Key Points
Plerixafor can be given safely to WHIM syndrome patients twice daily for a 6-month period and appears promising as a treatment.
Abstract
Warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome is a rare immunodeficiency disorder caused by gain-of-function mutations in the G protein–coupled chemokine receptor CXCR4. The CXCR4 antagonist plerixafor, which is approved by the US Food and Drug Administration (FDA) for stem cell mobilization in cancer and administered for that indication at 0.24 mg/kg, has been shown in short-term (1- to 2-week) phase 1 dose-escalation studies to correct neutropenia and other cytopenias in WHIM syndrome. However, long-term safety and long-term hematologic and clinical efficacy data are lacking. Here we report results from the first long-term clinical trial of plerixafor in any disease, in which 3 adults with WHIM syndrome self-injected 0.01 to 0.02 mg/kg (4% to 8% of the FDA-approved dose) subcutaneously twice daily for 6 months. Circulating leukocytes were durably increased throughout the trial in all patients, and this was associated with fewer infections and improvement in warts in combination with imiquimod; however, immunoglobulin levels and specific vaccine responses were not fully restored. No drug-associated side effects were observed. These results provide preliminary evidence for the safety and clinical efficacy of long-term, low-dose plerixafor in WHIM syndrome and support its continued study as mechanism-based therapy in this disease. The ClinicalTrials.gov identifier for this study is NCT00967785.
Introduction
WHIM syndrome is a rare primary immunodeficiency disorder whose name is an acronym of its main clinical features: warts, hypogammaglobulinemia, infections, and myelokathexis.1-3 Although myelokathexis refers specifically to bone marrow retention of neutrophils resulting in severe neutropenia, most patients are actually panleukopenic.4 The majority of cases are caused by autosomal dominant mutations truncating the carboxyl terminus of CXCR4.5,6 The mechanism appears to involve loss of negative regulatory elements,7,8 which is thought to increase CXCR4 signaling and the normal adhesion-promoting function of CXCR4 for neutrophils in bone marrow, among other effects.9-11
Granulocyte colony-stimulating factor (G-CSF) and intravenous immunoglobulin (IVIg) have been documented to prevent infections in patients with severe congenital neutropenia and hypogammaglobulinemia, respectively.12,13 Both treatments have been used in WHIM patients; however, no studies have documented clinical efficacy, and they are nonspecific, expensive, difficult to administer, and of questionable efficacy in controlling infections and warts.4,14 In contrast, the small molecule plerixafor (also known as Mozobil and AMD3100), which is approved by the US Food and Drug Administration (FDA) for mobilizing hematopoietic stem cells from bone marrow to blood for transplantation in cancer,15,16 is a highly specific antagonist of both wild-type and WHIM variants of CXCR4 and can rapidly mobilize all major subsets of mature leukocytes to blood in both healthy donors and patients with WHIM syndrome. Specifically, in two phase 1 clinical trials lasting 1 to 2 weeks in patients with WHIM syndrome, plerixafor was able to safely and rapidly increase absolute lymphocyte, monocyte, and neutrophil counts in the peripheral blood in a dose-dependent manner, including at the lowest dose tested, 0.02 mg/kg per day by subcutaneous administration, which is 8% of the FDA-approved dose for stem cell mobilization (0.24 mg/kg per day).17,18 These trials were not designed to test the effect of the drug on infection frequency or warts or long-term hematologic effects. Moreover, the suitability of CXCR4 as a drug target in chronic disease has been questioned on safety grounds, because mice lacking Cxcr4 are not viable and have defective myelopoiesis and B-cell lymphopoiesis as well as defects in cerebellar and vascular development.19-21 In addition, in a 10-day trial of up to 0.16 mg/kg per hour infusion of plerixafor administered to HIV-infected individuals (16 times higher than the FDA-approved dose), more than 20% of participants had gastrointestinal complaints, headaches, tachycardia, paresthesias, dizziness, or orthostatic hypotension, 5% had frequent premature ventricular contractions, and one individual of 40 developed severe thrombocytopenia.22 These concerns may be irrelevant in WHIM syndrome, since the therapeutic strategy is to use the drug at low doses in order to reduce CXCR4 signaling to normal rather than to completely block it. We have now tested this strategy in an open-label, 6-month phase 1 clinical trial of the safety and clinical efficacy of low-dose plerixafor in adults with WHIM syndrome.
Methods
Patients
All patients signed informed consent consistent with the Declaration of Helsinki under clinical protocols approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board prior to taking part in research at the National Institutes of Health Clinical Center. Three unrelated white adults with WHIM syndrome were recruited: a 44-year-old female (P1), a 30-year-old female (P2), and a 51-year-old male (P3). P1 was first reported in Wetzler et al,3 whereas P2 and P3 were first reported in McDermott et al.18 All 3 patients had the 4 major clinical features of WHIM syndrome and were heterozygous for CXCR4R334X, the most common mutation in the disease, which truncates the receptor by 19 amino acids. Each had previously participated in a 7-day, phase 1 dose-escalation study of plerixafor at the National Institutes of Health reported previously and had a hematologic response to the drug with an increase in most subsets of circulating leukocytes without significant side effects.18
P1 and P2 had completed the phase 1 dose-escalation study and had resumed G-CSF and monthly IVIg for 4.5 and 3 months, respectively, before beginning this trial. P3 began this study 2 days after the dose-escalation study18 ; he had not received G-CSF for >2 years because of severe bone pain but had been taking monthly IVIg. G-CSF was stopped on day −7, and IVIg was not given during the period of plerixafor treatment. Pretrial prophylactic antibiotic regimens were continued for P1 and P3 during and after the trial for bronchiectasis and chronic bronchitis, respectively. P2 did not receive prophylactic antibiotics before the trial. She did receive an oral antibiotic for 2 weeks starting on day 47 prophylactically after sustaining an accidental deep laceration with a kitchen knife contaminated with raw meat but never showed signs of infection. The wound was sutured and healed well without abnormal scarring.
Plerixafor pharmacokinetic analysis
Plerixafor was obtained under a Clinical Trials Agreement with Genzyme. Plasma concentrations were determined by Tandem Labs (Durham, NC) using a validated proprietary assay as previously reported.18
Flow cytometry
Flow cytometry was performed on whole blood and purified cells after staining for 30 minutes at 4°C with directly conjugated antibodies (BD Biosciences/Pharmingen, San Diego, CA) using standard techniques as previously reported.18
Statistics
Comparisons were made by using a two-sided paired Student t test by aligning data from each patient before and after treatment. P < .05 was considered significant.
Study design
The design of the trial is depicted in supplemental Figure 1 (available on the Blood Web site). After a minimum of 1 week washout of G-CSF and IVIg, patients self-administered plerixafor for 6 months. Dosing sought to achieve the following 4 goals: (1) use the minimal effective dose able to increase absolute neutrophil count (ANC) as a hematologic biomarker to a safe level; (2) minimize the number of injections required per day; (3) block the receptor at some level at all times, recognizing that plerixafor is a rapidly cleared drug; and (4) reduce CXCR4WHIM signaling to normal but not lower levels. To achieve these goals, dosage was based on the minimum effective dose determined in the dose-escalation study, defined as the dose that increased the ANC at least twofold above baseline and to a minimum of 500 cells per microliter.18 The minimum effective dose was the same for all patients (0.02 mg/kg) but was halved and given as 0.01 mg/kg twice daily subcutaneously because of plerixafor’s rapid pharmacokinetics (T1/2 = 5 hours).23-25 The dose could be doubled if the ANC remained <200 cells per microliter. Blood was drawn periodically for leukocyte dynamic measurements and safety throughout the study, as shown in supplemental Figure 1. Trough levels were measured in blood obtained just before the morning injection. Infections for 3 years before the study, during the 6-month treatment period, and for 1 year after treatment ended were identified by a retrospective review of all available medical records.
Results
Low-dose plerixafor corrects panleukopenia in patients with WHIM syndrome: early effects
Neither previous short-term dose-escalation study of plerixafor in WHIM syndrome evaluated hematologic effects of repeated administration of the same dose.17,18 Therefore, we studied leukocyte dynamics in detail after the morning dose during the first 4 days of long-term treatment. Both common and distinct responses were observed.
With regard to common responses, all 3 patients were panleukopenic at baseline but responded well to plerixafor with a rise in white blood cell count (WBC) peaking ∼3 hours after injection, consistent with previous reports in both healthy donors and in patients with WHIM syndrome (Figure 1).18,23,24,26 For all 3 patients, the peak response for lymphocytes, neutrophils, and monocytes occurred at ∼3 hours, with more persistent effects on neutrophils and monocytes, confirming our previous results (Figure 1).18
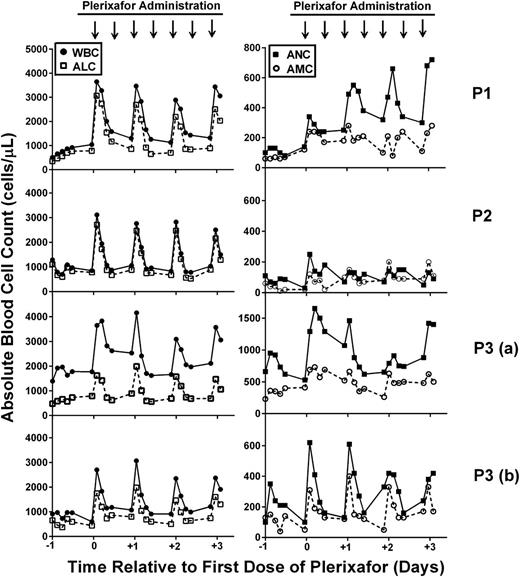
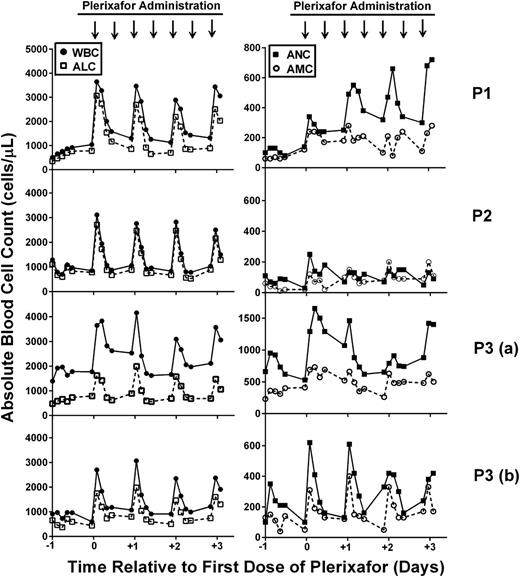
Leukocytes are mobilized to the blood by twice daily administration of plerixafor in WHIM syndrome patients: early phase. Patients were given 0.01 mg/kg plerixafor subcutaneously twice per day, and a complete blood count was determined before and every 3 hours after the morning dose for 12 hours. Left panels show the WBC (solid circles) and ALC (open squares), and right panels show the ANC (solid squares) and AMC (open circles) plotted against time, where day 0 is the first day of drug administration. Time of plerixafor administration is marked with arrows at the top of each column (note that data are missing after the evening dose because blood counts were obtained only during the day). Data for each patient are arranged in rows and designated on the right. Note that data were obtained twice for P3 (a and b) because treatment was interrupted for clinical care.
Leukocytes are mobilized to the blood by twice daily administration of plerixafor in WHIM syndrome patients: early phase. Patients were given 0.01 mg/kg plerixafor subcutaneously twice per day, and a complete blood count was determined before and every 3 hours after the morning dose for 12 hours. Left panels show the WBC (solid circles) and ALC (open squares), and right panels show the ANC (solid squares) and AMC (open circles) plotted against time, where day 0 is the first day of drug administration. Time of plerixafor administration is marked with arrows at the top of each column (note that data are missing after the evening dose because blood counts were obtained only during the day). Data for each patient are arranged in rows and designated on the right. Note that data were obtained twice for P3 (a and b) because treatment was interrupted for clinical care.
We also observed 3 distinct responses. First, P1 exhibited a progressive rise in ANC at both peak and trough, which was not observed for P2 or P3 or for other leukocyte subsets for P1. Second, the ANC of P2 responded poorly to the starting dose of plerixafor, whereas her total WBC and absolute lymphocyte count (ALC) responded well. In the dose escalation trial, the ANC of P2 had responded well to a single dose of 0.02 mg/kg, but she had not previously been given a 0.01 mg/kg dose. Per protocol, on day 15, her plerixafor dose was doubled, resulting in an acceptable increase in trough ANC (≥250 cells per microliter) by day 27, and this dose was maintained until the trial concluded.
Third, in this trial, the leukocyte subset rank order of responsiveness for P3 was ANC>>ALC>AMC vs ALC>>ANC>AMC (AMC, absolute monocyte count) for both P1 and P2 in this trial as well as for P1, P2, and P3 in the previous dose-escalation trial (Figure 1, row P3 [a]). Of note, baseline values for P1 and P2 were similar between the dose-escalation trial and this study for all subsets, whereas the initial baseline ANC for P3 was substantially higher in this study than in the dose-escalation study (735 vs 162 cells per microliter). Two factors may explain this difference. First, P3 began this study just 2 days after the dose-escalation trial, when his final recorded trough ANC was 660 cells per microliter, whereas P1 and P2 waited 4.5 and 3 months, respectively, after the dose-escalation trial ended before beginning this trial. Second, P3 may have been infected at baseline, and infection is known to increase ANC in WHIM patients. In particular, from a baseline of bronchiectasis and chronic airway colonization with Pseudomonas aeruginosa, he developed fever and a new pulmonary infiltrate during the first week of the trial and was treated with intravenous antibiotics. The course was complicated by persistent fever and headache. Cerebrospinal fluid contained 10 lymphocytes per milliliter but had normal glucose and protein levels, and multiple polymerase chain reaction assays and all cultures for micro-organisms were negative. Antibiotic coverage was broadened, and plerixafor was held starting on day 15 in case the aseptic meningitis picture was related to the study drug. Fever continued for 5 days and headaches resolved after 3.5 weeks. The trial was resumed at the same dose of plerixafor 114 days after the start (99 days after interrupting treatment). At this time, his leukocyte subset baseline values and rank order of responsiveness to plerixafor changed from the P3 (a) pattern (Figure 1) and were now aligned with P1 and P2 as well as with his own response during the dose-escalation trial (ie, ALC>>ANC>AMC); thus, most of the WBC response in all 3 patients was accounted for by the ALC response (Figure 1, row P3 [b]). Headache and fever did not recur in P3, and he completed the trial after receiving 194 days of plerixafor. Thus, the adverse event was judged not to be a side effect of plerixafor.
Low-dose plerixafor durably corrects panleukopenia in patients with WHIM syndrome
Throughout the trial, the total WBC and ALC continued to oscillate with large differences between peak and trough values, whereas ANC and AMC levels had less variation (Figure 2). Peak and trough ALC values increased only slightly with time (Figure 3), whereas increases in both ANC peak and trough values were more pronounced. ANC increases occurred as early as the first month for P3. Of note, the large differences between ALC peaks and troughs persisted throughout the study, whereas the ANC level increased and was maintained such that the peak/trough difference was relatively small for all 3 patients. There was no consistent temporal pattern for the AMC response. Three hours after the morning 0.01 mg/kg dose, plasma plerixafor concentrations for P1 were <5, 7.9, and 14.4 ng/mL on days 32, 88, and 178, respectively, of this trial in contrast to an average of 608 ng/mL for P1 and P2 1 hour after receiving 0.24 mg/kg in the dose-escalation trial.18
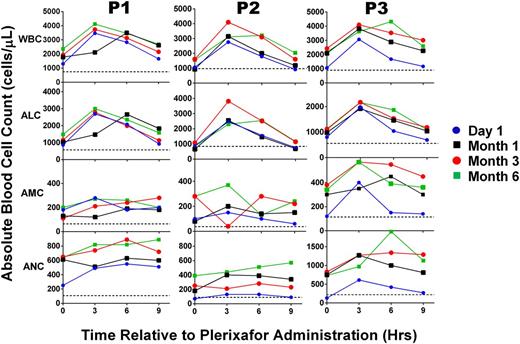
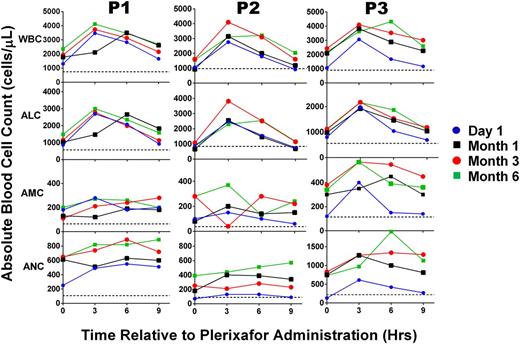
Leukocyte dynamics in response to plerixafor in WHIM syndrome patients are durable over time. For each panel, the leukocyte subset indicated to the left of the corresponding row was measured immediately before and every 3 hours after a dose for 9 hours on the dates indicated and color-coded according to the scheme at the right of the figure. Patients were treated with plerixafor 0.1 to 0.2 mg/kg twice per day for the entire 6-month period of study. Data for each patient are arranged in columns and identified at the top. Horizontal dotted lines indicate the average baseline values before starting treatment. Note that baseline values shown for P3 correspond to P3 (b) in Figure 1.
Leukocyte dynamics in response to plerixafor in WHIM syndrome patients are durable over time. For each panel, the leukocyte subset indicated to the left of the corresponding row was measured immediately before and every 3 hours after a dose for 9 hours on the dates indicated and color-coded according to the scheme at the right of the figure. Patients were treated with plerixafor 0.1 to 0.2 mg/kg twice per day for the entire 6-month period of study. Data for each patient are arranged in columns and identified at the top. Horizontal dotted lines indicate the average baseline values before starting treatment. Note that baseline values shown for P3 correspond to P3 (b) in Figure 1.
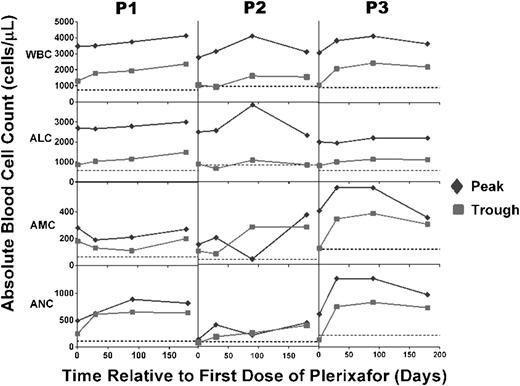
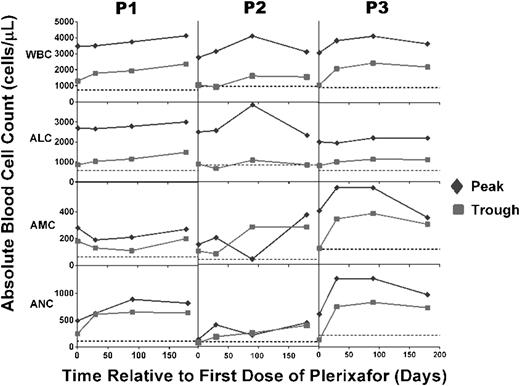
Plerixafor treatment durably maintains peak and trough leukocyte subset levels above baseline for at least 6 months. Patients had complete blood counts and differentials measured at the National Institutes of Health at the times indicated on the x-axis either just before a dose or every 3 hours for 9 hours after a dose, and peak (green diamonds) and trough (red squares) values were plotted. Horizontal dotted lines indicate the average baseline values before starting treatment. Note that baseline values shown for P3 correspond to P3 (b) in Figure 1.
Plerixafor treatment durably maintains peak and trough leukocyte subset levels above baseline for at least 6 months. Patients had complete blood counts and differentials measured at the National Institutes of Health at the times indicated on the x-axis either just before a dose or every 3 hours for 9 hours after a dose, and peak (green diamonds) and trough (red squares) values were plotted. Horizontal dotted lines indicate the average baseline values before starting treatment. Note that baseline values shown for P3 correspond to P3 (b) in Figure 1.
Consistent with the dose-escalation trial results,18 the increase in ALC was mainly comprised of naive (transitional) B cells (CD19+CD27–IgD+IgM+) and effector memory CD4+ and CD8+ T cells (CD45RO+CD62L–CCR7–), and the increase in AMC was predominantly from classical monocytes (CD14+CD16–), although inflammatory monocytes (CD14+CD16+) were also mobilized (Table 1).
Slow return to hematologic baseline after discontinuing chronic low-dose plerixafor in patients with WHIM syndrome
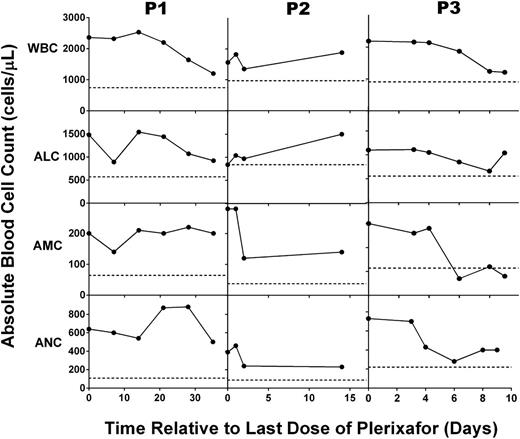
The ALC, AMC, and ANC declined surprisingly slowly (Figure 4) after plerixafor was discontinued, unlike our findings in the dose-escalation trial18 and on days 1 to 3 of this trial (Figure 1). As a result, G-CSF was not resumed for 2.5 to 5 weeks after stopping plerixafor, and we found that markedly lower doses of G-CSF (20% to 50% of pretrial doses) were effective for a prolonged period (2 to 6 months). We measured plerixafor concentrations in P1’s plasma and detected low concentrations: 8.2 and 6.6 ng/mL on days 10 and 17, respectively, after the final dose. By day 21, the drug level fell below the limit of detection (5 ng/mL).
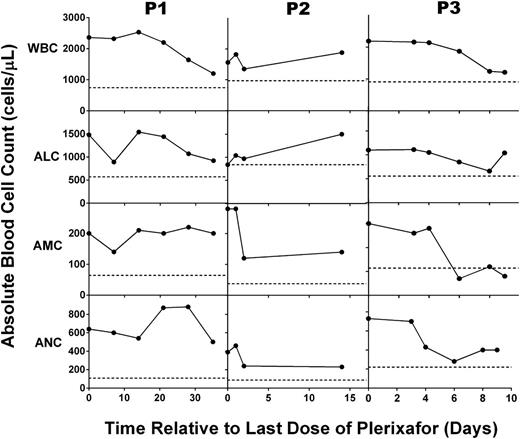
Chronic plerixafor treatment has prolonged effects on blood leukocyte levels in patients with WHIM syndrome. After plerixafor was discontinued and the 6-month trial was completed, the leukocyte subsets indicated to the left of each row were measured in the blood for up to 35 days for the patient indicated at the top of the corresponding column of panels. Horizontal dotted lines indicate the average baseline values before starting treatment. Note that baseline values shown for P3 correspond to P3 (b) in Figure 1.
Chronic plerixafor treatment has prolonged effects on blood leukocyte levels in patients with WHIM syndrome. After plerixafor was discontinued and the 6-month trial was completed, the leukocyte subsets indicated to the left of each row were measured in the blood for up to 35 days for the patient indicated at the top of the corresponding column of panels. Horizontal dotted lines indicate the average baseline values before starting treatment. Note that baseline values shown for P3 correspond to P3 (b) in Figure 1.
Chronic plerixaflor treatment did not enhance Ig levels or response to pneumococcal vaccine
Before the trial, all 3 patients had been receiving monthly IVIg, which was discontinued before plerixafor was started. On the last day of the trial, serum IgA, IgM, and IgG concentrations were on average 92%, 69%, and 63%, respectively, of the pretreatment values (supplemental Figure 2). In addition, pneumococcal and diphtheria/tetanus vaccinations were given at months 3 to 4 of plerixafor therapy. Measurement of titers just before and at 4 to 6 weeks postvaccination demonstrated that the diphtheria titers increased an average of 1.5-fold (range, −0.7- to 3.92-fold), tetanus titers increased 1.6-fold (range, 1.26- to 1.86-fold), and there were no antibodies to any pneumococcal serotypes that increased by more than fourfold. In particular, P1 had almost no response to pneumococcal vaccine, with only 1 of the 23 serotypes contained in the vaccine reaching a 1.3 μg/mL threshold level postvaccination. P2 and P3 had 22 of 23 and 20 of 23 serotypes, respectively, above the threshold prior to vaccination; the remaining serotypes did not increase above threshold postvaccination.
Plerixafor treatment was associated with fewer infections in WHIM patients
Before and after the trial, all 3 patients had frequent documented infections, mostly affecting the upper and lower airway (Figure 5 and supplemental Table 1). Compared with the infection frequencies before and after the trial, we observed a reduction in infection frequency during the trial for all 3 patients. Most strikingly, P1 and P2 had no infections while receiving plerixafor. Moreover, P1’s sputum production decreased by 95% from a baseline of ∼1 cup per day to <10 mL per day and was cleared of P aeruginosa for a year after therapy ended before her sputum was once again colonized. In addition to the apparent infectious episode at the start of the trial described above, P3 also had varicella zoster virus (VZV) infection confirmed by polymerase chain reaction assay affecting the left calf during week 5 of plerixafor treatment that resolved after a 10-day course of oral acyclovir. P3 also had one episode of bronchitis during month 6 of the trial that was treated on an outpatient basis with oral antibiotics without stopping plerixafor. These 3 infections still represented about a 40% reduction from his expected infection frequency compared with data collected for 3 years before and 1 year after the trial.
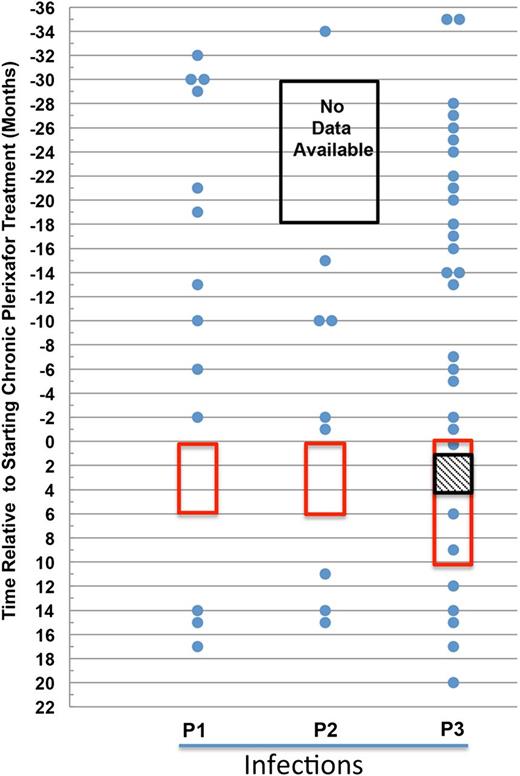
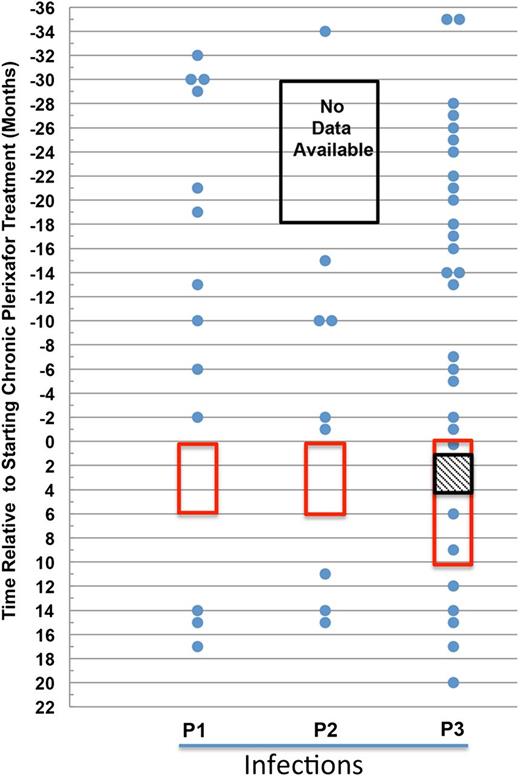
Plerixafor may reduce infection incidence in patients with WHIM syndrome. The data for each patient are presented in separate columns. Each infection is represented as a dot and plotted by the month it occurred in relation to the start of plerixafor therapy. The time on plerixafor is bounded by the red box. Note that data for P2 were unavailable for the 12-month period marked by the black box. For P3, the hatched box represents a gap in plerixafor administration for the purpose of clinical care.
Plerixafor may reduce infection incidence in patients with WHIM syndrome. The data for each patient are presented in separate columns. Each infection is represented as a dot and plotted by the month it occurred in relation to the start of plerixafor therapy. The time on plerixafor is bounded by the red box. Note that data for P2 were unavailable for the 12-month period marked by the black box. For P3, the hatched box represents a gap in plerixafor administration for the purpose of clinical care.
Comparing the average infection rate using 6 month intervals, we found a statistically significant reduction when the plerixafor dosing period was compared with the prior 3-year periods combined (average of 2.2 infections per 6-month period before treatment with plerixafor vs 0.92 per 6 months during treatment with plerixafor; P < .05). However, these results, while promising, should be interpreted with caution because the small number of participants may not be representative of all WHIM patients.
Plerixafor may be beneficial in treating warts in WHIM patients
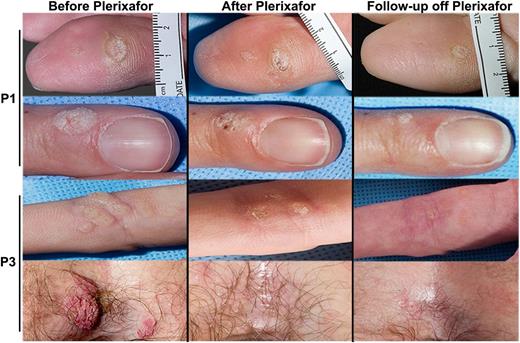
All 3 patients had had large numbers of cutaneous and anogenital warts since childhood that reportedly had failed to respond to multiple medical therapies including imiquimod and/or returned within a year of surgical removal. In both female patients, genital warts had developed into precancerous lesions and had required extensive surgical treatment, including vulvectomy in P1. When treatment of P1 for 4 months with plerixafor failed to affect wart burden, we began topical treatment with imiquimod on warts on the right side of her body 3 times per week for the final 2 months of the trial. With this limited treatment, cutaneous warts (Figure 6) and genital warts (not shown) improved bilaterally, suggesting an induced systemic anti–human papillomavirus (HPV) immune response. We subsequently administered topical imiquimod to P2 and P3 for all 6 months of plerixafor treatment. In addition, 2 months before beginning the trial, P2 underwent surgical removal of ∼300 condylomata acuminata lesions. While taking plerixafor and imiquimod, no new warts developed at the operative site of P2, and her remaining warts diminished in size. As for P3, multiple cutaneous and anogenital warts completely resolved on plerixafor and imiquimod without surgical treatment (Figure 6).
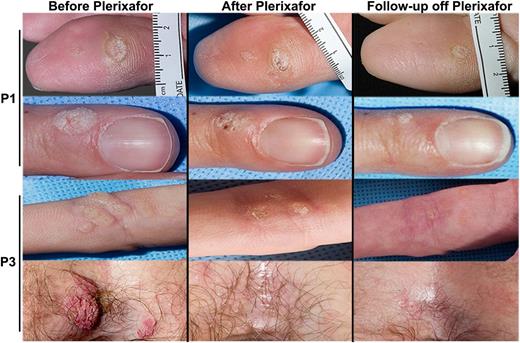
Plerixafor with imiquimod may improve warts in patients with WHIM syndrome. Images of representative warts that improved during the trial are shown before and after plerixafor treatment of P1 (rows 1 and 2) and P3 (rows 3 and 4). P1 received imiquimod for the final 2 months of the 6-month trial and continued this drug for 1 year after discontinuation of plerixafor. P3 received imiquimod from the start of the trial until 1 year after its conclusion. The patient is designated on the left of each row. “Before Plerixafor” pictures were taken before the start of the trial and “After Plerixafor” pictures were taken at the end of the trial after 6 months of treatment, while “Follow-up off Plerixafor” pictures were taken 9 months and 1 month after treatment with plerixafor for P1 and P3, respectively.
Plerixafor with imiquimod may improve warts in patients with WHIM syndrome. Images of representative warts that improved during the trial are shown before and after plerixafor treatment of P1 (rows 1 and 2) and P3 (rows 3 and 4). P1 received imiquimod for the final 2 months of the 6-month trial and continued this drug for 1 year after discontinuation of plerixafor. P3 received imiquimod from the start of the trial until 1 year after its conclusion. The patient is designated on the left of each row. “Before Plerixafor” pictures were taken before the start of the trial and “After Plerixafor” pictures were taken at the end of the trial after 6 months of treatment, while “Follow-up off Plerixafor” pictures were taken 9 months and 1 month after treatment with plerixafor for P1 and P3, respectively.
Long-term, low-dose plerixafor was well-tolerated in patients with WHIM syndrome
Only minor adverse effects could potentially be attributed to 6-month plerixafor administration, including occasional mild headaches, increased energy, and mild insomnia. Importantly, there were no local injection site reactions, anaphylactic or syncopal episodes, palpitations, premature ventricular contractions, other arrhythmias, electrocardiogram changes, or episodes of bone pain. Also not observed were changes in hematocrit, serum electrolytes, kidney function, or serum liver enzyme levels (supplemental Figures 3 and 4). All 3 patients had thrombocytopenia at baseline, which has been previously reported in WHIM syndrome, but this did not significantly change during low-dose long-term plerixafor treatment. P1 and P3 had histories of mild hyperglycemia, but both had mid-normal glucose levels while taking plerixafor. Splenic ultrasounds were performed before and after 6 months of therapy and did not reveal clear evidence of therapy-related enlargement (longitudinal measurements increased by less than 15% in all 3 patients, while transverse and height measurements decreased in P2 and P3) or focal lesions, and none of the patients experienced pain consistent with splenic pathology. All 3 patients expressed a strong interest in resuming plerixafor treatment.
Bone marrow data
Serial bone marrow biopsies and aspirates were performed before starting plerixafor and after 6 weeks and 6 months of treatment. We observed a decline in both neutrophil and CD19+ lymphocyte frequencies at 6 months compared with baseline in all 3 patients, consistent with these cells being mobilized into the blood. In contrast, monocyte frequency increased in all 3 patients, and CD3+ T-cell frequency increased in 2 of the 3 patients (supplemental Figure 5). All pretreatment marrows were markedly hypercellular for age (80% to 100%). The posttreatment marrows for P1 and P2 showed a relative reduction in overall marrow cellularity with areas of normocellularity (supplemental Figure 6). The frequency of CXCR4+ granulocytes increased in bone marrow from P1, but not from P2 or P3 (data not shown).
Discussion
In this study, we conducted the first investigation of the clinical safety and efficacy of long-term plerixafor therapy in any disease. We have focused on WHIM syndrome, because gain-of-function mutations in the G protein-coupled chemokine receptor CXCR4 are causal in this disease and because plerixafor is a specific CXCR4 antagonist already approved by the FDA for single high-dose use in stem cell mobilization. Our results extend our previous proof-of-concept phase 1 short-term dose-escalation study by showing that low-dose long-term plerixafor treatment appears to be safe and effective for at least 6 months in improving panleukopenia, the putative driver of clinical manifestations in the disease. Since correction of leukopenia serves as a simple biomarker for plerixafor action in vivo, these data illustrate the absence of drug desensitization long-term at the low dose used in this study. This study provides the first human chronic pharmacologic profile for considering plerixafor in the treatment of other chronic diseases, such as chronic inflammatory diseases or cancers that exhibit enhanced growth and metastasis via the CXCR4 pathway.27 One cannot infer, however, the safety of chronic high-dose plerixafor therapy, which should be approached with great caution, given the lethal phenotype of Cxcr4 deletion in mice19,21 and the toxicity reported during short-term high-dose human trials of plerixafor in cancer and HIV.15,22 Although only the most common CXCR4 mutation, R334X, was present in the 3 patients studied here, other disease-causing mutations are also in the same carboxyl-terminal region of CXCR4 and appear to lead to similar increases in receptor function.28 Therefore there is no reason to believe that the results reported here would not be generalizable to WHIM patients with other CXCR4 mutations or possibly even to the rare WHIM patients described who lack a CXCR4 mutation but have enhanced CXCR4 function.7
Our study did not attempt to optimize chronic plerixafor dosing in WHIM syndrome. However, the trial clearly shows that very low doses of plerixafor can be safe and effective long-term in some adult male and female patients with WHIM syndrome. The small difference between peak and trough ANC throughout the study and the unexpected slow fall to baseline leukocyte counts after drug was stopped at 6 months and slowly cleared from the circulation suggest that the dosage interval might be lengthened and yet maintain the ANC at a therapeutic level. Our results suggest that lengthening the dosing interval would result in a significant amount of time when the ALC is below the normal range because ALC continued to oscillate widely with each dose throughout the trial.
Our data also provide the first clinical end point efficacy data for plerixafor in WHIM syndrome, demonstrating a trend toward reduced infection frequency and decreasing the size of some warts. Although the effects we observed are promising, these data are limited to only 1.5 patient-years of study and must be confirmed with larger and longer clinical trials. The most striking observation was the absence of infection in P1 and P2 while they were taking plerixafor, a change from their pattern of frequent infections before and after the trial. The experience with P3 was less pronounced, although even his infection frequency during the trial was reduced by ∼40% compared with before and after. The marked reduction in sputum production of P1 and clearance of P aeruginosa colonization were also dramatic changes associated with plerixafor treatment. Since we have shown previously that neutrophils mobilized by plerixafor in WHIM syndrome patients have normal bactericidal and superoxide-generating capacity in vitro,18 these clinical effects suggest that the major problem in control of bacterial infection in the disease may relate to the number of circulating neutrophils. P3’s episode of dermatomal varicella zoster infection beginning at week 5 of treatment is interesting. He had a low baseline CD4+ T-cell count in the blood (154 vs 527 and 509 cells per microliter compared with P1 and P2, respectively), which remained low when evaluated at trough on plerixafor, and this could possibly have increased his susceptibility to VZV. Alternatively, VZV infection could represent a manifestation of immune reconstitution as reported in HIV/AIDS patients who have started antiretroviral treatment.29 Interestingly, his postinfection anti-VZV IgG antibody index was positive at 3.6 (>1.1 positive; Mayo Medical Laboratories Immunoassay) approximately 6 weeks after the infection, suggesting a humoral response.
We were unable to demonstrate improvement in Ig levels or vaccine responses in our patients, despite the fact that both B and T cells, including both CD4+ and CD8+ effector memory T cells, were mobilized to blood, and naive B cells appeared to be able to undergo class switching in vitro (data not shown). Perhaps dosing regimens that provide more sustained less oscillatory levels of these cells in blood are needed to enhance adaptive immune function. The full mechanism of action may be more complicated, since CXCR4 is also important in precisely directing B cell-T cell interaction in germinal centers, the site of antigen presentation, stimulation, and class switching, which could potentially be disrupted by plerixafor.30,31 Vaccination studies have identified effector memory T cells in mouse bone marrow that may serve as a reservoir,32,33 and this may also be true in humans.34 Thus, bone marrow is potentially a major source of all the major cell types mobilized to the blood by plerixafor. However, our serial biopsy/aspirate data were most consistent with bone marrow being a source for neutrophil and B-cell mobilization, since the frequency of these 2 cell types declined from baseline after 6 months of plerixafor treatment. In contrast, monocyte and T-cell frequencies appeared to rise. Other sources of mobilized cells are of course possible but difficult to define in human patient research.
Warts, which are caused by HPV, the signature pathogen in WHIM syndrome, may also respond to plerixafor therapy, since warts that were removed surgically from P2 before the trial did not recur during the trial and warts that were not removed surgically shrank or disappeared in P1 and P3 during treatment with plerixafor and imiquimod. Published and unpublished anecdotal observations suggest that surgically removed warts recur rapidly in WHIM syndrome and that imiquimod monotherapy for warts is ineffective.14 WHIM patients have been reported to have decreased circulating and skin dendritic cells, and CXCR4 and its ligand have been reported to be produced by infected keratinocytes.35,36 Thus, the effects of plerixafor may be complex; however, we believe that plerixafor stimulated the immune response against HPV because many lesions demonstrated an inflamed appearance prior to improvement or resolution. Some existing warts enlarged after treatment ended and new warts were seen. Although these results are encouraging, properly controlled clinical trials will be needed to determine whether plerixafor is an effective treatment of warts in this disease.
In conclusion, our study provides initial in vivo evidence supporting CXCR4 signaling as the critical molecular mechanism in patients with WHIM syndrome and justifies continued study of plerixafor as a potentially safe and effective mechanism-based treatment for adult patients with this disease. Side effects of plerixafor therapy were not observed in our patients, and plerixafor was better-tolerated than G-CSF. All 3 patients are alive and well at least 1 year after the trial ended and are eager to resume treatment. Future work will need to address long-term safety and efficacy in more patients, including children.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The investigators thank the patients with WHIM syndrome and their families who have volunteered to make this study possible.
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases and the Office of Rare Diseases Research, National Institutes of Health and was funded in part with federal funds from the National Cancer Institute, National Institutes of Health under Contract No. HHSN261200800001E. Plerixafor was provided at no cost for the study by Genzyme/Sanofi.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Authorship
Contribution: D.H.M., N.K., H.L.M., and P.M.M. performed protocol design; D.H.M., D.V., S.A.-O., J.U., N.K., J.S., M.A.M., R.L.G., M.O.E., P.L., M.M.M., D.H., R.D., G.J.G., S.T.H., S.P., K.R.C., P.S., E.W.C., H.L.M., and P.M.M. provided patient recruitment and care; Q.L., L.L., T.A.F., and D.A.L.P. generated and analyzed experimental data with supervision and analysis from D.H.M., P.M.M., D.B.K., and H.L.M.; and all authors participated (but D.H.M. and P.M.M. were principally responsible for) writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David H. McDermott, Building 10, Room 11N107, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; e-mail: dmcdermott@nih.gov.