Key Points
Persistent CLL cells during ibrutinib therapy show evidence of biochemical activation, but inhibited BCR and no proliferation.
Long lymphocytosis during ibrutinib therapy is not associated with adverse progression-free survival.
Abstract
The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib has outstanding activity in patients with chronic lymphocytic leukemia. Most patients experience lymphocytosis, representing lymphocyte egress from nodal compartments. This resolves within 8 months in the majority of patients, but a subgroup has lymphocytosis lasting >12 months. Here we report a detailed characterization of patients with persistent lymphocytosis during ibrutinib therapy. Signaling evaluation showed that while BTK is inhibited, downstream mediators of B-cell receptor (BCR) signaling are activated in persistent lymphocytes. These cells cannot be stimulated through the BCR and do not show evidence of target gene activation. Flow cytometry for κ and λ expression, IGHV sequencing, Zap-70 methylation, and targeted gene sequencing in these patients are identical at baseline and later time points, suggesting that persistent lymphocytes do not represent clonal evolution. In vitro treatment with targeted kinase inhibitors shows that they are not addicted to a single survival pathway. Finally, progression-free survival is not inferior for patients with prolonged lymphocytosis vs those with traditional responses. Thus, prolonged lymphocytosis is common following ibrutinib treatment, likely represents the persistence of a quiescent clone, and does not predict a subgroup of patients likely to relapse early.
Introduction
Chronic lymphocytic leukemia (CLL) is a common adult leukemia and is currently incurable outside of stem cell transplantation. Although chemoimmunotherapy has improved survival,1,2 patients who relapse have poor outcomes with additional standard therapies. Also, many standard therapies are associated with significant toxicities and sustained immunosuppression.3,4 Identifying effective therapies with better toxicity profiles is thus a high priority, and targeted therapies may allow attainment of this goal.
One broad target is the B-cell receptor (BCR) signaling pathway. In normal B cells, ligation of the BCR results in a signaling cascade that can lead to proliferation, apoptosis, or anergy depending on the stage of development and antigen ligated.5 In CLL cells, however, the BCR is dysregulated, and activation through antigen ligation or autostimulation results in the propagation of proliferative and prosurvival signals.6,7 Although multiple agents are in clinical development that target the BCR, one of the most exciting is the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib. Ibrutinib binds BTK irreversibly at the Cys481 residue in the active site, rendering it kinase inactive. This inhibition has been shown in vitro to induce modest CLL cell apoptosis and to abolish proliferation and BCR signaling.8,9 Clinical trial results with this agent have been outstanding, including an estimated 26-month progression-free survival (PFS) of 75% for patients with relapsed and refractory disease.10
Although PFS with ibrutinib is excellent, the overall response rate for this group of relapsed patients is only 71%,10 lagging behind the clinical benefit seen in 88% of patients because of lymphocytosis induced by this agent and all agents targeting the BCR pathway. BCR-associated lymphocytosis was first recognized with the SYK inhibitor fostamatinib and may be due to disruption of signaling through CXCR4-SDF1 and other adhesion factors in the marrow and nodal sites, leading to cell mobilization.11 Although this phenomenon has been recognized with fostamatinib, idelalisib,12 and now ibrutinib,13 the characteristics of these lymphocytes and the consequences of this lymphocytosis have been unexplored. In this report, we present the first data regarding the scope of lymphocytosis observed with ibrutinib and a detailed characterization of persistent lymphocytes relative to pretreatment lymphocytes. Also, we will report clinical outcomes associated with these patients to establish the clinical consequences of persistent lymphocytosis with ibrutinib.
Methods
Patient sample processing and cell culture
Blood was obtained from patients with relapsed CLL participating in institutional trials of ibrutinib who had provided informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the Institutional Review Board of The Ohio State University. All patients were treated with ibrutinib at doses of 420 or 840 mg daily and were on continuous therapy at the time when samples were collected. Peripheral blood mononuclear cells were isolated using methods detailed in the supplemental Methods on the Blood Web site. CD19+ cells were not specifically isolated; however, clinical flow cytometry was obtained in all patients at 6 and 12 months during the study. At 6 months, for the 19 patients whose samples were used in the experiments outlined, the average percentage of lymphocytes that were CLL cells was 93% (range, 83-99%), and at 12 months, the average was 88% (range, 72-96%).
Immunoblotting and real-time reverse-transcription–polymerase chain reaction
Whole cell lysates and RNA were prepared as previously described by our group.8 Nuclear and cytoplasmic lysates were prepared using the NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit (Thermo Scientific, Waltham, MA) according to manufacturer’s instructions.
Equivalent amounts of protein were separated on polyacrylamide gels and transferred onto nitrocellulose membranes. After antibody incubations, proteins were detected with chemiluminescent substrate (SuperSignal; Thermo Scientific).
Antibodies against phospho-BTK(Tyr223), phospho-AKT(Ser473), AKT, ERK1/2, phospho-PLCγ2(Tyr759), and PLCγ2 were obtained from Cell Signaling Technologies (Beverly, MA). Phospho-Erk(Thr202/Tyr204) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Millipore (Billerica, MA). BTK, lamin B, actin, and tubulin were purchased from Santa Cruz Biotechnologies (Dallas, TX).
RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's directions and converted to cDNA with the SuperScript First-Strand Synthesis System (Invitrogen). Real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed using Taqman primers from Applied Biosystems (Foster City, CA), and was performed on an Applied Biosystems Viia7 Real Time PCR System. The housekeeping gene GAPDH was used for normalization purposes. The expression of genes relative to GAPDH was calculated by plotting the cycle number (Ct) and the average relative expression for each group was determined using the comparative method (2ΔCt).
Flow cytometry
Peripheral blood mononuclear cells were thawed from frozen cryovials, washed, and suspended in staining media. Staining techniques can be found in the supplemental Methods. Cells were surface stained for 30 minutes and then for Ki67 staining were lysed and fixed prior to 30-minute incubation with κ and λ antibodies. Isotypes and appropriate controls were used in all cases and are outlined in the supplemental Methods. For κ/λ analysis, samples were run on a FACS-Calibur (Becton Dickinson, San Jose, CA) at the University of California, San Diego. For Ki67 analysis, samples were run on a FC500 flow cytometer equipped with CXP version 2B software (Beckman Coulter, Miami, FL) at The Ohio State University.
IGHV sequencing
The IGHV gene characterization and mutation status was assessed as previously described and detailed in the supplemental Methods.14,15 Most PCR products were sequenced directly, although in some cases, amplified products were cloned into pGEM-T (Promega, Madison, WI). Nucleotide sequences were analyzed using the ImMunoGenetic (IMGT) directory (European Bioinformatics Institute ImMunoGeneTics Informations System available at http://www.cines.fr/).16,17 The heavy chain complementarity-determining region was determined by the method of Kabat et al18 as defined by the number of amino acids between codon 94 at the end of framework 3 and the conserved Trp of position 102 at the beginning of framework 4.
Ion torrent sequencing
DNA was extracted from cryopreserved isolated CLL cells using a DNA extraction kit (QIAamp DNA Mini Kit; Qiagen) according to the manufacturer’s recommendations. DNA was quantified using the spectrophotometric method and standard 260/280 OD ratio. Analysis of 22 select genes and 2 additional select mutations was performed using the next-generation sequencing Ion Torrent platform and reagents from Life Technologies (Carlsbad, CA). The library was prepared with the Ion AmpliSeq Library kit2.0 with a custom-designed panel of AmpliSeq primers. DNA was amplified on GeneAmp PCR system 9700 Dual 96-well thermal cycler from Applied Biosystems. The PCR product was purified with Agencourt AMPure XP kit (Beckman Coulter). The library was quantified using real-time PCR with Ion Library TAQMAN Quantitation kit 44688022 on an Applied Biosystems ViiA7 Real Time PCR System to allow for optimal final dilution of the library for template preparation on a One Touch DL version instrument with a Ion One Touch 200Template Kit v2DL. The ion sphere particle enrichment and purification were performed on a One Touch ES using One Touch 200Template Kit v2DL. Purified ion sphere particles were analyzed on an Ion Torrent personal Genome Machine using the IonPGM 200 Sequencing kit and 316 chips. Data were collected and analyzed using the torrent server with torrent suite 3.6.2. Final analysis of sequence data was performed using a combination of software: Variant Caller v.3.6.63335, IonTorrent IGV3.6.033, and IonReporterUploader v.3.6.2-r62834. The entire length of sequences was reviewed manually using these programs to assess for deviation from reference sequence and to evaluate the quality of sequence and the depth of coverage. The depth of coverage ranged from 80 to 3000 for different amplicons.
Zap-70 methylation analysis
The methylation of ZAP-70 at CpG319 was assessed by pyrosequencing. Genomic DNA was bisulfite-treated using the Zymo EZ DNA Methylation-GoldKit (Zymo, Irvine, CA). Full details on PCR primers and conditions can be found in the supplemental Methods. The pyrosequencing assay was performed on a Pyromark Q96 MD machine (Qiagen, Gaithersburg, MD) with setting CDT0003 using 8 µL of the PCR product and the sequencing primer zap70_us_SEQ 5′-ATGAGTGAGAAATTTTGG-3′ following the standard Qiagen protocol with Qiagen Pyromark reagents, GE streptavidin Sepharose beads (GE Healthcare, Pittsburgh, PA), and the PyroMark Q96 Vacuum Workstation.
Statistical methods
Most experiments presented herein were conducted to test for differences in phosphorylation or total protein expression using densitometry, gene expression using RT-PCR, or percent viability of cells in patient samples with prolonged and persistent lymphocytosis, post-treatment with ibrutinib relative to pretreatment. Phosphorylated and total protein measures were log transformed, and differences in expression between the 2 time points were evaluated using random effects models with repeated measures; some patient samples were measured multiple times, and experiment number nested within the donor patient was included as a random effect. Differences in gene expression were evaluated on the δ CT scale using paired t tests. Results were transformed (2-∆CT) to represent fold changes post-treatment relative to baseline. Differences in percent viability between the 2 time points were also assessed using paired t tests. For all experiments with testing of multiple targets or agents, type I error was protected by adjusting P values using Holm’s method. Two-sided significance levels were set at α = 0.05.
Results
Prolonged lymphocytosis is common after ibrutinib and is associated with favorable prognostic features
Clinical data were obtained from all patients enrolled on PCYC-1102-CA, a multi-institutional phase 1b/2 trial of single agent ibrutinib in patients with relapsed or refractory CLL.10 We defined prolonged lymphocytosis as that which had not resolved to normal or <50% of baseline within 12 months to achieve a clear separation between patients with prolonged vs transient lymphocytosis. Among all patients (n = 85), lymphocytosis was common, occurring in 77%. The median time to lymphocyte normalization was 6.2 months (95% confidence interval [CI]: 4.4-8.1). At 12 months, 17 patients (20%) would be classified as a partial response (PR) except for lymphocytosis (PR-L), and an additional 49% of patients remained on drug with a documented PR or complete response (CR).
Baseline characteristics of these groups are outlined in Table 1. There was no difference between the groups in terms of age, gender, baseline WBC count, or Rai stage. Patients with PR-L were more likely to have favorable prognostic markers including a deletion of 13q (70.6% vs 35.7% for PR/CR patients, P = .010) and mutated IGHV (41.2% vs 11.9% for PR/CR patients, P = .015).
Persistent lymphocytes show evidence of BTK and PLCγ2 inhibition, but up-regulation of downstream mediators of BCR signaling
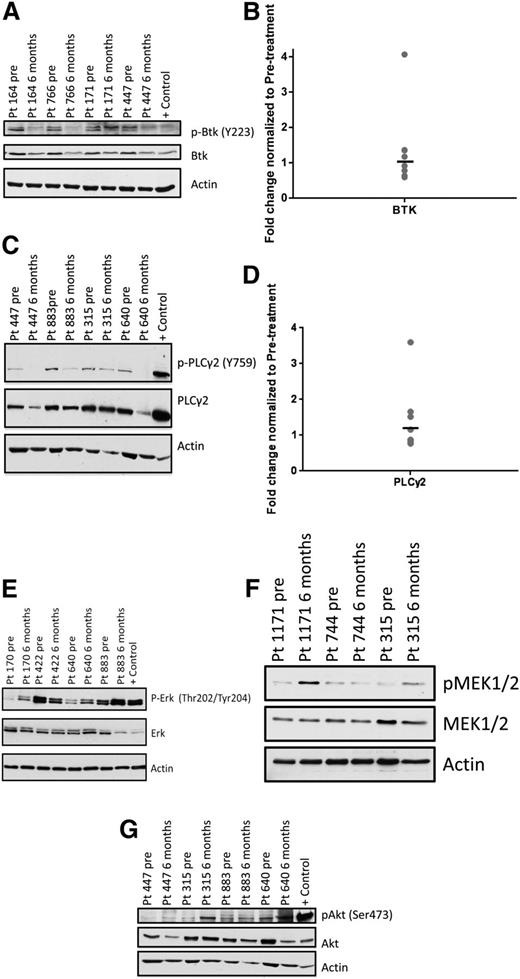
After 1 month of ibrutinib therapy, active forms of BCR signaling proteins are down-regulated (supplemental Figure 1). To determine whether BCR signaling is altered in persistent lymphocytes, we examined serial samples from patients with prolonged lymphocytosis by immunoblot at baseline and at 6 months after the initiation of ibrutinib. We found that BTK autophosphorylation at Y223 was down-regulated in 8 of 8 patients (Figure 1A), and BTK gene expression was not significantly changed (P = .65; Figure 1B). As well, PLCγ2, the immediate downstream target of BTK, showed diminished phosphorylation at Y759 compared with baseline in 7 of 9 patients (Figure 1C), whereas gene expression remained constant (P = .32; Figure 1D). These data confirm that BTK phosphorylation, a surrogate of kinase activity, and PLCγ2 phosphorylation remain inhibited by ibrutinib in most patients with persistent lymphocytosis.
Protein and gene expression of BCR signaling components in persistent lymphocytes compared with baseline. Phosphorylated and total protein expression of various signaling molecules of the BCR pathway and gene expression of BTK and PLCγ2 were investigated in patients with persistent lymphocytosis. Serial samples were taken at baseline and at 6 or 9 months after initiation of therapy. Each immunoblot shown is representative of multiple patients evaluated, and statistics presented in the text show the mean change of all evaluated patients. Number of patients evaluated was determined by amount of sample available. (A) pBTK (Y223), as well as (C) pPLCγ2 (Y759) decrease in the majority of patients after ibrutinib therapy. (E) pERK (Thr202/Tyr204), (F) pMEK1/2 (Ser217/221), and (G) pAKT (Ser473) increase in the majority of patients with prolonged lymphocytosis during ibrutinib therapy. Gene expression of (B) BTK and (D) PLCγ2 does not change over time in the majority of patients.
Protein and gene expression of BCR signaling components in persistent lymphocytes compared with baseline. Phosphorylated and total protein expression of various signaling molecules of the BCR pathway and gene expression of BTK and PLCγ2 were investigated in patients with persistent lymphocytosis. Serial samples were taken at baseline and at 6 or 9 months after initiation of therapy. Each immunoblot shown is representative of multiple patients evaluated, and statistics presented in the text show the mean change of all evaluated patients. Number of patients evaluated was determined by amount of sample available. (A) pBTK (Y223), as well as (C) pPLCγ2 (Y759) decrease in the majority of patients after ibrutinib therapy. (E) pERK (Thr202/Tyr204), (F) pMEK1/2 (Ser217/221), and (G) pAKT (Ser473) increase in the majority of patients with prolonged lymphocytosis during ibrutinib therapy. Gene expression of (B) BTK and (D) PLCγ2 does not change over time in the majority of patients.
We then evaluated the activity of ERK and AKT to determine whether the BCR signaling pathway remains inhibited distally. Surprisingly, we found that in 9 of 11 patients, ERK phosphorylation increases following ibrutinib (Figure 1E), with an average increase of 3.28-fold from baseline (95% CI: 1.75-6.14; P < .01). Also, proximal MEK1/2 shows increased phosphorylation after ibrutinib in 9 of 13 patients (Figure 1F), with an average increase of 2.34-fold from baseline (95% CI: 1.22-4.50; P = .02). Similarly, phosphorylated AKT is up-regulated in 6 of 7 patients (Figure 1G), with an average increase of 2.61-fold from baseline (95% CI: 1.12-6.11; P = .03). These data suggest that downstream signaling through ERK and AKT may be involved in the survival of these lymphocytes in vivo.
These immunoblot data are from baseline to a 6-month time point due to sample availability; however, we see that relative changes from baseline to 6 months are preserved in 12-month samples in a representative subset of patients (supplemental Figure 2).
In persistent lymphocytes, AKT and ERK continue to have mainly cytoplasmic localization and cannot be activated by proximal stimulation of BCR signaling, but can be activated by CD40L
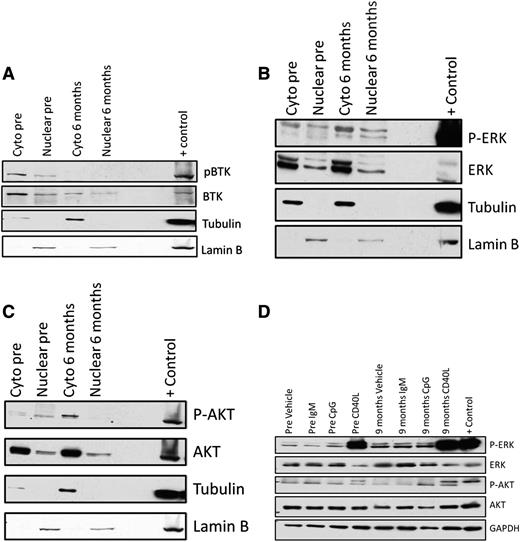
To determine whether cellular localization of BCR signaling proteins is altered in persistent lymphocytes, we prepared nuclear and cytoplasmic lysates on samples obtained at baseline and during ibrutinib therapy from 4 patients with persistent lymphocytosis. In all patients, phosphorylated BTK is present mainly in the cytoplasm prior to ibrutinib therapy and is significantly decreased or absent in persistent lymphocytes (Figure 2A). Consistent with whole cell lysates, phosphorylated ERK increased in all 4 patients and maintained the same localization pattern present pretherapy (Figure 2B). Phosphorylated AKT is more abundant in the cytoplasm in all patients, and pAKT increases in 2 of 4 patients (Figure 2C). These results show that, although downstream mediators of BCR signaling are amplified, cellular localization does not change, suggesting that there is not increased nuclear recruitment in these persistent cells.
Nuclear and cytoplasmic localization of BTK, ERK, and AKT in persistent lymphocytes following ibrutinib therapy and ability of persistent lymphocytes to stimulate. Nuclear and cytoplasmic localization of BCR signaling pathway proteins was evaluated using nuclear and cytoplasmic lysates from patients at baseline and after 6 months of ibrutinib therapy. In these representative immunoblots, there is no evidence of a shift toward nuclear localization of either phosphorylated or total (A) BTK, (B) ERK, or (C) AKT. (D) Patient samples at baseline and after 9 months of therapy were used to evaluate whether persistent lymphocytes can be stimulated through the BCR (15-minute exposure to plate-immobilized IgM; BD Pharmigen), TLR9 (3.2 μM CpG; Eurofins MWG Operon), or distal to BTK (1 μg/mL CD40L; PeproTech). (D) In this representative immunoblot, mild stimulation with CpG is seen at baseline along with robust stimulation following CD40L. After 9 months of ibrutinib therapy, CD40L alone is able to stimulate CLL cells.
Nuclear and cytoplasmic localization of BTK, ERK, and AKT in persistent lymphocytes following ibrutinib therapy and ability of persistent lymphocytes to stimulate. Nuclear and cytoplasmic localization of BCR signaling pathway proteins was evaluated using nuclear and cytoplasmic lysates from patients at baseline and after 6 months of ibrutinib therapy. In these representative immunoblots, there is no evidence of a shift toward nuclear localization of either phosphorylated or total (A) BTK, (B) ERK, or (C) AKT. (D) Patient samples at baseline and after 9 months of therapy were used to evaluate whether persistent lymphocytes can be stimulated through the BCR (15-minute exposure to plate-immobilized IgM; BD Pharmigen), TLR9 (3.2 μM CpG; Eurofins MWG Operon), or distal to BTK (1 μg/mL CD40L; PeproTech). (D) In this representative immunoblot, mild stimulation with CpG is seen at baseline along with robust stimulation following CD40L. After 9 months of ibrutinib therapy, CD40L alone is able to stimulate CLL cells.
With the up-regulation of ERK and AKT phosphorylation in persistent lymphocytes, we next investigated whether these cells can be stimulated through the BCR or TLR9, which both require BTK, or through CD40L, which activates downstream of BTK. In samples from 3 patients we stimulated cells obtained at baseline and 9 months into ibrutinib therapy. At baseline, these cells were often unable to be stimulated by IgM or CpG, and we found that persistent lymphocytes are unable to be stimulated by IgM or CpG, but can be stimulated by CD40L (Figure 2D), showing that BTK remains kinase inactive. This indicates that, although cells are unable to be stimulated by BTK-dependent pathways, they remain responsive to stimulation outside of BTK or the BCR.
Persistent lymphocytes do not show significant up-regulation of BCR target genes
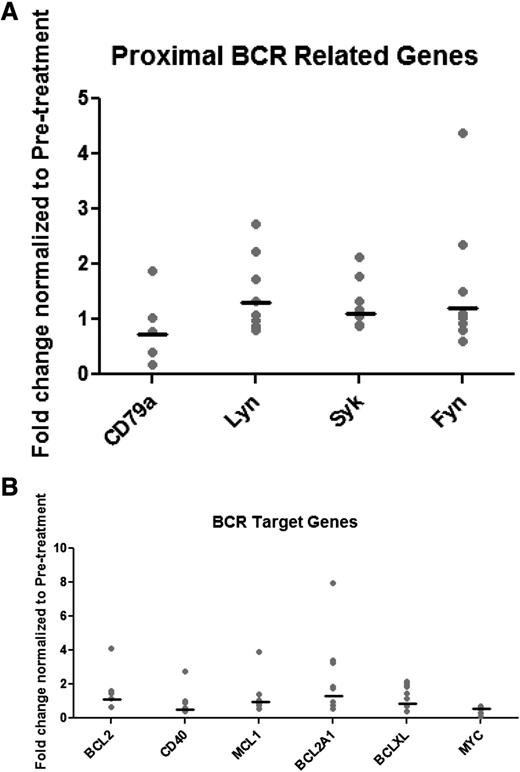
Next, we sought to determine whether there are distinct gene expression changes in cells from patients who experience persistent lymphocytosis after ibrutinib. From serial samples, we performed real-time RT-PCR to measure gene expression of proximal signaling molecules and targets of nuclear factor κB (NFκB). First, we evaluated gene expression of the components of the BCR signalosome: CD79, SYK, LYN, and FYN. We saw no significant differences between baseline and 12-month expression (Figure 3A). Next, we evaluated targets of ERK, AKT, and NFκB including BCL2 family genes, MCL1, XIAP, CFLAR, MYC, and CD40. Although we saw variability in the gene expression while on ibrutinib therapy, there were no consistent differences from baseline in the expression of these target genes (Figure 3B and supplemental Table 1). To validate, we also performed analysis of protein expression of MCL1, BCL2, and XIAP in 4 patients and again did not see consistent changes in target expression (supplemental Figure 3). These data suggest that, although we see evidence of signaling downstream of BTK, transcriptional consequences of this up-regulation are not apparent.
Real-time PCR of BCR pathway–associated genes in persistent lymphocytes. Patient samples at baseline and 12 months of ibrutinib therapy were evaluated for gene expression of proximal signaling molecules and target genes of ERK, AKT, and NFκB. There is no consistent change in gene expression of either (A) proximal BCR genes or (B) BCR target genes.
Real-time PCR of BCR pathway–associated genes in persistent lymphocytes. Patient samples at baseline and 12 months of ibrutinib therapy were evaluated for gene expression of proximal signaling molecules and target genes of ERK, AKT, and NFκB. There is no consistent change in gene expression of either (A) proximal BCR genes or (B) BCR target genes.
Persistent lymphocytes have no evidence of clonal diversification, clonal evolution, or differential Zap-70 methylation
To evaluate whether persistent lymphocytes represent an expanding subclone of the patient’s original CLL with different IGHV stereotypy or alternate κ/λ use, we performed flow cytometry for κ and λ expression and sequencing of the IGHV in 6 patients and targeted gene sequencing by Ion Torrent technology in 9 patients. κ/λ staining from baseline to the 12-month time point was identical in all patients (supplemental Table 2). Similarly, all patients had identical VH gene use and percent mutation of the IGHV both prior to ibrutinib and after 1 year of therapy (supplemental Table 2). Ion Torrent sequencing was used to detect mutations in 21 genes either known to be recurrently mutated in CLL or of interest with ibrutinib therapy. At 12 months, mutations and allelic frequency were compared with baseline, and none of the 9 patients had acquired additional mutations with a variant frequency of >5%. As well, no mutation at 12 months had a variant fraction that varied by >10% from baseline. Thus, these data suggest that there has been no global alteration in clonal diversity after ibrutinib treatment.
Methylation of Zap-70 at CpG3 has been shown to be an epigenetic modifier of Zap-70 transcription and therefore expression that serves as a strong biomarker of disease19 and is more reproducible than Zap-70 expression as assessed by intracellular flow cytometry. To determine whether Zap-70 at CpG3 was differentially methylated in persistent lymphocytes, we serially assessed for methylation of Zap-70 in 6 patients. In all patients, Zap-70 methylation did not change significantly after ibrutinib therapy (supplemental Table 3), showing that epigenetic alteration of Zap-70 is not altered in persistent lymphocytes.
Persistent lymphocytes are not actively proliferating and are not addicted to one signaling pathway
To determine whether persistent lymphocytes are actively proliferating, we performed flow cytometry for Ki67 on serial samples from 7 patients (supplemental Figure 4 and supplemental Table 4). Not surprisingly, Ki67 was low in all patients at baseline (median, 0.6%; range, 0.2%-3.3%). Ki67 was decreased from baseline in all patients at 9 months (median, 0%; range, 0%-0.1%; P < .05), demonstrating that persistent lymphocytes are not actively proliferating and indeed proliferation is inhibited similar to what is seen with ibrutinib in vitro and at early time points in vivo.8,20
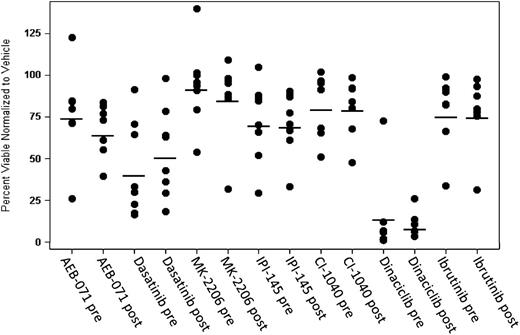
One area of interest surrounding the peripheral lymphocytosis with ibrutinib is whether this can be mitigated through combination therapy. Although combinations of ibrutinib and CD20 antibody therapy virtually eliminate lymphocytosis,21,22 this effect is almost certainly due to the single agent activity of the antibody and not due to a disruption of the underlying biology leading to lymphocytosis. To determine whether persistent lymphocytes are dependent for survival on 1 signaling pathway that could potentially be exploited by combination therapy, we took serial samples and exposed these cells in vitro to agents targeting the BCR pathway, looking for agents that were more cytotoxic after long-term ibrutinib therapy. LYN and BTK were targeted using dasatinib, PI3 kinase (p110γ and δ) was targeted with IPI-145, PKCβ was targeted with AEB-071, the MAP kinase pathway was targeted with the MEK1/2 inhibitor CI-1040, and AKT was targeted with MK-2206. Also, the cyclin-dependent kinase inhibitor dinaciclib was evaluated. As shown in Figure 4, none of these agents showed a differential effect pre- vs post-ibrutinib (P > .05 when adjusting for multiple tests; supplemental Table 5). Notably, dinaciclib showed significant cytotoxicity at both time points, suggesting that this could be a rational partner for combination therapy.
Persistent lymphocytes are not addicted to a single signaling pathway. To determine whether persistent lymphocytes were dependent on a single signaling pathway for survival, cells at baseline and 9 months from patients with persistent lymphocytosis were treated with various inhibitors of the BCR signaling pathway. AEB-071 was dosed at 1 μM, Dasatinib at 5 μM, MK2206 at 1 μM, IPI-145 at 1 μM, CI-1040 at 1 μM, and Dinaciclib at 1 μM. Dinaciclib was washed out at 2 hours. Annexin V/propidium iodide staining and flow cytometry were used to identify viable cells (Annexin negative/PI negative) after 72 hours. No single drug produced more effective cytotoxicity at a late time point compared with baseline. Dinaciclib, a cyclin-dependent kinase inhibitor, induced robust cytotoxicity both at baseline and at 9 months of ibrutinib therapy.
Persistent lymphocytes are not addicted to a single signaling pathway. To determine whether persistent lymphocytes were dependent on a single signaling pathway for survival, cells at baseline and 9 months from patients with persistent lymphocytosis were treated with various inhibitors of the BCR signaling pathway. AEB-071 was dosed at 1 μM, Dasatinib at 5 μM, MK2206 at 1 μM, IPI-145 at 1 μM, CI-1040 at 1 μM, and Dinaciclib at 1 μM. Dinaciclib was washed out at 2 hours. Annexin V/propidium iodide staining and flow cytometry were used to identify viable cells (Annexin negative/PI negative) after 72 hours. No single drug produced more effective cytotoxicity at a late time point compared with baseline. Dinaciclib, a cyclin-dependent kinase inhibitor, induced robust cytotoxicity both at baseline and at 9 months of ibrutinib therapy.
Prolonged lymphocytosis is not associated with adverse outcomes after ibrutinib therapy
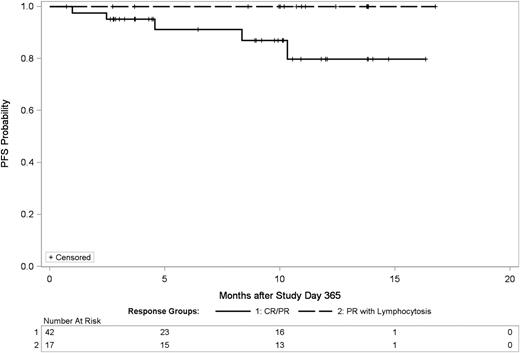
Given the minimal change in gene expression profile in persistent lymphocytosis and absent proliferation, we hypothesized that persistent lymphocytosis may have little impact in treatment outcome. This would contrast with what is typically observed with chemoimmunotherapy where gross disease typically is adversely associated with a shortened PFS. We examined PFS using a landmark analysis in patients achieving a PR-L at 365 days compared with those achieving an objective PR or CR by this time. This landmark analysis was performed at this time point to exclude patients with early death or drug discontinuation. As shown in Figure 5, PFS for patients with PR-L is not inferior to those patients who attain a traditional PR or CR prior to 12 months.
PFS of patients with persistent lymphocytosis is not inferior to those achieving complete or PR by 12 months. This is a landmark analysis at day 365 comparing patients with PR-L at 12 months vs those with CR/PR at 12 months. There is no statistical difference between these groups, although there is a trend toward improved survival in patients with PR-L.
PFS of patients with persistent lymphocytosis is not inferior to those achieving complete or PR by 12 months. This is a landmark analysis at day 365 comparing patients with PR-L at 12 months vs those with CR/PR at 12 months. There is no statistical difference between these groups, although there is a trend toward improved survival in patients with PR-L.
Discussion
In this study of patients who experience prolonged lymphocytosis following ibrutinib treatment, we report for the first time, patient characteristics, a detailed molecular characterization of persistent lymphocytes, and the PFS for patients with PR-L vs PR/CR. We see that prolonged lymphocytosis is relatively common following ibrutinib, and although there is some biochemical evidence of activation of these cells, the persistence of lymphocytosis ultimately does not appear to predict a group of patients likely to relapse.
Our laboratory data demonstrate that the majority of these cells exhibit constitutively active downstream BCR signaling, whereas BTK remains inhibited. Although these downstream pathways may indeed contribute to the survival of these cells, we do not see evidence of up-regulation of target genes and transcription factors implicated in survival and proliferation of CLL, and notably, proliferation is absent in these cells. It is also important that targeting of a single pathway in vitro does not lead to cell death, indicating that these cells are not addicted to a single survival pathway. We therefore postulate that these cells represent those CLL cells that are not dependent on proximal BCR signaling for survival and are thus resistant to ibrutinib-induced apoptosis. These cells do not represent the proliferative compartment, however, so their persistence does not lead to rapid disease progression. Additionally, our serial Ion Torrent analysis demonstrates no evidence of new recurring mutations or alternatively increasing clonal aberrations.
Phenotypically, these cells bear some resemblance to anergic B cells with constitutive phosphorylation of Erk1/2.23 However, the cells described here also show evidence of constitutive Akt activation, which is not a feature of this group. Despite this, given the benign clinical course of these patients, the phenotype that we describe here also likely represents a subgroup of quiescent anergic cells. Supporting this are recent data showing that primary CLL cells contain intraclonal subgroups that vary in the level of IgM responsiveness and that ibrutinib preferentially inhibits the most IgM-responsive cells,24 suggesting that these persistent lymphocytes may represent the subclone of cells that are the most anergic. Also, our baseline data from patients who develop persistent lymphocytosis demonstrate a lack of response to IgM stimulation, which is a hallmark of molecular anergy and may indicate that patients with an anergic phenotype at baseline are more likely to develop persistent lymphocytosis. However, the question of which patients are more likely to develop persistent lymphocytosis will likely not be answered until ibrutinib is more widely available and we are able to evaluate larger numbers of patients who have persistent vs transient lymphocytosis.
An alternative to the hypothesis that these persistent lymphocytes represent a group of cells that are resistant to ibrutinib-induced apoptosis is that these cells are those that formerly resided in the lymph nodes or bone marrow and thus represent the phenotype of more activated cells because of their former niche. This is unlikely, however, as previous studies demonstrated lymph node extrusion as early as 24 hours,20 yet analysis of signaling at early time points demonstrates reduced ERK phosphorylation (supplemental Figure 1).
Most interesting is the finding that patients with persistent lymphocytosis over 1 year have a similar PFS, with a trend toward a superior outcome compared with those who achieve an objective response within this time frame. Although more patients and longer follow-up will be necessary to validate these findings, these results are significant and worthy of further investigation. This could indicate that baseline characteristics that predict a favorable response are the same as those that predict persistent lymphocytosis. Alternatively, the persistence of a quiescent CLL clone may inhibit resistant subclone formation or expansion. At this time, combination therapies with ibrutinib are under investigation with the goal of hastening response and improving the rate of CR. The data presented here, however, suggest that these goals are not necessarily relevant. Although there is convincing evidence that the attainment of a CR and minimal residual disease negativity is important for long-term PFS with chemoimmunotherapy,25 with ibrutinib the elimination of MRD and even gross disease may not be necessary.
These findings also highlight a need to revise consensus response criteria in CLL. The preliminary success of ibrutinib in this disease is likely to lead to a paradigm shift toward targeted small molecules. The BCR pathway is now a validated target in this disease, and because we have seen that lymphocytosis is a class effect, it is very likely that more agents will come into clinical trials that also induce lymphocytosis. As these agents become more widely available and even standard, it is important that physicians understand that isolated progression of lymphocytosis, even when persistent for many months, is not a sign of disease progression, to prevent unnecessary drug discontinuation.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported in part by the Four Winds Foundation, the Leukemia and Lymphoma Society, D Warren Brown Foundation, Mr and Mrs Michael Thomas, Harry Mangurian Foundation, National Institutes of Health grants K23 CA178183-01, P50 CA140158, K12 CA133250-05, and R01 CA177292, and the Tissue Core of the CLL Research Consortium. Ibrutinib for in vitro experiments was provided by Pharmacyclics.
Authorship
Contribution: J.A.W., G.L., A.J.J., and J.C.B. designed and performed research, analyzed data, and wrote the manuscript; K.S., L.L.S., A.L., Y.Z., R.M., and K.W. performed research; D.L., W.Z., L.R., E.G., and T.J.K. designed and performed research and analyzed data; J.J., J.F., K.M., S.O., R.R.F., and J.C.B. contributed patients, collected data, and performed research; D.F.J. contributed aggregate data, collected data, and analyzed data; and A.S.R. and F.C. analyzed data.
Conflict-of-interest disclosure: J.A.W. and J.C.B. have served as unpaid consultants for Pharmacyclics. J.J., R.R.F., and T.J.K. have served as consultants for Pharmacyclics. T.J.K. has received research funding from Janssen. D.F.J. and F.C. are employees of Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Jennifer Woyach, 455A Wiseman Hall, 410 W 12th Ave, Columbus, OH 43210; e-mail: jennifer.woyach@osumc.edu.
References
Author notes
G.L., A.J.J., and J.C.B. contributed equally to this work.