Abstract
Thromboses limited to infrapopliteal leg deep veins (isolated distal deep vein thrombosis [IDDVT]) are frequently diagnosed in subjects with suspected pulmonary embolism or DVT and account for one-fourth to one-half of all diagnosed leg DVTs. Despite their frequency, the natural history of IDDVTs and their real risk of thromboembolic complications are still uncertain because of the scarcity of prospective, blind, nonintervention studies. Therefore it is still debated whether they warrant diagnosis and treatment. Diagnosis is based on ultrasonographic examination, which is more operator dependent and less sensitive in distal than in proximal veins. The available data seem to support the view that most IDDVTs are self-limiting and inconsequential for patients, though in some cases they can be associated with complications and warrant diagnosis and treatment. The available guidelines for treatment of IDDVTs give different indications ranging from serial imaging of the deep veins for 2 weeks to detect and treat only in case of proximal extension, to giving oral anticoagulation in all IDDVT patients for 3 months. I review this issue, focusing on possible and suggested treatments in symptomatic IDDVT patients, and describe our current therapeutic approach to these patients.
Case 1: M.P.B.
M.P.B. is a white woman who last year, at 27 years old, presented to to our vascular emergency department for a pain in the left calf. The week before, she had a minor road accident when she was driving her motorcycle, with trauma to the left leg. Her personal history showed no important diseases or previous venous thromboembolism (VTE). At physical examination, her body mass index was 20.7, there was no edema in the symptomatic leg, but the pain, which was moderate at rest, increased at gentle palpation of the calf muscles. One point was attributed by the Wells score (interpreted as moderate risk).1 Blood was sampled for d-dimer testing (STA Liatest d-dimer; Diagnostica Stago, Asnieres, France) and scored just above the upper normal limit (540 ng/mL; normal values = <500 ng/mL). Ultrasonography (US) examination was then performed and showed isolated thrombus in the posterior tibial veins. A full anticoagulant dose of low-molecular-weight heparin (LMWH) was prescribed for 10 days, followed by half of the daily dose to complete one month of treatment; the wearing of a below-knee elastic stocking (class II) was recommended. At the end of treatment, no symptoms were reported and no US signs of thrombus seen.
Case 2: R.P.
R.P. is a 46-year-old white man who in July of this year was referred to our unit for a pain in the right calf that appeared 5 days prior. The day before the visit, ankle and calf swelling also appeared with the pain so his doctor advised him to come to our outpatient service. His personal history showed no important diseases, no previous thrombotic event, and no predisposing triggering factors. At physical examination, his body mass index was 27.7; the right calf measured 2 cm more than the contralateral; and the pain was present at rest, increasing at palpation. Two points were attributed by the Wells score (moderate risk). The d-dimer test was clearly abnormal (835 ng/mL; normal values = <500 ng/mL). At US examination, thrombi were detected in one gastrocnemius vein and in one peroneal vein. A full anticoagulant treatment with LMWH was started together with warfarin administration, with a program of 3 months anticoagulation (international normalized ratio [INR] 2.0-3.0); a below-knee elastic stocking (class II) was also prescribed. The treatment is still underway and the patient, who is currently feeling well, is waiting to finish anticoagulation therapy to repeat US examination.
Although both patients described here had thrombosis limited to infrapopliteal veins of the lower limbs (isolated distal deep vein thrombosis [IDDVT]), our therapeutic approach was different in the 2 cases. Although IDDVTs occur frequently, their clinical significance is still unclear; major disparities exist between countries (and between professionals within the same country) over their management both regarding how (and whether) to diagnose and how to treat. Indeed, IDDVT is presently one of the most debated issues in the field of venous thromboembolism (VTE) on account of the scarcity of scientific evidence available
In this manuscript, I briefly review what we know of the issue, focusing especially on the clinical risk in patients with IDDVT and proposed treatments, and I outline my current therapeutic approach to treating such patients.
The “distal” or “calf” deep veins
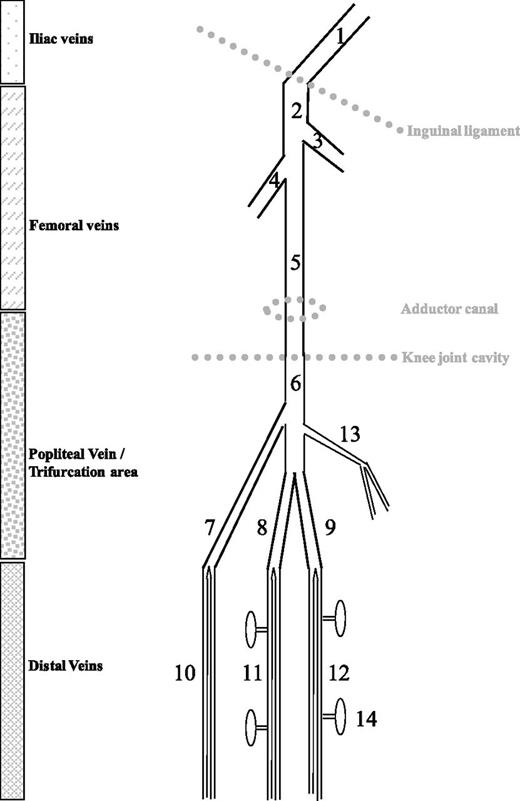
The word “distal” refers to the deep veins below the knee. Though anatomical variability is often the case, these veins include the paired peroneal, posterior tibial, and anterior tibial veins that accompany the corresponding arteries of the lower leg (Figure 1). These paired veins unify proximally into confluent segments, which form the trifurcation area before joining to become the popliteal vein. Because the confluent segments are easily identified at US examination of knee hollow, it is now standard to classify the trifurcation area as proximal.
Schematic representation of leg veins as discussed in this review. 1, External iliac vein; 2, common femoral vein; 3, greater saphenous vein; 4, profound femoral vein; 5, (superficial) femoral vein; 6, popliteal vein; 7, anterior tibial confluent segment; 8, posterior tibial confluent segment; 9, peroneal confluent segment; 10, anterior tibial veins; 11, posterior tibial veins; 12, peroneal veins; 13, gastrocnemius muscle veins (medial head); 14, soleus muscle veins. (From Palareti G, Schellong S. Isolated distal DVT: what we know and what we are doing. J Thromb Haemost. 2012;10:11-19)
Schematic representation of leg veins as discussed in this review. 1, External iliac vein; 2, common femoral vein; 3, greater saphenous vein; 4, profound femoral vein; 5, (superficial) femoral vein; 6, popliteal vein; 7, anterior tibial confluent segment; 8, posterior tibial confluent segment; 9, peroneal confluent segment; 10, anterior tibial veins; 11, posterior tibial veins; 12, peroneal veins; 13, gastrocnemius muscle veins (medial head); 14, soleus muscle veins. (From Palareti G, Schellong S. Isolated distal DVT: what we know and what we are doing. J Thromb Haemost. 2012;10:11-19)
The calf deep veins also include 2 groups of muscle veins: the soleal muscle veins, which are connected with the posterior tibial or peroneal veins, and the gastrocnemius muscle veins that drain into the popliteal vein. Although some authors2-4 claim that isolated calf muscle vein thrombosis (ICMVT) should be distinguished from IDDVT (thrombosis in the paired veins), most clinical contributions consider these anatomically distinct veins as a whole (and refer to them as IDDVT).
Diagnosis of proximal and distal DVT
Venous US investigation, based on the compression of veins (compression ultrasonography[CUS]), helped by color flow duplex imaging, is currently the method almost exclusively adopted for diagnosing DVT in routine clinical practice. However, 2 CUS strategies are currently performed in subjects with suspected leg DVT:
(1) Serial CUS examination of only proximal veins; this strategy is based on the premise that distal DVT may result in the risk of complications and may deserve treatment only if and when it expands to involve more proximal veins, an eventuality expected to occur in a minority of cases and within 1 to 2 weeks from initial symptoms. For this reason, CUS should be repeated in patients at risk during this interval period to detect possible extension of calf DVT to popliteal or more proximal veins. Obviously, this strategy does not look for and does not detect IDDVTs.
(2) Complete CUS examination of all deep veins examined in a single procedure able to detect all IDDVTs. This procedure is less simple than the former and requires more skillful, specially trained operators; a significantly lower sensitivity of CUS for IDDVT than for proximal DVT has been reported.5,6 However, the accuracy of CUS for IDDVT diagnosis greatly depends on the examination protocol used. As detailed elsewhere,7,8 after examining the proximal veins, the patient must sit upright with legs hanging down to maximize venous filling of the lower leg veins. In this position, the following segments are examined with CUS (5-MHz linear transducer, the color Doppler can be used to anatomically identify the posterior tibial and peroneal arteries) starting from the trifurcation of the popliteal vein, including the posterior tibial and the peroneal veins, the gastrocnemius (medial and lateral) veins, and the soleal (lateral and medial) veins. The anterior tibial veins are usually not investigated because they are rarely affected by thrombosis.8 All of these venous segments are investigated in transverse planes, gently compressing the probe every 1-cm interval from the proximal calf down to the ankle. Diagnosis of thrombosis is based on lack of compressibility of one or more of these venous segments.
Epidemiology
A series of factors accounts for the large variation of IDDVT prevalence in the literature. First is the diagnostic method and procedure adopted: venography, or US. Second are the different clinical settings: asymptomatic patients examined for DVT in clinical studies on surgical or medical patients; in- or outpatients examined for suspected DVT or pulmonary embolism (PE) or after diagnosis of PE, searching for a possible embolic source.
In studies investigating deep veins in the whole leg to assess the incidence of DVT in situations at risk, IDDVT was the most prevalent finding in asymptomatic patients. Interestingly, in the few studies comparing results obtained with venography or US in populations at risk, the prevalence of IDDVT was higher in US.11 In patients examined for suspected PE or leg DVT, the prevalence of IDDVT ranged between 7% to 11% in cases with suspected PE and 4% to 15% in suspected DVT, while it ranged between 23% and 59% in patients who had received a diagnosis of DVT.12 This large variation in the prevalence of IDDVT in patients with DVT may be, at least partially, attributed to the different patient populations investigated, as well as to the different diagnostic strategies and examination protocols adopted. If all patients with suspected DVT are examined with a complete CUS procedure, IDDVTs should account for about half of all DVTs diagnosed.10 Conversely, very few IDDVTs can be diagnosed if the diagnostic strategy is based on proximal vein examination only. Recent data from the Worcester VTE study showed an IDDVT prevalence of 11.1% in patients with leg DVT.13
Natural history and clinical relevance of IDDVT
Proximal extension
It is generally accepted, though with some exceptions,14 that most symptomatic DVTs start in the calf, from where they can extend to proximal veins, then become (more) symptomatic and at a higher risk for PE.15-18 The rate of extension of IDDVTs to proximal veins seems to be crucial because the embolic potential of these DVTs is generally considered to be much lower than that of proximal DVTs.19 Unfortunately, evidence on this issue is scanty, especially because in most studies, diagnosed symptomatic IDDVTs received anticoagulant treatment, masking the natural history of the disease. It has been reported that about one-quarter to one-third of IDDVTs extend proximally in the absence of treatment,20 rates that seem however excessive if seen in the light of recent reviews in the literature, which have reported extension rates of 10%,21 or of 8% to 15%22 in untreated patients. A recent prospective study showed that >90% of IDDVTs that were diagnosed but left untreated and monitored with serial CUS had complete resolution, whereas a proximal extension rate as low as about 3% was detected 5 to 7 days after diagnosis23 ; this rate is consistent with indirect data (1%-5.7%) deriving from clinical studies that adopted serial proximal CUS.24
The risk of pulmonary embolism
With regard to the reported risk of PE-associated IDDVT, studies in which calf DVTs were diagnosed in cases with PE at presentation should be distinguished from studies in which PE was detected during surveillance of patients with diagnosed IDDVT. In the first case PE association may be seriously overestimated.22 In the Italian Master registry, the presence of PE at presentation was paradoxically more frequently associated with IDDVT than proximal DVT (26.5% vs 19.9%, P < .05), a clear result of overestimation.9 Several reasons can account for this. First, many IDDVTs associated with PE may have formerly involved proximal veins. Furthermore, it cannot be excluded that a more thorough US investigation by examiners determined to detect a source of emboli after PE diagnosis, and looking extra carefully for distal thrombi, may lead to more calf DVT diagnosis. It is clear, therefore, that only prospective and blind studies can give reliable answers about the risk of PE associated with IDDVT. In our CALTHRO study, where the presence of IDDVT was kept blind to both patients and doctors in charge, only one patient (of 64 with untreated IDDVT, 1.6%) had a PE complication during the 3 months of follow-up.23 Similar rates of PE in IDDVT or proximal DVT were found in recent data from the Worcester VTE study (2.6% vs 1.8%, respectively).13
Recurrences
Many studies,25-28 though not all,13 have reported that IDDVT is associated with a lower risk of recurrence than proximal DVT or PE. Bilateral IDDVT, often associated with malignancy,29 seems to have a worse prognosis as evidenced by more frequent recurrences29,30 and increased mortality.29-31 The cumulative rate of recurrent VTE at 5-year follow-up was found to be 4.8-fold higher in patients with proximal than in those with distal DVT in a recent patient-level meta-analysis.32
Muscular or deep calf vein thromboses
The natural history of isolated calf muscle vein thrombosis (ICMVT) has not been fully elucidated, and some authors, though not all,33 have reported differences vs IDDVT in rates of extension or evolution toward postthrombotic syndrome (PTS), and in the need for anticoagulation.2-4 Special anatomical features of muscle vs deep veins may plausibly account for some of the differences. First, their diameter and length are smaller, which means the thrombus volume is smaller too; furthermore, soleal veins are connected with distal deep veins, instead of with popliteal veins, and so are farther away from proximal veins. Also, some specific risk factors seem to be more important for muscle vein thrombosis than for deep veins, including a particularly high rate of flight-associated ICMVT.34 However, the OPTIMEV study found no differences in risk factors, affected patient population characteristics, and prognosis at 3 months between ICMVT and IDDVT.31
Late sequelae
In the long term, IDDVT may lead to PTS disease.35-37 Signs and symptoms of venous insufficiency were detected after long-term follow-up in 37% of subjects with venographically diagnosed symptomatic IDDVT.38 Signs of PTS (comprehensive classification system/CEAP class 4-6) were found in 11% of patients after 5 years of follow-up.39 In a study on determinants of PTS, patients with IDDVT had a PTS score significantly lower than did those with femoral or iliac DVT.40 A recent prospective study showed that patients with proximal DVT had a relative risk of developing PTS of 2.3 (95% confidence interval [CI], 1.0–5.6) compared with patients with IDDVT, although the latter were not free from this late complication.41
In conclusion, it should be recognized that, because of the scarcity of prospective, blind, nonintervention studies, both the natural history of IDDVT and its clinical relevance are still not clearly understood. In general, however, it can be said that the proximal extension of IDDVT, although at rates much lower than previously reported, is not rare, and the disease—with or without extension—is not always free from acute and late complications.
Treatment
What the guidelines say
The high degree of uncertainty about the clinical relevance of and risks associated with IDDVT is clearly at the basis of the disagreement regarding the need for their diagnosis and treatment. The Consensus Conference of the American College of Chest Physicians published in 2008 recommended the same immediate and long-term (for at least 3 months) anticoagulant treatment of all diagnosed DVT (without any distinction between proximal and IDDVT).42 In contrast, the last published edition43 suggested serial imaging of the deep veins for 2 weeks over initial anticoagulation (grade 2C) in patients with acute IDDVT not presenting severe symptoms or risk factors for extension, which may include: positive d-dimer, thrombosis extensive, or close to the proximal veins (>5 cm in length, involves multiple veins, >7 mm in maximum diameter), no reversible provoking factor for DVT, active cancer, history of VTE, and inpatient status. In the presence of severe symptoms or any of the aforementioned conditions, anticoagulation is suggested (grade 2C) using the same approach as for patients with acute proximal DVT (Grade 1B). The last published (June 2012) guideline on venous thromboembolic diseases from the National Clinical Guideline Centre did not mention the treatment of IDDVT because the guideline “… focused on proximal DVT rather than isolated calf vein DVT as the latter is less likely to cause PTS than proximal DVT and also less likely to embolize to the lungs.”44 Conversely, in the International Consensus Statement on Prevention and Treatment of Venous Thromboembolism,45 it is stated that evidence “… indicates that oral anticoagulants should be given to all patients with symptomatic isolated calf DVT and that three months seems to be sufficient.”
What is done in studies and in clinical practice
These important guideline differences also give rise to a wide variability in management strategies adopted in clinical practice running from US screening alone to treatment with various heparin-derivative dosages and duration and conventional prolonged anticoagulation.
Only a few studies, of different design (retrospective or prospective) and in different clinical settings (post-surgery, in- or outpatient, thrombosis limited to the deep muscle veins), have addressed the issue of management in patients with confirmed IDDVT (see a recent review by Masuda46 ), often with inconsistent results. A recent meta-analysis of available controlled studies on anticoagulation (at least 1 month of a therapeutically dosed anticoagulant drug) in patients with IDDVT included only 8 studies (most of them judged to be of poor methodological quality) and concluded that anticoagulation therapy may reduce the incidence of PE and thrombus propagation, whereas bleeding events (that were reported sparsely in the studies) seemed to favor controls.47
Furthermore, study results are often discordant, as is the case with some recent studies on treatment of ICMVT, a condition that may be found in 20% to 40% of patients with calf vein DVT. Retrospective studies have signaled that these thromboses have a risk of extension to proximal veins.2,48 Schwarz et al in 2 subsequent studies reported different results. In the first study,49 they found few complications in patients receiving therapeutic doses of LMWH for 10 days plus compression therapy vs those receiving compression alone. In the second,4 no difference in 3-month progression rates was detected in low-risk patients randomized to 10 days of therapeutic LMWH doses and compression therapy or to compression therapy only. Finally, a very high rate of muscle vein thrombosis was recorded in a study involving patients who were hospitalized, and so whom by definition were at higher risk.3
Many studies reviewed by Righini et al21 and 2 more recent randomized studies in symptomatic patients50,51 compared the results of the 2 different diagnostic strategies—serial proximal CUS or single whole-leg vein examination—with only the latter being able to diagnose and treat IDDVT. They consistently found that 3-month thromboembolic risk did not differ in the 2 diagnostic approaches, indicating that it is not indispensable to diagnose and treat IDDVTs. We only partially agree with that conclusion, especially on the basis of the results of our recent CALTHRO study, which, to my knowledge, is the only one to date to have left patients untreated after calf DVT diagnosis and hence was able to provide insight into the natural history of the disease. In fact, although it was shown that the rate of proximal extension at one week of diagnosed but untreated IDDVT was much lower than expected (3.1%), the rate of complications at 3 months was nevertheless significantly higher in subjects with vs without calf DVT (7.8% vs 0.8%, P = .003), although the difference was not very significant after excluding 2 subjects with proximally extending calf DVT picked at the second serial CUS (4.7% vs 0.8%, P = .049).23 These data seem to support the view that, although most IDDVTs are self limiting and inconsequential for patients, a few are not free of risk and warrant diagnosis and treatment. The problem is that it is not easy to single out the symptomatic patients at higher risk for complications.
Personal views
In what follows, I would like to give some personal opinions on how my coworkers and I select diagnostic procedures in patients with suspected DVT and what sort of treatment we offer those with diagnosed IDDVT.
(1) I find it hard to agree with what was suggested by the American College of Clinical Pharmacy (ACCP) guideline regarding diagnosis and treatment of IDDVT: “If isolated distal DVT is detected on whole-leg US, we suggest serial testing to rule out proximal extension over treatment (Grade 2C)”.52 It is extremely difficult in everyday clinical practice—if not impossible, at least in my country—to diagnose and inform the patient (and his general practitioner) that he has venous thrombosis, albeit limited to a calf vein, without giving him appropriate therapy. First, the patient is already symptomatic and referred to the emergency vascular room with symptoms we ascribe to the presence of thrombosis; he expects to receive some kind of treatment to improve such symptoms. Second, after being informed of the presence of thrombosis, the patient should also be informed of the risks associated with this condition: the possible extension of the thrombosis and , although rare, occurrence of PE (with or without extension). Imparting this information without giving any treatment would cause anxiety in the patient and would disappoint the physician, who would probably in the meantime administer some therapy (in most cases heparin or derivatives). We know that this kind of treatment, if not given at therapeutic doses, is not fully curative and may interfere with serial US testing, because thrombus proximal extension can only be delayed but not completely averted.
It should be recognized, however, that ACCP guideline recommendations may have also been prompted by economic reasons (to limit the cost of anticoagulant treatment, which in some cases may even be unnecessary), and above all by a need to improve the lifestyle of patients, which is inevitably compromised by an anticoagulant course. I am therefore convinced that after IDDVT is diagnosed, proper treatment should be given. The main problem is the best diagnostic procedure to adopt in symptomatic outpatients because this will determine the number of IDDVTs diagnosed. Obviously, when whole-leg examination is used in all symptomatic patients, a consistent number of IDDVTs are diagnosed and 6% to 14% more thromboses can be expected.50,51
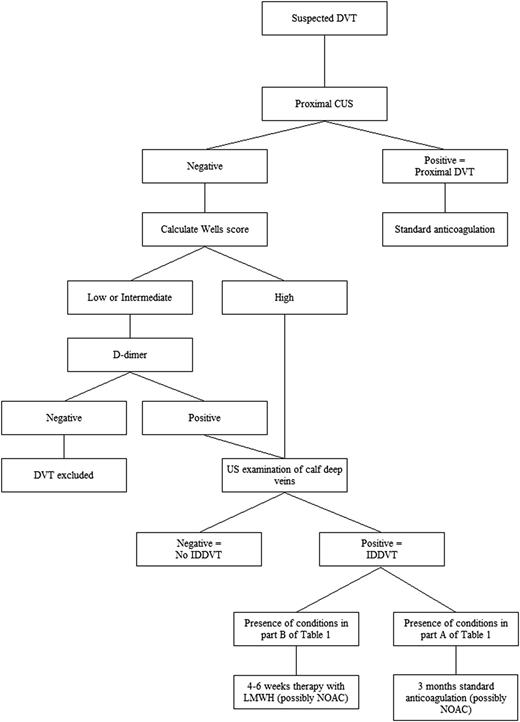
We believe that a better solution is to select a single diagnostic strategy based first on exclusion of proximal DVT in all symptomatic outpatients by using CUS, and then by extending US to calf veins only in those patients deemed to be at higher risk on the basis of probability scores and/or risk markers, such as increased d-dimer levels (Figure 2). This strategy would eliminate the need for serial CUS, reducing the chances of diagnosing a number of small distal thrombi that would expose patients to often unnecessary anticoagulant treatment. This procedure needs however to be tested by a prospective management study to assess the risk of DVT after a single negative whole-leg US examination,10,53 and to evaluate the value of the Wells score,54 the clinical decision-making rule most widely adopted, which was drawn up for proximal and not distal DVT and of d-dimer assay that may also have some problems in this regard.55 We recently found that Wells pretest clinical probability has low diagnostic accuracy for IDDVT, whereas d-dimer has a better negative predictive value.56
A similar strategy is currently under investigation by an international, multicenter trial (the Palladio study, proposed by Paolo Prandoni [Padua, Italy] and Walter Ageno [Varese, Italy]), and we will have to wait for its results to know whether it is effective and safe.
(2) Heparin, LMWH, or fondaparinux can be used for acute treatment of patients with IDDVT; they can be overlapped with vitamin K antagonists (VKAs), preferably in 3-month treatment periods, or they can be used alone in special conditions (such as the presence of cancer) or when the foreseen period of treatment is shorter. The cost of the drugs differs widely among countries and is often expensive (even after the introduction of generic enoxaparin, where available). The final choice may therefore be influenced by the cost-effectiveness of the drugs in relation to local marketing conditions that can make a drug less expensive than others.
(3) Several phase 3 clinical studies have confirmed the efficacy and safety of new oral anticoagulant drugs (direct thrombin [dabigatran] or factor Xa inhibitors [(rivaroxaban, apixaban, edoxaban]) for acute—monotherapy or after initial treatment with parenteral anticoagulants—and extended VTE treatment. Unfortunately, only proximal DVT has been included in these trials and therefore no data are currently available on the clinical outcome of IDDVTs treated with these drugs. It may be surmised, however that, when and where licensed and available in clinical practice for treatment of DVT, these new anticoagulants will also be used in IDDVT for a series of reasons: a single oral drug for the whole treatment (no need for initial parenteral anticoagulants, for some of these drugs), fixed dose, and no need for routine laboratory tests. In countries where one or more of these drugs is approved for the treatment of symptomatic DVT/PE, it is not stipulated that DVT must be proximal and so their use for symptomatic IDDVT is not considered to be “off-label.” Unfortunately, at the moment (December 2013) only one drug (rivaroxaban; Xarelto) has been licensed (since only October 2013) for VTE treatment in Italy; hence we have almost no experience at all on its use in IDDVT. My personal view is that the use of a direct oral anticoagulant such as monotherapy would be highly preferable to parenteral anticoagulation for 4 to 6 weeks, or for initial anticoagulation therapy followed by warfarin for 3 months. However, the necessary doses and duration of treatment remain to be assessed in patients with secondary or unprovoked IDDVT. Studies specifically devoted to the use of these drugs in patients with IDDVTs are needed to assess their efficacy and safety in treating these patients and to limiting as much as possible the burden for patients while keeping costs for health services to a necessary minimum.
Suggested diagnostic/therapeutic algorithm in outpatients with suspected acute deep vein thrombosis of the lower limbs. The algorithm is based on the common availability of US investigation in vascular units and on placing a high value on avoiding the inconvenience of repeat imaging in the days after the first examination. NOAC, new oral anticoagulants.
Suggested diagnostic/therapeutic algorithm in outpatients with suspected acute deep vein thrombosis of the lower limbs. The algorithm is based on the common availability of US investigation in vascular units and on placing a high value on avoiding the inconvenience of repeat imaging in the days after the first examination. NOAC, new oral anticoagulants.
How I treat IDDVT
Once diagnosed in symptomatic outpatients, all IDDVTs should receive anticoagulant treatment. In our institution my coworkers and I regulate the type, dose, and duration of treatment depending on a series of factors: history of VTE, nature of the event (idiopathic or secondary), extension of thrombosis, and presence of important predisposing diseases (Figure 2). All of these conditions, risk factors for extension, and potential complications are similar to those mentioned by the authors of the last ACCP guidelines,43 the only difference being that they suggest using the presence or absence of these factors as a criterion for deciding whether to give anticoagulation or to proceed with serial imaging alone. They recommend using the same approach (for duration and intensity) as in patients with acute proximal DVT. In contrast, our approach is based on the use of these conditions as criteria for regulating the type and duration of anticoagulation, because we believe there is evidence for shorter anticoagulation (and probably even less intense) in many IDDVTs.26,29,57
Our usual practical therapeutic approach to outpatients with IDDVT is first to exclude contraindications for anticoagulant treatment—such as major or nonmajor but clinically relevant bleeds or serious bleeding diseases, marked thrombocytopenia, or renal insufficiency—and then to start the following treatment.
(1) In the presence of one of the conditions listed in Table 1, part A, we start immediately with a full dose of one parenteral anticoagulant, usually LMWH, and overlap this with VKAs (INR 2.0-3.0), for a 3-month period. In patients with cancer and in pregnant women, we do not give VKAs and continue to administer full-dose LMWH for the first month before lowering the dose to 50% to 70% for the next 2 months. Then if antithrombotic protection is still deemed necessary, a prophylactic LMWH dose is administered.
(2) In patients with the conditions shown in Table 1, part B, we start with a full dose of one parenteral anticoagulant, usually LMWH, for the first 7 to 10 days and then we lower the dose to 50% for the next days to cover 1 month total of therapy.
(3) All patients are recommended to wear a below-knee class II elastic stocking in the symptomatic leg, a measure reported to be highly curative in itself.4,23 The patients undergo US examination at the end of anticoagulation to assess IDDVT evolution, to have a basal picture of deep calf veins in the case new symptoms/signs have occurred, suggesting possible DVT recurrence.
Conditions or risk factors that should be considered when selecting the therapeutic approach in symptomatic outpatients with diagnosed IDDVT
| A) Conditions favoring full anticoagulation for 3 mo (as for proximal DVT) . |
|---|
| Previous VTE events |
| Idiopathic event |
| Secondary event but with persistently hampered complete mobilization |
| Event occurring during pregnancy or puerperium |
| Distal thrombosis involving the popliteal trifurcation |
| Thrombosis involving >1 calf vein |
| Distal thrombosis present in both legs |
| Active cancer or chemotherapy |
| Presence of predisposing diseases (eg, inflammatory bowel diseases) |
| Known thrombophilic alterations |
| B) Conditions favoring shorter anticoagulation |
| First thrombotic event, if: |
| secondary to surgery or to other removable risk factors (eg, plasters, immobilization, trauma, long trip) |
| event occurring during contraceptive or replacement hormonal therapy (provided the therapy has been interrupted) |
| with subsequent full mobilization |
| no difference between muscular or tibial-peroneal veins |
| A) Conditions favoring full anticoagulation for 3 mo (as for proximal DVT) . |
|---|
| Previous VTE events |
| Idiopathic event |
| Secondary event but with persistently hampered complete mobilization |
| Event occurring during pregnancy or puerperium |
| Distal thrombosis involving the popliteal trifurcation |
| Thrombosis involving >1 calf vein |
| Distal thrombosis present in both legs |
| Active cancer or chemotherapy |
| Presence of predisposing diseases (eg, inflammatory bowel diseases) |
| Known thrombophilic alterations |
| B) Conditions favoring shorter anticoagulation |
| First thrombotic event, if: |
| secondary to surgery or to other removable risk factors (eg, plasters, immobilization, trauma, long trip) |
| event occurring during contraceptive or replacement hormonal therapy (provided the therapy has been interrupted) |
| with subsequent full mobilization |
| no difference between muscular or tibial-peroneal veins |
The treatment approaches in cases 1 and 2 were different because in case 1 a trigger factor was present (a trauma in the calf, albeit minor), only one deep vein was affected, there was no immobilization or walking impairment; thus we gave the treatment described in paragraph 2. In contrast, in case 2 the event was idiopathic, more than one deep vein was involved, symptoms were relatively important, and walking and mobilization was at least in part hampered, which led us to prescribe complete anticoagulation, starting with therapeutic LMWH doses overlapped with VKAs for a duration of 3 months.
Finally, I expect the very recent availability of direct oral anticoagulants for acute treatment of DVT (at the moment only rivaroxaban is available in my country for this indication) will shortly prompt a change in our therapeutic approach to patients with IDDVT. As I have mentioned, monotherapy is highly preferable to parenteral anticoagulation followed or not followed by a 3-month course of warfarin. However, I believe the initial and long-term doses and duration of treatment in these patients, issues not covered at all by phase 3 clinical trials, remain to be assessed. I would therefore prefer to offer these patients a treatment within studies specifically devoted to assess use of these drugs in this particular type of venous thrombosis.
Authorship
Contribution: G.P. wrote and approved the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Gualtiero Palareti, University Hospital S. Orsola-Malpighi, Bologna, Via Massarenti 9, 40138 Bologna, Italy; e-mail: Gualtiero.palareti@unibo.it or gualtiero.palareti@gmail.com.