast group was also demonstrated in the IMRAW database from which the initial IPSS was generated.
Scoring the depth of cytopenias by subdivision at clinically and statistically relevant cutpoints rather than solely counting their presence was demonstrated as being useful. The degree of anemia is an important correlate of poor clinical outcomes in MDS27 and appears to be a good surrogate for RBC transfusion dependence.28 In this regard, low hemoglobin levels have recently replaced RBC transfusion dependence as a prognostic parameter of the WPSS.28 Underlying this finding, chronic anemia may contribute to the high nonleukemic mortality related to cardiac disease in MDS patients.2,3,28
The other cytopenia cutpoints also have clinical relevance. The ANC of 0.8 × 109/L (in IPSS-R) is associated with higher potential infectious risk rather than that of 1.8 × 109/L (in the IPSS).29,30 Severe thrombocytopenia has been associated with increased morbidity and poor survival in MDS patients.29,31-33
The impact of age was a major prognostic parameter for overall survival, although not for AML evolution. This effect has previously been shown with the IPSS and in other studies.1,29,34-36 In the IPSS-R prognostic model, the data are shown for age 70 years (the near median age of our patient cohort). However, to incorporate the model for different patient ages (IPSS-RA), we provide a formula (in “Results”), which permits statistical adjustment of survival prognosis for patients of all ages. This formula and the Figure 6 nomogram for calculating the impact of age for modifying the risk score/category provide resources for clinicians and for trial design and analysis. These age-related survival data are also shown in Table 6, Figures 5 and 6, and supplemental Figure 5. Our approach herein for providing age as an important, although optional, feature to assess predicted survival permits this variable to be used if total mortality risk is the aim but to not be used if solely disease-related risk is the objective. Age as a variable has some prognostic influence in all risk groups, but with more impact in lower than in higher-risk patients.
Additional differentiating features in the IPSS-R were additive to the 5 major parameters for predicting survival, albeit not for AML evolution: performance status, serum ferritin, and LDH levels. Serum LDH,9-11 ferritin,12 and β2-microglobulin13,14 have previously been shown to have prognostic importance for survival in MDS. Thus, our analyses have helped determine that these clinical features, of the many previously reported, were also reproducible in our large patient cohort after multivariate analysis. Relevant is that, although these features had some additive impact on survival (often moving patients either into a higher or lower risk category based on dichotomized values), this effect was relatively minor for determining prognostic risk categories compared with that of the 5 major features (see gains in Dxy's and score points shown in supplemental Table 1, which would be added to the basic IPSS-R prognostic score values seen in Table 3). None of these features had additive prognostic impact on the potential for AML transformation.
Our data indicated the importance of performance status as contributing to prognosis for survival in MDS. Other studies have demonstrated the impact of comorbidities on survival in MDS,5,18-20,37-39 which may in part be reflected by performance status.
The negative impact of elevated serum ferritin levels for survival in our patients may relate to prior RBC transfusions contributing to iron overload and its complications or may reflect the severity of the anemia and degree of ineffective erythropoiesis because of the patients' poorer innate marrow function.2,12,40 In addition, as serum ferritin is an inflammatory marker and this value was obtained early in our patients' disease courses (ie, before high RBC transfusion burden), this abnormality may reflect the effects of inflammatory cytokines in MDS.41,42
Although high serum β2-microglobulin levels had significant negative impact on survival in our patients, as this feature was essentially reported from only one institution in our cohort, we have included this as a provisional predictive parameter. In addition, renal dysfunction alters these levels and could confound these results.
Marrow fibrosis did not show incremental prognostic value for clinical outcomes in our study despite previous reports demonstrating poor prognosis of this morphologic feature.15-17 The absence of this variable as an additive factor could relate to the low number of patients assessed for this feature (19%) as well as the variable ways the degree of fibrosis was reported from the different institutions in our study.
Comparison of the IPSS-R with the IPSS categorizations of our IWG-PM patients indicated that a substantial proportion of patients within the IPSS lower-risk group (27%) would be “upstaged” with the IPSS-R categorization, and 18% in the higher risk group would be “downstaged” by IPSS-R. Such findings have implications for more precisely evaluating patient prognosis and their potential management.
Our data showed that the risk of dying related to leukemic evolution, for our patients observed until death, was increased in those with more advanced prognostic risk categories, Table 7). Thus, mortality from complications of bone marrow failure (without leukemic evolution) and patient comorbidities plays a major role in the clinical outcome of the lower-risk patients in contrast to the more prominent role of leukemic evolution in the higher-risk patients. The proportion of patients dying is lower in our patent cohort compared with the patient group within the original IMRAW patient sample,1 predominantly because of a lower proportion of our patients dying after AML transformation. This was probably related to the more stringent entry criteria used in our current patient cohort.
Regarding the stringent inclusion criteria in our study, to be more precise with the diagnostic entity of MDS, and as recommended by National Comprehensive Cancer Network practice guidelines for MDS, relative stability of peripheral blood counts for 1-2 months was required to exclude other possible etiologies for their cytopenias, such as drugs, other diseases, or incipient evolution to AML.43 Exclusion of these patients had minimal influence on the estimates of survival and time to AML evolution (data not shown). In addition, we excluded patients with secondary MDS as their clinical and biologic features (higher degree of AML progression, decreased survival and differing distribution, incidence and types of aberrant and poor risk cytogenetics) distinctively differ from those of primary MDS patients.44
An external validation cohort of untreated primary MDS patients from the Medical University of Vienna was evaluated and demonstrated that the IPSS-R model also fit these data well. In particular, the validity of the model for this cohort was indicated by the high prognostic power (ie, Dxy's) and clearly differing temporal medians and hazard ratios between prognostic risk categories for both survival and AML evolution, including age-related survival. Further validation of the IPSS-R in other patient cohorts is warranted.
Some of the patients in the IWG-PM project were also assessed by the WPSS parameters.2,3 However, because of the relatively low proportion of our patients having several of this system's parameters reported (cellular dysplasia, RBC transfusions), these clinical variables were not included in our analysis. Modification and refinement of the WPSS (WPSS-R) based on the additional features present in the IWG-PM database will be the subject of a separate publication.
In conclusion, the IPSS-R retained continuity with the IPSS and was shown to possess improved prognostic ability for survival and AML evolution compared with the IPSS along with determining additional predictive features, particularly age, having significant impact on survival in primary untreated MDS patients. As such, the IPSS-R should prove beneficial for determination of prognostic status of untreated patients with this disease and aid design and analysis of clinical trials for this disease. Given recent molecular45-47 and flow cytometric studies48,49 showing prognostic value in MDS, further investigations to determine the impact of these technologies on the IPSS-R are warranted and ongoing.
The online version of this article contains a data supplement.
Presented in preliminary form at the 11th International Symposium of MDS, Edinburgh, Scotland, May 19, 2011.50
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the MDS Foundation Inc, for logistical support; Ms Tracey Iraca for helpful logistical assistance for the IWG-PM project; Sherry Pierce, Canan Alhan, Norene Keenan, Ann Hyslop, Michael Groves, Rosa Sapena, Fatiha Chermat, Friedrich Wimazal, and Susanne Herndlhofer for aid in preparing institutional databases; and Barbara Hildebrandt for aid in performing cytogenetic analyses.
Investigators and institutions providing data from the Spanish, French, Piemonte (Italy), and Brazilian MDS Registries are listed in supplemental Table 4.
This work was supported by Celgene Inc and Amgen Inc (unrestricted grants), the Tayside Leukemia Research Endowment Fund for enabling the Tayside Registry (S.T.), Associazione Italiana per la Ricerca sul Cancro (grant 1005) “Special Program Molecular Clinical Oncology 5 × 1000,” and Fondazione Cariplo, Milan, Italy (L.M. and M.C.).
The URLs for a Web-based calculator tool to the IPSS-R are located at http://www.ipss-r.com and at http://www.mds-foundation.org/calculator/index.php.
Authorship
Contribution: P.L.G. designed, performed, and coordinated the research, collected, contributed, analyzed and interpreted the data, and wrote the manuscript; H.T. designed and performed the research, performed the statistical analyses, produced the figures, and edited the manuscript; J.S. collected and contributed data, performed the research, and analyzed and interpreted the data; G.S., G.G.-M., F.S., D.B., P.F., A.L., J.C., O.K., M.L., J.M., S.M.M.M., Y.M., M.P., M.S., W.R.S., R.S., S.T., P.V., T.V., A.A.v.d.L., and U.G. collected and contributed data, analyzed the results and critically revised the paper; J.M.B. collected, contributed, analyzed, and interpreted the data and critically revised the paper; C.F., M.M.L.B., and M.L.S. analyzed and interpreted the data and critically revised the paper; F.D., H.K., A.K., L.M., and M.C. collected and contributed data and reviewed the manuscript; the Cytogenetics Committee members reviewed the cytogenetics data and formulations; and D.H. collected, contributed, analyzed, and interpreted data, designed and performed the research, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter L. Greenberg, Hematology Division, Stanford University Cancer Center, 875 Blake Wilbur Dr, Rm 2335, Stanford, CA 94305-5821; e-mail: peterg@stanford.edu.


![Figure 6. Age-adjusted IPSS-R risk categories. The nomogram describes predicted survival based on patient age and IPSS-R risk status (IPSS-RA). To determine an age-adjusted risk categorization, for example, follow the horizontal line, starting at the IPSS-R risk score 3.5 on the vertical axis (Int [Intermediate] risk category per Table 4) to the age of the patient and record the color at that point. If the patient is 45 years, the 3.5′-line and the vertical 45-year line cross in the gray field, placing the patient in the Low risk category, whereas if the patient is 95 years the 3.5′-line and the 95-year line cross in the yellow field, placing the patient in the Intermediate risk category. As indicated, for most patients in the Very high risk category there is no change of risk group, whereas for most patients in the lower risk categories there is greater possibility of category change. Note the “dotted” vertical line at 70 years, which is at the median age of the IWG-PM patient cohort from which the basic risk category scores were calculated (ie, without need for age correction for these patients). The formula to generate the age-adjusted risk score in the figure: (years − 70) × [0.05 − (IPSS-R risk score × 0.005)]. Example: For the 45-year-old patient with an IPSS-R risk score of 3.5 (Intermediate risk): (45-70) × [0.05 − (3.5 × 0.005)] = −0.81. Thus, 3.5-0.8 = 2.7 [age-adjusted IPSS-R score, IPSS-RA: Low risk].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/12/10.1182_blood-2012-03-420489/4/m_zh89991295030006.jpeg?Expires=1770644524&Signature=gV5PEnmmz0iCHj7pDqhVH10vOecGlSXLOO7FDLISuGYRpPLhHkuxMva2T8VbIEBSluGH274GCQQ1Y2FHn7uA9luYiBmAoVcH8khM3yI4JuAsEpf4KGOGLIJQQ5iKcuiiGbdPp~CoA2YI-PPQq3tLpKkwcHPOa2dLbuDqyqr4T0pdRmHI4TRMG9t8acY6ECT5ck0gCD0DmXCT~yhx4krVBvkgJQrQ9nTw6hLOg6c36966tO6MF4zCjCfYGuj8J~x2VY06YodUujz2eAEtr1dKEYaMZhT9OKbaZlfjH8tQYWYDwbaXDBdduOpCbIU2vNafMuJf462p4I-xRjNUQbrHuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)