In this issue of Blood, Woyach et al clarify that prolonged lymphocytosis is composed of biologically inert leukemic cells and does not anticipate poor outcome or relapse. Prolonged lymphocytosis may be perceived as a failure of chronic lymphocytic leukemia (CLL) treatment with ibrutinib.1
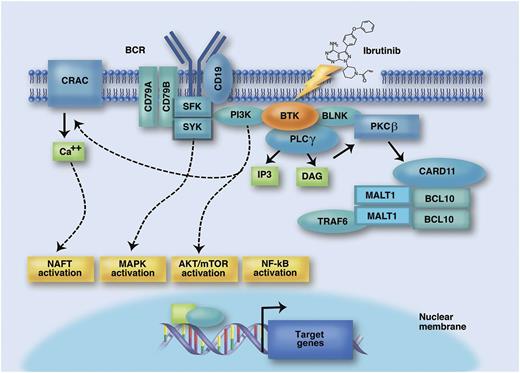
The BCR signaling pathway as a therapeutic target for the BTK inhibitor ibrutinib. The BCR is composed of a membrane immunoglobulin bound to CD79A and CD79B. Binding of the BCR by antigens/autoantigens recruit tyrosine kinases, including spleen tyrosine kinase (SYK) and Src family kinases (SFK), to the immunoreceptor tyrosine-based activation motif domain of CD79A/CD79B. This initial step translates into activation of a number of signal transduction molecules, including rat sarcoma viral oncogene homolog (RAF)-murine leukemia viral oncogene (RAF)-ERK/MAPK, PI3K, and BTK. In the PI3K arm of the pathway, AKT and mTOR relay PI3K activation to downstream targets and cell cycle regulation. On activation by the BCR, PI3K also promotes a sustained calcium uptake. One of the targets regulated by calcium elevation is the transcription factor nuclear factor of activated T cells (NFAT), which activates prosurvival genes in B cells. The BTK arm of the pathway induces phosphorylation of phospholipase C-gamma (PLCγ), which in turn promotes the production of the signaling mediators diacylglycerol (DAG) and inositol trisphosphate (IP3) and thus activates protein kinase C (PKC)β. Subsequently, PKCβ leads to phosphorylation of caspase recruitment domain family, member 11 (CARD11), recruitment of mucosa associated lymphoid tissue lymphoma translocation gene 1 (MALT1) and B-cell CLL/lymphoma 10 protein (BCL10) into a multiprotein complex, and initiation of NF-κB signaling, that ultimately activates a transcriptional program of survival, proliferation, and migration. Ibrutinib is a selective tyrosine kinase inhibitor that covalently and irreversibly binds BTK and consequently blocks BCR signaling and survival, proliferation, and migration of CLL cells. Professional illustration by Marie Dauenheimer.
The BCR signaling pathway as a therapeutic target for the BTK inhibitor ibrutinib. The BCR is composed of a membrane immunoglobulin bound to CD79A and CD79B. Binding of the BCR by antigens/autoantigens recruit tyrosine kinases, including spleen tyrosine kinase (SYK) and Src family kinases (SFK), to the immunoreceptor tyrosine-based activation motif domain of CD79A/CD79B. This initial step translates into activation of a number of signal transduction molecules, including rat sarcoma viral oncogene homolog (RAF)-murine leukemia viral oncogene (RAF)-ERK/MAPK, PI3K, and BTK. In the PI3K arm of the pathway, AKT and mTOR relay PI3K activation to downstream targets and cell cycle regulation. On activation by the BCR, PI3K also promotes a sustained calcium uptake. One of the targets regulated by calcium elevation is the transcription factor nuclear factor of activated T cells (NFAT), which activates prosurvival genes in B cells. The BTK arm of the pathway induces phosphorylation of phospholipase C-gamma (PLCγ), which in turn promotes the production of the signaling mediators diacylglycerol (DAG) and inositol trisphosphate (IP3) and thus activates protein kinase C (PKC)β. Subsequently, PKCβ leads to phosphorylation of caspase recruitment domain family, member 11 (CARD11), recruitment of mucosa associated lymphoid tissue lymphoma translocation gene 1 (MALT1) and B-cell CLL/lymphoma 10 protein (BCL10) into a multiprotein complex, and initiation of NF-κB signaling, that ultimately activates a transcriptional program of survival, proliferation, and migration. Ibrutinib is a selective tyrosine kinase inhibitor that covalently and irreversibly binds BTK and consequently blocks BCR signaling and survival, proliferation, and migration of CLL cells. Professional illustration by Marie Dauenheimer.
Beside the accumulation of genetic lesions,2 active signaling through the B-cell receptor (BCR) plays a central role in CLL pathogenesis and progression.3 The restricted and frequently stereotyped repertoire of the BCR in CLL has been interpreted as a result of BCR-driven selection initiated by specific antigens or autoantigens that may promote the expansion of the CLL clone and has been taken as the major proof of the addiction of CLL to BCR signaling.4 The Bruton’s tyrosine kinase (BTK) is at the crossroad of the BCR pathway (see figure).3 On BCR activation by the microenvironment, BTK transduces the signal to the phosphatidylinositol 3-kinase (PI3K)/murine thymoma viral oncogene homolog (AKT), mitogen-activated protein kinase (MAPK), and nuclear factor (NF)-κB pathways, resulting in prevention of apoptosis, as well as in promotion of cell adhesion, cell migration, and other cellular processes that can help survival and proliferation of leukemic cells.3 This strong biological rationale makes BTK an ideal target for therapy of CLL and other B-cell malignancies. Ibrutinib is a selective tyrosine kinase inhibitor that covalently and irreversibly binds BTK and consequently blocks survival, proliferation, and migration of CLL cells in in vitro models of the tumor microenvironment.3 Recently, ibrutinib has been shown to exert impressive clinical activity in CLL. In relapsed and refractory patients, ibrutinib monotherapy induces an estimated 26-month progression-free survival (PFS) in 75% of cases.5 In treatment-naïve patients, the drug leads to a projected 24-month PFS in 95% of patients.6 Beside antiproliferative activity, ibrutinib induces the redistribution of tissue-resident CLL cells into the blood with rapid shrinkage of the lymph nodes.5,6 In turn, this effect of cellular redistribution across anatomic compartments causes an increase of the absolute lymphocyte count in the peripheral blood.5,6 Such ibrutinib-induced lymphocytosis is transient in most patients, resolving within 8 months, but may be more prolonged in a fraction of CLL patients, lasting >12 months.1,5,6 The biological characteristics of the CLL cells egressed from the lymph nodes and sustaining ibrutinb-induced lymphocytosis, as well as the clinical impact of this phenomenon, have not been investigated in detail and are a matter of current debate.
By taking advantage of cases enrolled on the PCYC-1102-CA phase 1b/2 trial of single agent ibrutinib in patients with relapsed or refractory CLL, Woyach et al identify 20% of CLL cases that responded to ibrutinib but showed a prolonged lymphocytosis, defined as an elevation of the tumor lymphocyte count that had not resolved to normal or <50% of baseline within 12 months.1 Patients with prolonged lymphocytosis were more likely to carry mutated immunoglobulin genes, which is a favorable prognostic marker in CLL, and deletion of 13q. The genetics and immunogenetics profile of the leukemic cells composing the prolonged lymphocytosis did not vary significantly over time, indicating the lack of emergence of clonal diversity and selection in this clinical context. In patients with prolonged lymphocytosis, the delay in the clearance of the peripheral blood from leukemic cells was not due to a suboptimal inhibition of BTK activity by ibrutinib or to the selection of a resistant clone, because biochemical assays proved the effectiveness of ibrutinib in blocking phosphorylation of BTK targets.1 In contrast to the biochemical inhibition of BTK, downstream pathways, namely AKT and extracellular signal-regulated kinase (ERK), were still activated at enhanced levels in the lymphocytes of patients with prolonged lymphocytosis, conceivably because CLL cells still remain responsive to stimulation outside of BTK or the BCR.1 As suggested by Woyach et al,1 such BTK-independent activation of AKT and ERK might provide a biological explanation for the survival of lymphocytes in the peripheral blood of patients displaying prolonged lymphocytosis. Despite these clues of signaling downstream of BTK, lymphocytes composing the prolonged lymphocytosis observed during ibrutinib treatment appear to be transcriptionally inert, as revealed by the absence of variations in the BCR signalosome and BCR target gene expression program.1 Consistent with the biological features described above, CLL cells of prolonged lymphocytosis patients had a low mitotic index and were not actively proliferating, thus simulating a state of anergy and quiescence.1 The minimal change in gene expression profile combined to the absence of proliferation provide a biological rationale to the clinical observation that persistent lymphocytosis has no impact on ibrutinib treatment outcome and does not represent a risk factor for progression.1
Published guidelines define the outcome of CLL therapy based on surrogate markers of tumor burden reduction, including the decrease of the peripheral blood lymphocyte count, which enable the physician to assess the clinical benefit of a given CLL therapy.7 Under ibrutinib treatment, the emergence of lymphocytosis and its persistence for a long time, even in the presence of a clear clinical benefit for the patient, may confound response classification according to the current criteria and can generate the impression of resistance to treatment. The advent of new guidelines for ibrutinib-treated CLL is therefore desirable and important.8
As inhibitors of the BCR signaling become more widely available and enter the standard of care of CLL patients, it is important that physicians understand that lymphocytosis, even when persistent for many months, is not a sign of treatment resistance or disease aggressiveness, to prevent unnecessary drug discontinuation. The study by Woyach et al provides the proof of principle that prolonged lymphocytosis produced by ibrutinib is composed by quiescent leukemic cells and provides a biological rationale in support to the current revision of CLL response criteria.1,8
Conflict-of-interest disclosure: The authors declare no competing financial interests.