Key Points
Pretransplant imatinib improved both relapse and nonrelapse mortality in patients with BCR-ABL–positive acute lymphoblastic leukemia.
Abstract
We aimed to evaluate the impact of pretransplant imatinib administration on the outcome of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in adults with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). We retrospectively analyzed 738 patients with Ph+ ALL that underwent allo-HSCT between 1990 and 2010 using data from the Transplant Registry Unified Management Program of the Japan Society of Hematopoietic Cell Transplantation. We compared the allo-HSCT outcomes between 542 patients who received imatinib before allo-HSCT during the initial complete remission period (imatinib cohort) and 196 patients who did not receive imatinib (non-imatinib cohort). The 5-year overall survival after allo-HSCT was significantly higher in the imatinib cohort than in the non-imatinib cohort (59% vs 38%; 95% confidence interval [CI], 31-45%; P < .001). Multivariate analysis indicated that pretransplant imatinib administration had beneficial effects on overall survival (hazard ratio [HR], 0.57; 95% CI, 0.42-0.77; P < .001), relapse (HR, 0.66; 95% CI, 0.43-0.99; P = .048), and nonrelapse mortality (HR, 0.55; 95% CI, 0.37-0.83; P = .005). In conclusion, our study showed that imatinib administration before allo-HSCT had advantageous effects on the clinical outcomes of allo-HSCT in patients with Ph+ ALL.
Introduction
The treatment of Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) has changed dramatically since the introduction of imatinib. Most imatinib-treated patients achieve complete remission (CR), and hematopoietic stem cell transplantation (HSCT) can be performed in a substantial proportion of patients who have achieved major or complete molecular remission.1-4 Several studies have shown improvements in overall survival (OS) since the incorporation of imatinib-based therapy.5-9 However, the possible benefits of imatinib administration before HSCT have not been extensively examined. In Japan, imatinib was initially used to treat Ph+ ALL in the Japan Adult Leukemia Study Group (JALSG) ALL202 study, which began in February 2002, and has been widely used since 2005.4 A comparison of the clinical outcomes of the 60 patients enrolled in the JALSG ALL202 study with those of patients from the pre-imatinib era strongly suggested that Ph+ ALL patients who received imatinib before allogeneic HSCT (allo-HSCT) during the initial CR period had significantly improved OS compared with those who did not receive imatinib.10 In the present study, we used data from the Transplant Registry Unified Management Program of the Japan Society of Hematopoietic Cell Transplantation (JSHCT) to perform a large retrospective analysis of the clinical impact of imatinib administration before allo-HSCT.11,12
Methods
Data source and patient selection criteria
For this retrospective observational study, patient data were provided by the JSHCT, the Japan Marrow Donor Program, and the Japan Cord Blood Bank Network.11 In the Transplant Registry Unified Management Program, patient survival, disease status, and long-term complications, including chronic graft-versus-host disease (GVHD) and secondary malignancies, are reviewed annually using follow-up forms.12 Ph+ ALL was diagnosed by the presence of the Ph chromosome using cytogenetics and/or fluorescence in situ hybridization analysis and the determination of BCR-ABL fusion transcript positivity via real-time quantitative polymerase chain reaction (PCR) analysis. Grafts from unrelated donors were exclusively bone marrow derived because peripheral blood stem cell donation from unrelated donors was not approved in Japan during the study period. The timing and procedure of allo-HSCT, including the conditioning regimens, GVHD prophylaxis, and BCR-ABL transcript level assessments, were determined at each institution. BCR-ABL transcript levels were not compensated with a correction factor. In most laboratories, BCR-ABL mRNA copy numbers were normalized relative to glyceraldehyde-3-phosphate dehydrogenase mRNA copy numbers and expressed as copies per microgram of RNA. The quantification threshold was 50 copies/μg RNA, which corresponded to a minimal sensitivity of 10−5; nondetection of BCR-ABL or samples below this threshold was designated as “not detected” or “<50 copies/μg” (presented herein as PCR negative). Minimum residual disease (MRD) was evaluated using real-time quantitative PCR within a 30-day period before transplantation. Therapeutic decisions regarding tyrosine kinase inhibitor (TKI) administration after allo-HSCT were made at each institution. This study was approved by the data management committees of the JSHCT, the Japan Marrow Donor Program, and the Japan Cord Blood Bank Network and by the Institutional Review Board of the Fujita Health University. This study was conducted in accordance with the Declaration of Helsinki.
Patient selection
To attain an adequate level of comparability in terms of the allo-HSCT regimens, the following inclusion criteria were used: (1) presence of de novo Ph+ ALL; (2) age of 16 to 59 years; (3) allo-HSCT during the first CR; and (4) initial HSCT between 1990 and 2010. Additional data on pretransplant imatinib administration and MRD at the time of allo-HSCT were also collected for this study. Of the 865 patients who fulfilled these criteria, information on pretransplant imatinib administration was available for 739 patients. One patient was excluded because of missing information on the date of relapse. Finally, 738 patients with Ph+ ALL who underwent allo-HSCT during the initial CR were analyzed.
Statistical considerations
The primary end point of our study was OS after allo-HSCT. Secondary end points included the incidence of nonrelapse mortality (NRM) and relapse. The observation periods for OS were calculated from the date of transplantation until the date of the event or the last known date of follow-up. The OS probabilities were estimated according to the Kaplan-Meier product limit method. The cumulative relapse and NRM incidences were estimated while considering the competing risk, as described elsewhere.13 For each estimate of the cumulative event incidence, death without an event was defined as a competing risk. Risk factors were evaluated using a combination of univariate and multivariate analyses. The following variables were evaluated: imatinib use before HSCT (yes vs no), age group in years (40-54 and 55-59 vs <40), donor and stem cell source (bone marrow from unrelated donor, peripheral blood from related donor or cord blood vs bone marrow from related donor), human leukocyte antigen (HLA) disparity (matched [HLA identical siblings or 6/6 allele-matched unrelated] vs mismatched), performance status (PS) at allo-HSCT (0 vs 1-4), time from diagnosis to allo-HSCT (<180 vs ≥180 days), BCR-ABL subtype (major vs minor vs major and minor), donor-recipient gender match (male-male vs male-female vs female-male vs female-female), conditioning regimen (decreased intensity vs myeloablative), white blood cell (WBC) count at diagnosis (<30 000/μL vs ≥30 000/μL), GVHD prophylaxis (CyA/methotrexate vs tacrolimus/methotrexate), cytogenetics [t(9;22) only vs more/other abnormalities], and ABO blood type compatibility (match, minor mismatch, or major mismatch). Continuous CR was defined as the absence of any hematological recurrence. We defined the following dosages as decreased-intensity regimens: busulfan, <9 mg/kg; melphalan, ≤140 mg/m2; and total body irradiation, <500 cGy (single or fractionated) or 500 to 800 cGy (fractionated).14 Donor and recipient pairs were considered matched when the HLAs were matched at the A, B, and DRB1 loci, as determined by low-resolution HLA typing in allo-SCT from a related donor or cord blood. For unrelated allo-HSCT, matching at the HLA-A, B, Cw, and DRB1 loci in HLA high-resolution molecular typing was considered matched cases. Mismatches were defined by the presence of ≥1 disparity among these loci. Univariate analysis was performed using Cox regression models or a log-rank analysis. Multivariate analysis was performed using the Cox proportional hazards regression model or the competing risk regression model,15 as appropriate. Demographic differences among groups were evaluated using the χ2 or Wilcoxon rank-sum tests as appropriate. All statistical analyses were performed with STATA 11 software (STATA Corp., College Station, TX).
Results
Patient characteristics
The 738 study patients included 402 men and 336 women with a median age of 41 years (range, 16-59 years). HLA matching information was not available for 2 patients. The donor sources included HLA-identical sibling donors (n = 280), unrelated donors (n = 439), and other related donors (n = 19). There were no significant differences between the imatinib and non-imatinib cohorts with respect to gender, PS at allo-HSCT, interval between diagnosis and allo-HSCT, donor-recipient gender match, WBC count at diagnosis, or donor-recipient ABO compatibility, whereas significant differences were observed with respect to the age distribution at allo-HSCT, donor status, HLA disparity, stem cell source, BCR-ABL subtype, conditioning regimen, GVHD prophylaxis, and cytogenetics (Table 1). Of the 196 patients in the non-imatinib cohort, 183 (93%) underwent allo-HSCT between 1990 and 2005. In contrast, 403 of the 542 (74%) patients in the imatinib cohort underwent allo-HSCT between 2006 and 2010.
Outcomes
Overall survival.
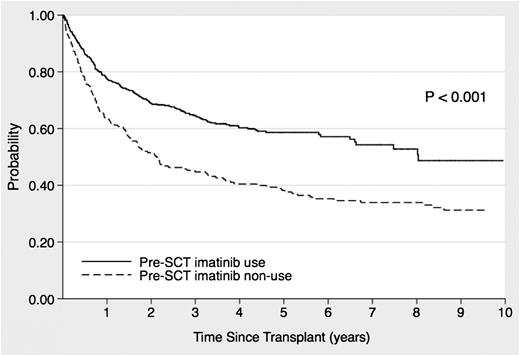
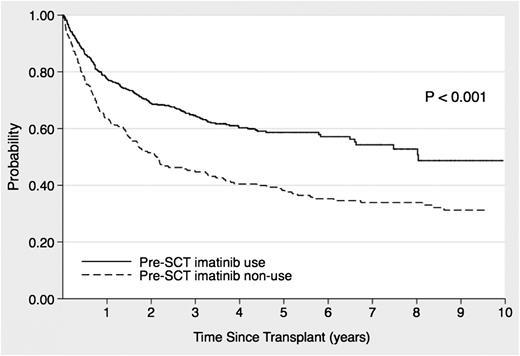
The median follow-up duration of the allo-HSCT survivors was 1551 days (range, 66-6648 days), and the 3- and 5-year OS rates for all patients were 59% (95% confidence interval [CI], 55-63%) and 53% (95% CI, 49-56%), respectively. The 5-year OS in the imatinib cohort was 59% (95% CI, 54-63%), which was significantly higher than that in the non-imatinib cohort (38%; 95% CI, 31-45%; P < .001; Figure 1). Table 2 shows the OS risk factor analysis. Imatinib administration before allo-HSCT had a significantly favorable effect on OS, as revealed by univariate analysis (hazard ratio [HR], 0.56; 95% CI, 0.45-0.70; P < .001] and confirmed by multivariate analysis (HR, 0.57; 95% CI, 0.42-0.77; P < .001). In addition, age, interval between diagnosis and HSCT, and WBC count at diagnosis were significant prognostic factors for OS in the multivariate analysis.
Effects of imatinib administration before stem cell transplantation on the overall survival of patients with Ph+ ALL who underwent allo-HSCT during the initial CR period.
Effects of imatinib administration before stem cell transplantation on the overall survival of patients with Ph+ ALL who underwent allo-HSCT during the initial CR period.
Relapse.
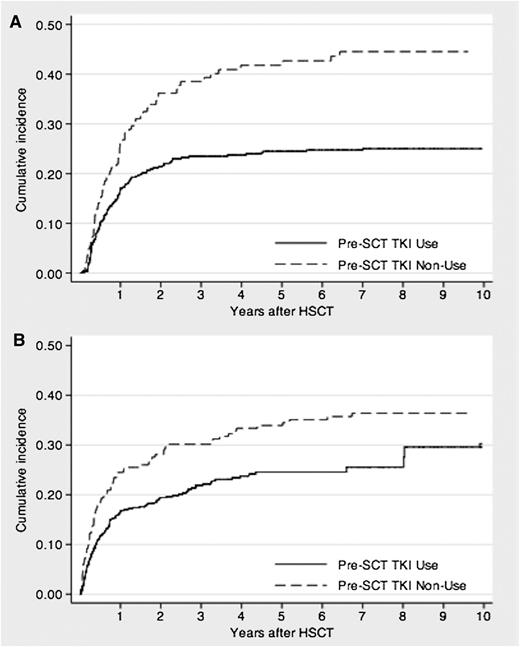
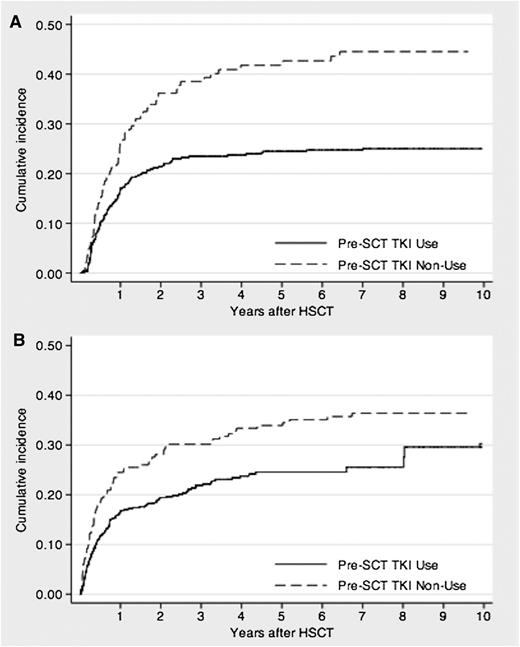
Relapse after allo-HSCT occurred in 116 (21%) and 66 (34%) patients in the imatinib and non-imatinib cohorts, respectively, after median periods of 232 (range, 19-2560 days) and 258 days (range, 42-2350 days), respectively. In the imatinib cohort, the estimated 3-year cumulative incidence of relapse was 23% (95% CI, 20-27%), which was significantly lower than that in the non-imatinib cohort (39%; 95% CI, 31-47%; P < .001; Figure 2). Table 3 shows the relapse risk factor analysis. Imatinib administration before allo-HSCT had a significantly favorable effect on relapse, as determined by univariate analysis (HR, 0.52; 95% CI, 0.39-0.71; P < .001) and confirmed by multivariate analysis (HR, 0.66; 95% CI, 0.43-0.99; P = .048). In addition, the following were significant prognostic factors for relapse: age, 30 to 54 years; HLA disparity; and female-male donor-recipient matching.
Cumulative incidence of relapse- or nonrelapse-related mortality of patients with Ph+ ALL who underwent allo-HSCT during the initial CR period. (A) Relapse mortality. (B) NRM.
Cumulative incidence of relapse- or nonrelapse-related mortality of patients with Ph+ ALL who underwent allo-HSCT during the initial CR period. (A) Relapse mortality. (B) NRM.
NRM.
Overall, 207 (38%) patients in the imatinib cohort and 131 (67%) in the non-imatinib cohort died after allo-HSCT within median periods of 178 (range, 8-2935 days) and 177 days (range, 5-4549 days), respectively. Of these, 124 (23%) and 71 (36%) deaths in the former and latter cohorts, respectively, were not related to relapse after allo-HSCT. The major causes of all deaths and their respective frequencies in the imatinib and non-imatinib cohorts were as follows: relapse (40% vs 47%), infection (18% vs 9%), organ failure (12% vs 9%), interstitial pneumonia (6% vs 4%), GVHD (5% vs 9%), transplantation-associated thrombotic microangiopathy (2% vs 2%), bleeding (2% vs 5%), sinusoidal obstruction syndrome (1% vs 5%), and others (13% vs 11%). The estimated cumulative incidence of NRM at 3 years was significantly lower in the imatinib cohort (22%; 95% CI, 18-26%) than in the non-imatinib cohort (30%; 95% CI, 24-37%; P = .002; Figure 2). Table 4 shows the NRM risk factor analysis. Imatinib administration before allo-HSCT had a significantly favorable effect on NRM, as determined by univariate (HR, 0.65; 95% CI, 0.49-0.88; P < .001) and multivariate analyses (HR, 0.55; 95% CI, 0.37-0.83; P = .005). Age was also found to be a significant prognostic factor in multivariate analysis.
MRD.
Data regarding MRD status before allo-HSCT were available for 67 (34%) patients in the non-imatinib cohort and 400 (74%) patients in the imatinib cohort (Table 1). Among the 467 patients, the MRD negativity rate before allo-HSCT was significantly higher in the imatinib cohort than in the non-imatinib cohort (64%; 95% CI, 59-69% vs 34%; 23-47%; P < .001). The estimated cumulative incidence of relapse at 3 years was significantly lower in the MRD-negative patients than in the MRD-positive patients (20%; 95% CI, 15-25% vs 32%; 95% CI, 25-40%, respectively; P = .0017), and this tendency was significant in the imatinib cohort (19%; 95% CI, 15-25% vs 34%; 95% CI, 25-42%, respectively; P = .0016), but not in the non-imatinib cohort (27%; 95% CI, 10-48% vs 28%; 95% CI, 15-43%, respectively; P = .566). There was no significant difference in NRM between the MRD-negative and MRD-positive patients (19%; 95% CI, 14-24% vs 22%; 95% CI, 16-28% at 3 years, respectively; P = .0642).
Discussion
Although many studies have confirmed the beneficial effects of imatinib on the clinical outcomes of patients with Ph+ ALL,1-6 the potential benefits of pretransplant imatinib administration has not been investigated in a sufficient number of patients. In our study, which is the largest of its type to date, we analyzed the records of 738 patients during a long-term follow-up period to analyze the benefits of pretransplant imatinib administration in patients with Ph+ ALL. We observed significant improvements in the relapse rate and NRM in patients who received imatinib before allo-HSCT compared with those who did not receive imatinib (23% vs 39%; P < .001 and 22% vs 30%; P = .002, respectively). In the MVA, pretransplant imatinib administration was shown to have a significant favorable effect on both relapse and NRM after allo-HSCT.
Some investigators have reported that MRD before HSCT can serve as a powerful predictor of a lower relapse rate. In an analysis of the outcomes of 95 patients with Ph+ ALL who received pretransplant imatinib-based therapy, Lee et al showed that the strongest predictor of relapse was the patient’s MRD status at the end of 2 courses of pretransplant imatinib-based chemotherapy.16 In the present study, patients who were MRD negative before HSCT had a significantly lower relapse rate after HSCT compared with those who were initially MRD positive (20.0% vs 32%, P = .0017), and this tendency was remarkable in the imatinib cohort (19% vs 34%, P = .0016). Moreover, the MRD negativity rate for BCR-ABL patients before allo-HSCT was significantly higher in the imatinib cohort than in the non-imatinib cohort (62% vs 37%, P < .001). These data suggest that in the imatinib cohort, the powerful antileukemia activity associated with pretransplant imatinib administration extensively decreased the MRD before allo-HSCT and prevented subsequent relapse after allo-HSCT.
The Ph chromosome is an adverse prognostic factor in patients with ALL, and only allo-HSCT offers a curative option for patients with Ph ALL. However, the probability of NRM in patients who undergo transplantation during the initial CR is relatively high; therefore, methods to decrease NRM were investigated. Recently, the UKALLXII/ECOG2993 study confirmed the superiority of allogeneic transplantation over chemotherapy on the basis of prospective outcome data from 267 unselected adult patients and reported that high NRM remained a significant problem in the pre-imatinib era.17 Patient age, donor status, and HLA disparity are well-known prognostic factors for NRM after allo-HSCT.1,3,17-19 In the present study, the risk of NRM was significantly lower in the imatinib cohort than in the non-imatinib cohort (P = .002), despite the former comprising significantly larger proportions of older recipients and unrelated and/or HLA-mismatched donors (P < .001, P < .001, and P = .01, respectively). Imatinib-based therapy has increased the proportion of patients who achieve sustained remission, thus providing additional time for suitable donor selection and allo-HSCT and enabling individualized treatment approaches.1-3 These secondary benefits may have contributed to the lower NRM in the imatinib cohort. Moreover, several recent studies have reported improved NRM following the incorporation or dose escalation of imatinib before allo-HSCT.18-22 Given these findings, we believe that imatinib administration has allowed more patients with Ph+ ALL to undergo allo-HSCT while in a better condition, resulting in the achievement of a lower NRM.
Over the last few decades, there have been many attempts to improve patient outcomes after allo-HSCT, including changes in the conditioning regimens and donor selection and the prophylaxis and treatment of organ complications, GVHD, and infectious diseases. In Japan, the period of 1990 to 2005 marked a pioneering era of cord blood transplantation, during which the relevance of cell doses and HLA matching had not yet been recognized. Laport et al reported their experiences with 79 patients with Ph+ ALL who underwent allo-SCT with matched sibling donors; in these patients, the 5-year OS and NRM were examined according to the decade in which SCT was performed (1985-1995 vs 1996-2005), and no significant difference were observed between these 2 time periods.23 In Japan, Kurosawa et al used a nationwide registry database of >6000 patients to retrospectively assess changes in the incidence and causes of NRM during 3 consecutive 4-year periods (1997-2000, 2001-2004, and 2005-2008).24 The authors reported that the incidence of NRM after allo-HCT had significantly decreased during the entire 12-year period, which led to improvements in OS and decreases in NRM in subgroups comprising older patients (50-70 years of age) and/or those who received unrelated bone marrow transplants.24 According to the present study, patients who underwent allo-HSCT with alternative donors and/or elderly patients would benefit from recent improvements in transplantation procedures, and this progress in transplantation may have partly contributed to the improved NRM in the imatinib cohort.
A strength of the present study was its large sample size; this permitted a more accurate estimation of the end points and added statistical power to the analyses. However, because this was a retrospective multicenter study, our results may be susceptible to the disadvantages of any retrospective study, such as heterogeneity in the treatment strategies selected by the physicians. With regard to patient selection bias, changes in patient selection and transplantation procedures throughout the study period (1990-2010) should also be considered. In Japan, the widespread use of alternative donors after 2000 facilitated the extension of allo-HSCT eligibility. Furthermore, cord blood cells were more frequently used in the imatinib cohort (20%) than in the non-imatinib cohort (5%). These discrepancies resulted in different donor status, HLA disparity, and stem cell source frequencies in the present study.
An important difference in the pretransplant chemotherapy regimens should also be noted. Although detailed information about pretransplant chemotherapy was not available, the majority of the non-imatinib cohort was likely treated according to the JALSG ALL9325 or JALSG ALL97 protocols,26 whereas most of the imatinib cohort was likely to be treated according to the JALSG ALL202 protocols,4 in which the chemotherapeutic regimen was similar to that used in the earlier protocols, except for the use of imatinib, because these were widely used regimens in Japan during the study period. Therefore, the influence of pretransplant chemotherapy appears to be limited.
In conclusion, our study involving a large number of patients observed over a long-term follow-up period clearly demonstrates that imatinib administration before allo-HSCT had advantageous effects on the clinical outcomes of patients with Ph+ ALL. This finding encourages us to consider allo-HSCT for patients with Ph+ ALL even during the imatinib era; however, we should continue to investigate alternative treatment options for patients who are not eligible for allo-HSCT because of older age and/or comorbidity. For example, in recent years, MRD monitoring has been increasingly used as an independent prognostic factor in response to a number of studies that have demonstrated its importance. Ravandi et al analyzed the clinical outcomes of patients with Ph+ ALL treated with TKI combined chemotherapy without allogeneic SCT and demonstrated that the achievement of a major molecular response status at 3 months (and beyond) after treatment initiation was associated with a decreased likelihood of relapse and a longer OS.27 Bachanova et al used data from the Center for International Bone Marrow Transplant Research to analyze 197 patients with Ph+ ALL and reported that the achievement of a MRD-negative status may lead to a low relapse rate and prolonged survival in response to either myeloablative conditioning or decreased-intensity conditioning HSCT. They also reported that MRD status may be more helpful than a predefined age cutoff in guiding decisions regarding the conditioning intensity before allo-HSCT.28 In the TKI era, the potential of MRD monitoring via PCR was demonstrated; this technique allows us to identify patients who would benefit from treatment intensification and to select continued therapy without transplantation in older patients with poorer conditions. In addition, recent studies have shown that imatinib therapy before autologous HSCT is also beneficial.7,29 The clinical relevance of autologous HSCT in patients with Ph+ ALL should also be investigated as an alternative stem cell source in the TKI era.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all physicians and staff members of the collaborating institutes of the Japan Society for Hematopoietic Stem Cell Transplantation.
This work was supported by a research grant for cancer from the Japanese Ministry of Health, Labour and Welfare.
Authorship
Contribution: S.M., S.N., K.I., and J.T. designed the study and wrote the manuscript; S.M., Y.A., and K. Matsuo performed the statistical analysis and interpreted the data; H.K., K.O., T.F., Y.O., K. Miyamura, S.T., and M.O. provided the patient data; and Y.A., R.S., Y.M., K.K., and H.S. collected the patient data.
Conflict-of-interest disclosure: S.T. received research funding and honoraria from Novartis Japan. H.S. received honoraria from Novartis Japan. The remaining authors declare no competing financial interests.
Correspondence: Shuichi Mizuta, Department of Hematology, Fujita Health University Hospital, 1-98 Dengakugakubo, Kutsukake-cho, Toyoake 470-1192, Japan; e-mail: mizuta@mb.ccnw.ne.jp.