Abstract
It takes about 3 months to complete “active treatment” of venous thromboembolism (VTE), with further treatment serving to prevent new episodes of thrombosis (“pure secondary prevention”). Consequently, VTE should generally be treated for either 3 months or indefinitely (exceptions will be described in the text). The decision to stop anticoagulants at 3 months or to treat indefinitely is dominated by the long-term risk of recurrence, and secondarily influenced by the risk of bleeding and by patient preference. VTE provoked by a reversible risk factor, or a first unprovoked isolated distal (calf) deep vein thrombosis (DVT), has a low risk of recurrence and is usually treated for 3 months. VTE associated with active cancer, or a second unprovoked VTE, has a high risk of recurrence and is usually treated indefinitely. The decision to stop anticoagulants at 3 months or to treat indefinitely is more finely balanced after a first unprovoked proximal DVT or pulmonary embolism (PE). Indefinite anticoagulation is often chosen if there is a low risk of bleeding, whereas anticoagulation is usually stopped at 3 months if there is a high risk of bleeding. The decision to continue anticoagulation indefinitely after a first unprovoked proximal DVT or PE is strengthened if the patient is male, the index event was PE rather than DVT, and/or d-dimer testing is positive 1 month after stopping anticoagulant therapy.
Anticoagulant therapy is the mainstay for the treatment of venous thromboembolism (VTE). Once treatment is started, the question arises as to how long patients should be treated, which is the focus of this perspective. Our recommendations build on those of the American College of Chest Physician’s Evidence-Based Clinical Practice Guidelines for the Treatment of VTE (hereafter referred to as “ACCP guidelines”), and we thank our copanelists for helping to shape our thoughts on this topic.1 Those guidelines also provide recommendations for duration of anticoagulant therapy in patients with upper limb deep vein thrombosis (DVT), superficial vein thrombosis, and thrombosis in unusual sites; topics that will not be addressed here.
Patients should either stop anticoagulants when the acute episode of VTE has been adequately treated or remain on treatment indefinitely
This section summarizes evidence that it takes a finite period, generally 3 months, to complete treatment of an acute episode of VTE; we will refer to this as “active treatment.”1,2 The goal of active treatment is to suppress the acute episode of thrombosis, whereas the aim of subsequent anticoagulation is to prevent new episodes of VTE that are unrelated to the index event; we will refer to this latter treatment as “pure secondary prevention.” Active treatment and secondary prevention overlap; initially, however, treatment of the acute episode of VTE is the priority.
The concept of 2 overlapping phases of anticoagulation for VTE has important management implications. If the goal is to reduce the risk of recurrence after a time-limited course of anticoagulation to as low a level as possible, treatment should be stopped once active treatment is completed. If, however, the risk of recurrence after completion of active treatment remains unacceptably high, indefinite anticoagulation is indicated (termed “extended anticoagulation” in the ACCP guidelines1 ). Indefinite anticoagulation refers to continued treatment without a scheduled stopping date; treatment is stopped only if the risk of bleeding increases or anticoagulation becomes excessively burdensome.
Three months completes “active treatment” and should usually be the duration of “time-limited” treatment
If anticoagulants are stopped before active treatment is completed, the risk of recurrent VTE is higher than if treatment was stopped after its completion.2,3 The excess episodes are due to reactivation of the initial thrombus. Extending anticoagulation beyond “active treatment” prevents recurrence while patients are treated, but does not further reduce the risk of recurrence after treatment is stopped. Randomization of patients to different time-limited durations of anticoagulation, with subsequent follow-up to determine the rate of recurrence in each group after anticoagulants are stopped, provides the best evidence on the duration required to complete “active treatment.” These trials are summarized in the following sections.
Four or 6 weeks vs 3 months or longer
Five randomized trials compared 4 to 6 weeks of anticoagulation with 3 to 6 months of therapy.4-8 Meta-analysis of their findings found that the shorter course of therapy was associated with about a twofold increase in recurrence during 9 to 24 months of follow-up (relative risk, 1.83; 95% confidence interval [CI], 1.39-2.42; follow-up included the period when 1 group was on, and the other was off, anticoagulants).1 Analysis of individual patient data from 4 of these trials4,6-8 demonstrated that the risk of recurrence after stopping anticoagulant therapy was higher in patients who were treated for 4 to 6 weeks than in those treated for 3 months or more (hazard ratio, 1.52; 95% CI, 1.14-2.02).3 Furthermore, the excess recurrences with 4 to 6 weeks of therapy were confined in the first 6 months after stopping therapy3 and, in those with a DVT, the extra recurrences were in the same leg as the initial event.9 These data indicate that 4 to 6 weeks of anticoagulation is insufficient for “active treatment” and support the concept that early recurrences reflect inadequate suppression of coagulation at the site of the initial thrombus.
Three months vs 6 months or longer
Four randomized trials compared 3 months of anticoagulation with 6 to 12 months of therapy.6,10-12 Meta-analysis of their findings found a similar risk of recurrence with 3 months compared with 6 to 12 months of therapy during 1 to 3 years of follow-up (relative risk, 1.12; 95% CI, 0.88-1.45).1 Analysis of individual patient data from these 4 trials, and another study that compared 3 months with 27 months of anticoagulation,13 also found no convincing increase in the risk of recurrence after treatment was stopped in patients treated for 3 months (hazard ratio, 1.19; 95% CI, 0.86-1.65).3 These data suggest that 3 months of anticoagulation is long enough to complete “active treatment.”
Subgroups for which <3 months might be adequate for “active treatment”
It is logical that it may not take as long to complete active treatment in patients with small thrombi provoked by a factor that rapidly resolves. Consistent with this hypothesis, patients with isolated distal DVT provoked by a temporary risk factor, such as recent surgery, did not appear to have a higher risk of recurrence if treatment was stopped at 4 or 6 weeks compared with at 3 months or longer (hazard ratio, 0.36; 95% CI, 0.09-1.54).3 Although 4 or 6 weeks of anticoagulation may complete active treatment in patients with a small thrombus and a reversible provoking factor, this was not evident when only 1 of these 2 factors applied.3
We generally treat patients with isolated distal DVT provoked by a transient risk factor for 3 months because: (1) there is uncertainty whether 4 to 6 weeks of treatment is adequate and (2) we only look for and treat isolated distal DVT if patients have severe leg symptoms.
Subgroups for which >3 months might be required to complete active treatment
It is also logical that it may take longer to complete active treatment in patients with more extensive thrombosis who do not have reversible provoking factors. Consistent with this hypothesis, patients with unprovoked proximal DVT or pulmonary embolism (PE) may have a lower risk of recurrence if they stop treatment after 6 or more months compared with at 3 months (hazard ratio, 0.59 [95% CI, 0.35-0.98] for the first 6 months, and a hazard ratio of 0.72 [95% CI, 0.48-1.04] for the first 24 months of follow-up).3 The duration required to complete active treatment in patients with iliac DVT or cancer-associated VTE has not specifically been evaluated.
Many patients with a first unprovoked proximal DVT or PE are treated indefinitely (see “Unprovoked VTE: recommendations”).1 Reasons not to treat indefinitely include a lower than average risk of recurrence, a high risk of bleeding, and patient preference. These are also factors that support treatment of 3 rather than 6 months in patients who are not treated indefinitely. Furthermore, the trials that compared 3 months with 6 to 12 months of anticoagulation (mostly patients with unprovoked VTE)6,10-12 found more major bleeding (relative risk, 2.49; 95% CI, 1.20-5.16) with longer therapy.1 For these reasons, if patients with a first unprovoked proximal DVT or PE are not treated indefinitely, we generally stop anticoagulants at 3 rather than 6 months.
Benefits and risks of indefinite anticoagulant therapy
Type of evidence available
No trial has randomized patients with VTE, with or without cancer, to stop or continue anticoagulants and then followed patients indefinitely (eg, for 10 or more years). Available studies anticoagulated all patients for 3 or 6 months, randomized half to stop and half to continue anticoagulants from that time point, and followed the 2 groups while the extended therapy group was being treated (ie, 1-4 years). If patients in the extended therapy group then stopped anticoagulants, which was often the case, they were not subsequently followed. The studies were heterogeneous with respect to: when randomization and follow-up started (at diagnosis or after the initial common period of treatment); study populations; type and intensity of anticoagulant; use of placebo; assessment of bleeding in the nonanticoagulated group, including if they had a recurrent VTE and restarted anticoagulants; and whether patients were followed for the same or for a variable length of time.
These studies were designed to assess efficacy of treatment for prevention of recurrent VTE; they were not powered to assess mortality. Consequently, evidence for or against indefinite anticoagulation in different subgroups of patients with VTE is based on estimating the absolute reduction in recurrent VTE and the increase in major bleeding with indefinite anticoagulation, and then estimating their combined effect on mortality. However, many of the assumptions used in these calculations are uncertain.
Reduction of recurrent VTE with indefinite anticoagulation: efficacy
Indefinite anticoagulation with a vitamin K antagonist (VKA; dose-adjusted to achieve a target international normalized ratio [INR] of 2.5) reduces recurrent VTE by ∼90% (based on meta-analysis of 4 studies13-16 : relative risk, 0.12; 95% CI, 0.05-0.25),1 with about half of the recurrent episodes occurring in patients who had prematurely stopped therapy. Direct and indirect comparisons have found similar reductions in recurrent VTE with extended anticoagulation using dabigatran (150 mg twice-daily),17 rivaroxaban (20 mg daily),18 or apixaban (2.5 mg or 5 mg twice-daily).19,20 Extended treatment with low-molecular-weight-heparin (LMWH) is also very effective, and is more effective than a VKA in cancer patients.1,21,22
Increase in major bleeding with extended anticoagulation: safety
Anticoagulation with VKAs is associated with about a 2.6-fold increase in major bleeding (based on 4 studies13-16 : relative risk, 2.63; 95% CI, 1.02-6.78). Compared with VKAs, the new oral anticoagulants are associated with about half the risk of intracranial bleeding, a smaller reduction in all extracranial bleeding, and no reduction or an increase in gastrointestinal bleeding (∼50% higher with dabigatran and rivaroxaban).20,23-25
Consequences of a recurrent VTE or a major bleed
The most important consequence of a recurrent VTE or a major bleed is that it may be fatal. In prospective studies, case fatality has been estimated as 3.6% for a recurrent VTE and 11.3% for a major bleed on a VKA.26 There is uncertainty about these estimates. Fatal PE may occur more often outside of prospective studies because early detection and treatment of recurrent DVT and PE is less likely, and the 11.3% estimate for the case fatality of major bleeding is based on data from initial rather than extended therapy. Also, because a recurrence is 3 times as likely to be a PE if the initial event was a PE rather than a DVT, case fatality for recurrent VTE may be substantially higher (perhaps double) when the initial VTE was a PE.27,28
Which patients should stop anticoagulants at 3 months and which should remain on anticoagulants indefinitely?
This decision is dominated by the risk of recurrent VTE. Risk of bleeding is secondary because: (1) with a low risk of recurrent VTE (eg, patients with a reversible provoking factor), anticoagulants are stopped at 3 months even if the bleeding risk is low; (2) with a high risk of recurrent VTE (eg, patients with cancer), anticoagulants are usually continued even if bleeding risk is high; (3) with the exception of advanced age, risk factors for bleeding are not common in patients with unprovoked VTE, the subgroup in whom bleeding risk is most influential33,34 ; and (4) the risk of bleeding is difficult to predict.35,36
Risk of recurrent VTE in individual patients if anticoagulants are stopped
Primary estimator: provoking factor for the VTE
VTE provoked by a major reversible risk factor, such as recent surgery, has a very low risk of recurrence that is estimated to be 1% within 1 year and 3% within 5 years of stopping therapy.1,3,37 Although the risk of recurrence in patients with VTE provoked by a nonsurgical trigger (eg, estrogen therapy, pregnancy, leg injury, flight of longer than 8 hours) is higher than in patients with VTE provoked by surgery, the risk is still low and is estimated at 5% within 1 year and 15% within 5 years.1,37 Unprovoked VTE, for which there is no apparent or only a trivial risk factor, has a moderately high risk of recurrence and is estimated at 10% within 1 year and 30% within 5 years.1,3,37 VTE provoked by a persistent or progressive factor, such as cancer, has a high risk of recurrence, perhaps equivalent to 20% in a year, with the risk expected to be lower if the cancer is in remission and higher if it is rapidly progressing, metastatic, or being treated with chemotherapy.38-40
Secondary estimators: isolated distal DVT or previous VTE
The risk of recurrence in patients with isolated distal DVT is about half that of proximal DVT or PE.3,6,7,28,41 A second episode VTE is estimated to be associated with about a 50% higher risk of recurrence compared with a first event.41-43 These factors often influence the risk of recurrence enough to modify treatment decisions, particularly in patients with unprovoked VTE.
Secondary estimators after an unprovoked proximal DVT or a PE: sex and d-dimer
Men have a higher risk of recurrence than women (1.5- to 2-fold).44,45 Men and women with a positive d-dimer test 1 month after stopping anticoagulants have a higher risk of recurrence than those with a negative test (1.5- to 2.5-fold46 ; difference appears to diminish with longer follow-up47 ), and the influence of these 2 factors on recurrence is at least partly additive.45 However, exactly how sex and d-dimer testing (choice of assay, discriminatory value, single or serial tests) should modify treatment decisions remains unclear.48
Other factors that influence the risk of recurrence
Factors that are associated with recurrence, but rarely strongly or consistently enough to influence treatment decisions once the primary and secondary estimators have been considered, include: antiphospholipid antibody (relative risk, ∼2)49 ; hereditary thrombophilia (relative risk, ∼1.5)46,50-53 ; Asian ethnicity (relative risk, ∼0.8)54 ; and ultrasound evidence of residual thrombosis in the proximal veins (relative risk, ∼1.5).55 PTS may increase the risk of recurrent VTE,53,56 and recurrent ipsilateral DVT increases the risk of PTS32 ; these considerations may prompt indefinite anticoagulation in patients with severe PTS.48
Clinical prediction rules for risk of recurrence
Three clinical prediction rules have been developed to estimate the risk of recurrence in patients with unprovoked VTE. They take into account, with some differences, combinations of sex, d-dimer levels (continuous or binary; on or off anticoagulants), site of initial thrombosis, age when VTE occurred, and signs of PTS (1 rule).53,57,58 Ability to predict the risk of recurrence, and to improve patient outcomes, has yet to be prospectively demonstrated for all 3 rules.
Risk of major bleeding in individual patients if anticoagulants are continued
Many factors are associated with bleeding during anticoagulant therapy including: older age (>65 years and particularly >75 years), previous bleeding (particularly if the cause was not correctable), cancer (particularly if metastatic or highly vascular), renal insufficiency, liver failure, diabetes, previous stroke, thrombocytopenia, anemia, concomitant antiplatelet therapy, recent surgery, frequent falls, alcohol abuse, reduced functional capacity, and poor control of VKA therapy.1 With an increase in the severity of individual factors, and with the number of factors present, the risk of bleeding is expected to increase (both at baseline and while on anticoagulants). However, there are no validated prediction rules for bleeding during extended anticoagulation for VTE, and the rules that are available have demonstrated limited discriminatory capacity in VTE patients.35,36,59 That, however, does not mean that it is impossible to stratify patients’ risk of bleeding; young (eg, <65 years) healthy patients with good VKA control will have a low risk of major bleeding (≤1% per patient-year), those with less severe factors have an intermediate risk, and elderly patients with severe or multiple factors are at high risk for major bleeding (>4% per patient-year).1,33,59
Risk of recurrent VTE that justifies strong and weak recommendation for either 3 months or indefinite anticoagulation
Indefinite anticoagulant therapy is indicated if its benefits (reduction in VTE) outweigh its harms (increase in bleeding) enough to offset the burden and cost of treatment. As shown in Table 1, which is based on assumptions previously noted in this perspective and originally described in the ACCP guidelines,1 in patients with a low risk of bleeding (including age <65 years), a risk of recurrent VTE of >13% in the first year results in a strong recommendation and a risk of 8% to 13% in the first year results in a weak recommendation for indefinite therapy.
Risks of recurrent VTE after stopping anticoagulant therapy which justify strong or weak recommendations to either stop anticoagulants at 3 months or to treat indefinitely
| Effect of 5 y of anticoagulation on mortality* . | Recommendation . | Risk of recurrent VTE without anticoagulation† (%) . | |||
|---|---|---|---|---|---|
| Low bleeding risk‡ . | Intermediate bleeding risk§ . | ||||
| 5 y . | First y . | 5 y . | First y . | ||
| Any increase | Strong for 3 mo | <9 | <3 | <18 | <6 |
| 0%-0.5% decrease | Weak for 3 mo | 9-24 | 3-8 | 18-33 | 6-11 |
| 0.5%-1% decrease | Weak for indefinite | 24-39 | 8-13 | 33-48 | 11-16 |
| >1% decrease | Strong for indefinite | >39 | >13 | >48 | >16 |
| Effect of 5 y of anticoagulation on mortality* . | Recommendation . | Risk of recurrent VTE without anticoagulation† (%) . | |||
|---|---|---|---|---|---|
| Low bleeding risk‡ . | Intermediate bleeding risk§ . | ||||
| 5 y . | First y . | 5 y . | First y . | ||
| Any increase | Strong for 3 mo | <9 | <3 | <18 | <6 |
| 0%-0.5% decrease | Weak for 3 mo | 9-24 | 3-8 | 18-33 | 6-11 |
| 0.5%-1% decrease | Weak for indefinite | 24-39 | 8-13 | 33-48 | 11-16 |
| >1% decrease | Strong for indefinite | >39 | >13 | >48 | >16 |
Assumptions as described in text and in the ACCP guidelines1 for: case fatality of recurrent VTE (3.6%) and major bleeding (11.3%); proportion of major bleeds attributable to anticoagulation (62%); risk reduction for VTE with anticoagulation (88%).
Net effect of decrease in recurrent VTE and increase in bleeding.
Calculations based on a 5-year period, with one-third of recurrences in the first year and two-thirds in the next 4 years.
Risk of major bleeding of 0.8% for each of the 5 years.
Risk of major bleeding of 1.6% for each of the 5 years.
How to apply a strong or a weak recommendation
Consistent with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) nomenclature and the ACCP guidelines, a strong recommendation indicates a high degree of confidence that following the recommendation will result in substantial benefits for most patients.1,60 Strong recommendations, which are usually based on high-quality evidence, have been described as “just do it”; given the evidence, almost all patients would chose that option (ie, decisions are not sensitive to patient values and preferences). A weak recommendation indicates a lower degree of confidence that following the recommendation will result in substantial benefits for patients, usually because the quality of evidence is poorer, the benefits and risks are more closely balanced, or because differences among patients may shift that balance. Weak recommendations, therefore, are sensitive to differences in patient values and preferences.
VTE provoked by a reversible factor: recommendations
Whereas the ACCP guidelines divided patients with VTE provoked by a reversible risk factor into 2 categories (provoked by surgery or a nonsurgical trigger), while acknowledging there is a higher risk of recurrence in the later subgroup, we will consider this as a single category. This is because both subgroups have sufficiently low risks of recurrence to recommend stopping anticoagulants at 3 months (strongly for VTE provoked by surgery; weakly for VTE provoked by a nonsurgical trigger if there is a low or intermediate risk of bleeding). We discourage indefinite therapy if there is a convincing reversible risk factor (Table 2). However, if patients are still recovering from the VTE, or if the provoking factor is incompletely resolved, it is appropriate to treat for longer than 3 months.
Additional issues relating to duration of anticoagulant therapy for VTE
| Issues . | Considerations . |
|---|---|
| What is a reversible provoking factor? | The magnitude (or severity) of VTE risk factors, and the reversibility of risk factors, are on a continuum. Therefore, the distinction between a “trivial provoking factor” (consistent with being an unprovoked VTE) and a nonsurgical trigger (or minor reversible provoking factor) is arbitrary. We suggest that VTE can be considered provoked if there was a major reversible risk factor within 3 mo, or a minor reversible risk factor within 6 wk (eg, any general anesthesia; soft tissue injury that causes a limp; flight of >8 h; illness that renders the patient bed-bound for a day or chair-bound for 3 d). |
| Catheter-based thrombus removal | These patients should be treated for at least 3 mo. As the acute DVT is often severe, and symptoms may have become chromic (ie, PTS), anticoagulation for 6 mo is often desirable, and patients may be more likely to opt for indefinite anticoagulation if the DVT was provoked by a minor reversible risk factor. Placement of an iliac vein stent does not necessarily mean that patients should be treated indefinitely, but residual thrombus or extrinsic compression encourages that option. |
| Permanent vena cava filter | Vena cava filters appear to reduce PE and increase recurrent DVT.61 The presence of a permanent filter should not influence the duration of anticoagulant therapy. |
| Chronic thromboembolic pulmonary hypertension | These patients are generally treated with indefinite anticoagulation, whether or not they undergo endarterectomy or if known previous episodes of VTE were provoked by a reversible risk factor. |
| Hereditary thrombophilias | Hereditary thrombophilias are weak risk factors for recurrent VTE, although this is uncertain for antithrombin deficiency. Testing for hereditary thrombophilias in order to guide decisions about treatment duration does not appear to be justified. |
| Antiphospholipid antibodies | It is unclear if, independent of other clinical factors, an antiphospholipid antibody justifies indefinite anticoagulant therapy. For this reason, we do not routinely test for antiphospholipid antibodies in patients with VTE, including those with an unprovoked episode. |
| Inflammatory bowel disease | Inflammatory bowel disease (and probably other chronic inflammatory conditions) can serve as a persistent or intermittent risk factor for recurrent VTE.62 However, it is also possible that inflammatory bowel disease can serve as a reversible risk factor (eg, if it becomes inactive). |
| Estrogens | Estrogens serve as a reversible risk factor for VTE. It may be acceptable, however, for patients to remain on oral contraceptives during anticoagulant therapy.48 We then stop estrogen therapy at least a month before stopping anticoagulants. |
| Confidence to stop anticoagulants | It may take >3 mo for patients to be ready to consider stopping anticoagulant therapy. |
| Issues . | Considerations . |
|---|---|
| What is a reversible provoking factor? | The magnitude (or severity) of VTE risk factors, and the reversibility of risk factors, are on a continuum. Therefore, the distinction between a “trivial provoking factor” (consistent with being an unprovoked VTE) and a nonsurgical trigger (or minor reversible provoking factor) is arbitrary. We suggest that VTE can be considered provoked if there was a major reversible risk factor within 3 mo, or a minor reversible risk factor within 6 wk (eg, any general anesthesia; soft tissue injury that causes a limp; flight of >8 h; illness that renders the patient bed-bound for a day or chair-bound for 3 d). |
| Catheter-based thrombus removal | These patients should be treated for at least 3 mo. As the acute DVT is often severe, and symptoms may have become chromic (ie, PTS), anticoagulation for 6 mo is often desirable, and patients may be more likely to opt for indefinite anticoagulation if the DVT was provoked by a minor reversible risk factor. Placement of an iliac vein stent does not necessarily mean that patients should be treated indefinitely, but residual thrombus or extrinsic compression encourages that option. |
| Permanent vena cava filter | Vena cava filters appear to reduce PE and increase recurrent DVT.61 The presence of a permanent filter should not influence the duration of anticoagulant therapy. |
| Chronic thromboembolic pulmonary hypertension | These patients are generally treated with indefinite anticoagulation, whether or not they undergo endarterectomy or if known previous episodes of VTE were provoked by a reversible risk factor. |
| Hereditary thrombophilias | Hereditary thrombophilias are weak risk factors for recurrent VTE, although this is uncertain for antithrombin deficiency. Testing for hereditary thrombophilias in order to guide decisions about treatment duration does not appear to be justified. |
| Antiphospholipid antibodies | It is unclear if, independent of other clinical factors, an antiphospholipid antibody justifies indefinite anticoagulant therapy. For this reason, we do not routinely test for antiphospholipid antibodies in patients with VTE, including those with an unprovoked episode. |
| Inflammatory bowel disease | Inflammatory bowel disease (and probably other chronic inflammatory conditions) can serve as a persistent or intermittent risk factor for recurrent VTE.62 However, it is also possible that inflammatory bowel disease can serve as a reversible risk factor (eg, if it becomes inactive). |
| Estrogens | Estrogens serve as a reversible risk factor for VTE. It may be acceptable, however, for patients to remain on oral contraceptives during anticoagulant therapy.48 We then stop estrogen therapy at least a month before stopping anticoagulants. |
| Confidence to stop anticoagulants | It may take >3 mo for patients to be ready to consider stopping anticoagulant therapy. |
Unprovoked VTE: recommendations
Patients with a first unprovoked proximal DVT or PE who do not have a high risk of bleeding are expected to derive a modest mortality benefit from extended therapy, resulting in a weak recommendation for indefinite anticoagulation. As the risk of recurrence is expected to be higher in men (∼12% at 1 year and 36% at 5 years) than in women (∼8% at 1 year and 24% at 5 years), and as a new PE is more likely after a PE than after a DVT, being male or having had a PE strengthens the argument for indefinite therapy.
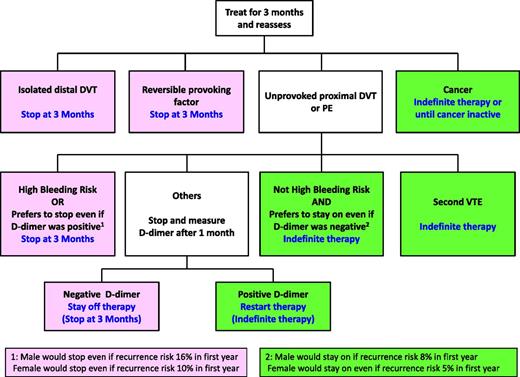
As previously noted, current evidence suggests that d-dimer levels a month after stopping anticoagulant therapy can help to predict the risk of recurrence in patients with a first unprovoked VTE, with a first-year risk of recurrence of ∼5% for women with a negative d-dimer, 10% for women with a positive d-dimer, 8% for men with a negative d-dimer, and 16% for men with a positive d-dimer. However, because these finding are preliminary, it appears equally acceptable to either use, or not use, d-dimer levels to help decide about duration of therapy. If the intention is to use d-dimer testing in this way, it should first be established with the patient that d-dimer results will influence treatment decisions (Figure 1). This applies if a woman would choose to remain on anticoagulants if she had a first-year recurrence risk of 10%, but would choose to stop treatment if this risk was 5%; if a 10% risk would not justify staying on treatment, anticoagulants should be stopped without d-dimer testing. It would also apply if a man would choose to stop anticoagulants if he had a first-year recurrence risk of 8%, but would choose not to stop treatment if his risk was 16%; if an 8% risk would not justify stopping treatment, anticoagulants should be continued without d-dimer testing.
Patients with VTE who should be treated for 3 months and who should be treated indefinitely. Use of d-dimer testing to guide treatment decisions in patients with a first unprovoked proximal DVT or PE is optional. If d-dimer is not used, the decision is based on risk of bleeding and patient preference (estimated risk of recurrence in the first year of 12% for men and 8% for women).
Patients with VTE who should be treated for 3 months and who should be treated indefinitely. Use of d-dimer testing to guide treatment decisions in patients with a first unprovoked proximal DVT or PE is optional. If d-dimer is not used, the decision is based on risk of bleeding and patient preference (estimated risk of recurrence in the first year of 12% for men and 8% for women).
If the first unprovoked VTE was an isolated distal DVT, the risk of recurrence is estimated to be low enough (5% in the first year; similar to a proximal DVT or PE associated with a nonsurgical trigger) to justify stopping anticoagulants at 3 months (weak recommendation if bleeding risk is low or intermediate; strong recommendation if bleeding risk is high).
If this is a second or subsequent episode of unprovoked VTE, the risk of recurrence is estimated to be high enough (15% in the first year and 45% at 5 years) to justify indefinite anticoagulation, provided there is not a high risk of bleeding (strong recommendation if bleeding risk is low; weak recommendation if bleeding risk is intermediate). The predictive value of patient sex and posttreatment d-dimer levels has not been evaluated after a second unprovoked VTE.
Duration of anticoagulation in patients with VTE and cancer
Patients with VTE and cancer have a high risk of recurrence and are expected to derive substantial benefit from extended anticoagulant therapy (strong recommendation, reduced to weak if bleeding risk is high).1 Anticoagulation is usually with LMWH, particularly if there is rapid cancer progression, metastatic disease, or patients are receiving chemotherapy.1,22,63-66 Anticoagulants can be stopped if patients have been treated for at least 3 months and the cancer is thought to have been cured (eg, successful resection). If there is uncertainty, our practice is to continue treatment until 6 months have passed without recurrent disease. If the cancer is in remission but not cured, and there is indirect evidence for a lower risk of recurrence (such as 2 of: VTE was associated with a risk factor that has resolved [eg, surgery or chemotherapy]; absence of metastases; not receiving chemotherapy; calf DVT), it is reasonable to stop anticoagulants (at least temporarily) or to treat with an oral agent, particularly if that is the patient’s preference. In addition to considering the usual contraindications, we avoid using the new oral anticoagulants in patients who are receiving chemotherapy.
Influence of patient preferences and cost
Some patients resent, whereas others are reassured by, anticoagulant therapy. Consequently, patient preferences should influence decision-making, particularly when there is a weak recommendation for indefinite therapy.30,67 To solicit preferences, patients first need to be informed of the risks and benefits with different options, areas of uncertainty, and why the decision is sensitive to their preferences and values.68 Health care providers should be prepared to say which option they think is best, and to explain why. Some patients may indicate that they do not want to be involved with decision-making, and care should be taken to avoid adding to the burden of their illness. Costs (ie, to patients, health care systems, third-party payers) and available treatment options (eg, licensing) may further influence decisions at a patient or societal level.
Should duration of treatment be influenced by type of anticoagulant?
It is not known whether the time needed to complete active treatment differs with the type of anticoagulant. For now, it is reasonable to assume that this is not the case. Because the new oral anticoagulants are less burdensome than VKA and cause less bleeding, more patients with unprovoked VTE are expected to opt for indefinite therapy.
Antiplatelet therapy
After anticoagulation for unprovoked VTE, aspirin reduces the risk of recurrence by about one-third.20,69,70 This is a minor reduction compared with the 90% reduction with anticoagulants and, although bleeding with aspirin should be less than with a VKA, there may be a similar risk of bleeding with aspirin and the new oral anticoagulants. Therefore, rather than considering aspirin as an alternative to anticoagulation, if a decision has been made to stop anticoagulants, the reduction in recurrent VTE with aspirin can be factored into the overall assessment of aspirin’s long-term benefits.
Follow-up of patients on extended therapy
Patients who are treated indefinitely should be reviewed regularly (eg, annually) to ensure that: (1) they have not developed contraindications to anticoagulant therapy; (2) their preferences have not changed; (3) they can avail of improved ways to predict risk of recurrence and the possibility of safely stopping therapy; and (4) they are being treated with the most suitable anticoagulant regimen.
Acknowledgments
The authors thank Drs Sarah Takach Lapner, Jeffrey Weitz, Jeffrey Ginsberg, and Sam Schulman for their constructive comments, and thank copanelists of the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines for the Treatment of Venous Thromboembolism who helped to shape our thoughts on this topic.
C.K. is supported by the Jack Hirsh Professorship in Thromboembolism and an Investigator Award from the Heart and Stroke Foundation of Ontario.
Authorship
Contribution: C.K. drafted the article; and C.K. and E.A.A. developed the concepts included in the article, revised the article, and approved the final version.
Conflict-of-interest disclosure: C.K. has served as a consultant to Boehringer Ingelheim and to Bayer Inc. E.A.A. declares no competing financial interests.
Correspondence: Clive Kearon, Juravinski Hospital, Room A3-73, 711 Concession St, Hamilton, ON, L8V 1C3, Canada; e-mail: kearonc@mcmaster.ca.