Key Points
Freshly isolated arterial/venous endothelial cells differ in their gene signature, which is only partially controlled by the Notch pathway.
Eight transcription factors codetermine the arterial fingerprint in a complementary and overlapping fashion.
Abstract
Endothelial cells (ECs) lining arteries and veins have distinct molecular/functional signatures. The underlying regulatory mechanisms are incompletely understood. Here, we established a specific fingerprint of freshly isolated arterial and venous ECs from human umbilical cord comprising 64 arterial and 12 venous genes, representing distinct functions/pathways. Among the arterial genes were 8 transcription factors (TFs), including Notch target HEY2, the current “gold standard” determinant for arterial EC (aEC) specification. Culture abrogated differential gene expression in part due to gradual loss of canonical Notch activity and HEY2 expression. Notably, restoring HEY2 expression or Delta-like4–induced Notch signaling in cultured ECs only partially reinstated the aEC gene signature, whereas combined overexpression of the 8 TFs restored this fingerprint more robustly. Whereas some TFs stimulated few genes, others boosted a large proportion of arterial genes. Although there was some overlap and crossregulation, the TFs largely complemented each other in regulating the aEC gene profile. Finally, overexpression of the 8 TFs in human umbilical vein ECs conveyed an arterial-like behavior upon their implantation in a Matrigel plug in vivo. Thus, our study shows that Notch signaling determines only part of the aEC signature and identifies additional novel and complementary transcriptional players in the complex regulation of human arteriovenous EC identity.
Introduction
Endothelial cells (ECs) lining different vessels differ in morphology, function, and gene expression.1-3 EC heterogeneity is due to exposure to variable microenvironments and regulation by intrinsic genetic programs during development.1 Molecular mechanisms determining arteriovenous specification have predominantly been unveiled in developing mice and zebrafish. Current reports on arteriovenous EC identity in humans are limited to cultured cells.4 Collectively, these studies make a case for Notch signaling, involving ligands DLL1/4, receptors Notch1/4, and transcription factors (TFs) Hey1/2 as a major determinant of arterial EC (aEC) identity.5-8 In zebrafish, Notch is part of a cascade in which sonic hedgehog induces vascular endothelial growth factor (VEGF) expression from the somites, which in turn activates Notch in endothelial precursors. Recent findings show that hedgehog signaling can also induce arterial differentiation independent of VEGF.9 Arteriovenous specification happens before the first heart beat, suggesting that it is in part blood flow independent. Nr2f2 (COUP-TFII) confers a venous identity upon ECs by blocking Notch1 and Hey1/2.10-12 Notch induces ephrinB2 and blocks EphB4 expression in aECs, whereas in venous ECs (vECs) Nr2f2 mediates the opposite effect.8,11,12 This ephrinB2-EphB4 differential expression establishes a polarity that assists in segregating arteries from veins down to the capillary level.13
Additional molecules, such as neuropilins, forkhead box (Fox)c1/2, adrenomedullin, calcitonin receptor-like receptor, Sprouty protein with EVH-1 domain (Spred)s, bone morphogenetic proteins, gap junction protein-alpha5, microRNA (mir)27b, Wnt, Sry-related HMG box (Sox), transforming growth factor-β, phosphatidylinositol-3 kinase, and ETS factors, play a role in arteriovenous specification.8,9,14-21 It remains largely unknown whether these pathways are also important in established vessels for maintenance of the arteriovenous phenotype. The plastic nature of this phenotype is apparent in certain situations, such as during angiogenic sprouting or venous bypass grafting.8,22 Although most studies focused on one or a few regulatory elements, Chi et al4 used a genome-wide approach to document heterogeneity between aECs and vECs. As mentioned, they used cultured ECs. However, studies in microvascular23,24 and lymphatic ECs25,26 revealed that the culture process erases their specific expression profile. This identity loss upon culture has not been systematically studied for aECs/vECs. Therefore, our aim was to perform a stringent genome-wide profiling study on ECs isolated from human arteries and veins, including cultured and freshly isolated cells, to discover new regulators of arteriovenous EC heterogeneity.
Methods
Extended supplemental “Methods” are available online.
EC isolation and culture
The following commercial EC lines were used: human aortic ECs, human coronary artery ECs (Lonza), human iliac arterial ECs, human pulmonary artery ECs, human iliac vein ECs, and human pulmonary vein ECs. Human hepatic artery ECs, human hepatic vein ECs, human umbilical cord vein ECs (HUVECs), and human umbilical cord artery ECs (HUAECs) were isolated at the University of Navarra (after informed consent from donors in accordance with the Declaration of Helsinki and approval by the ethics committee). For analysis of fresh cells or short-term culture for time-course analysis, ECs from umbilical arteries and veins were selected using anti-hCD34 magnetic beads (Miltenyi Biotec) or fluorescence-activated cell sorting based on positive selection for Tie2 and CD31 and negative selection for CD45 (supplemental Figure 1).
RNA isolation, qRT-PCR, western blot, and immunofluorescence staining
RNA was extracted using TRIzol or RLT lysis buffer (Qiagen). RNA integrity and quality were determined with a Bioanalyzer-2100 (Agilent Technologies). Messenger RNA was reverse-transcribed and complementary DNA (cDNA) underwent 40 amplification rounds. Quantitative reverse-transcription polymerase chain reaction (RT-PCR) primers are listed in supplemental Table 1. Messenger RNA levels were normalized using GAPDH or ACTB. Western blot and immunofluorescence staining were done as described online.
Microarray and time course analysis
Microarray was done using the Affymetrix HG-U133 Plus-2.0 GeneChip Oligonucleotide Microarray, and differential gene, fingerprint, and hierarchical cluster analysis was performed as described online. Functional/pathway enrichment analysis was done using Ingenuity Pathway Analysis software (http://www.ingenuity.com). Microarray data are accessible at the NCBI Gene Expression Omnibus website (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE43475. For time-course analysis, TaqMan low-density array plates for the arteriovenous fresh profile were obtained from Applied Biosystems and analyzed as described online.
nCounter analysis
DLL4-Fc-activated or bovine serum albumin (BSA)-treated HUAECs (n = 3) and HUVECs transduced with Cherry, each of the 8 TFs, or their combination (n = 4-6) were used for nCounter analysis (Nanostring Technologies), as described online. Some results were confirmed by qRT-PCR, and genes for which the probe intensity value was low were analyzed by qRT-PCR. The probe list is provided in supplemental Table 2. Heatmap and hierarchical cluster analysis was performed as described online.
siRNA knockdown and Notch activity assays
Small interfering RNA (siRNA) knockdown was performed using Silencer Select predesigned siRNA from Applied Biosystems for RBPJκ (ID: s7251/s7253), Negative Control-1 (ID: am4636) as described online. The canonical Notch pathway was induced by immobilized DLL4 ligand, as described online, and was blocked by 3 μM γ-secretase inhibitor DAPT (Calbiochem).
Lentivirus production and overexpression
The lentiviral construct for constitutively overexpressing human HEY2 was from Genecopoeia. Open reading frames for human MSX1, EMX2, NKX2-3, TOX2, and murine Aff3 and Prdm16 were cloned from cDNA-containing plasmids (Thermo Scientific) or total human cDNA (Gentaur) after the cytomegalovirus (CMV) promoter in pRRL2-CMV-PGK-Cherry. The lentiviral construct for human Sox17 was provided by C. Verfaillie (Stem Cell Institute, KU Leuven). For regulable overexpression, EMX2 or NKX2-3 were cloned in the pRRL3-TetONCMV-hUbiquitin-rtTA3-IRES-Cherry vector (supplemental Figure 2A). Lentiviral transduction was performed as described online. For constitutive overexpression, transduced cells were cultured for 6 to 28 days. For conditional overexpression, half of the cells were kept for 6 days on doxycycline (2 μg/mL) and the other half were divided into 2 groups: one with continued exposure to doxycyline up to 10 days and another in media without doxycylin to switch off TF overexpression (supplemental Figure 2B).
In vivo Matrigel assay
A total of 0.5 × 106 HUVECs transduced with a lentivirus encoding Cherry or each of the 8 TFs were mixed with Matrigel containing VEGF165 and basic fibroblast growth factor (R&D Systems) and subcutaneously injected in the back of 8-week-old athymic nu/nu mice (n = 5 per group). Two weeks later, mice were sacrificed and the Matrigel plug was dissected out and divided in 2 pieces: one was processed for cryo and the other for paraffin sectioning. Human cells were detected by the Cherry signal or by human-specific CD31 staining (Dako). Smooth muscle coverage was analyzed on α-smooth muscle actin (αSMA)-stained (Sigma) sections and collagen deposition was quantified on Sirius red–stained sections (examined under polarized light). Animal studies were approved by the ethics committee at KU Leuven.
Results
Freshly isolated, but not cultured, aECs and vECs differ in gene expression profile
To identify an arteriovenous fingerprint in human ECs across different vascular beds, we used microarrays on RNA from 38 human EC samples (supplemental Figure 3) corresponding to 6 cultured aEC types (human hepatic artery ECs, n = 3; human aorta ECs, n = 2; human coronary artery ECs, n = 2; human iliac arterial ECs, n = 2; human pulmonary artery ECs, n = 3; and HUAEC-C, n = 5), 4 cultured vEC types (human hepatic vein ECs, n = 3; human iliac vein ECs, n = 3; human pulmonary vein ECs, n = 2; and HUVEC-C, n = 5), freshly isolated HUAECs (HUAEC-F, n = 4), and freshly isolated HUVECs (HUVEC-F, n = 4). Due to the difficulty of obtaining biopsy specimens from healthy donors, we did not have access to freshly isolated aECs or vECs matched for all cultured EC types. Despite the consistency within EC subtypes, when considering all 30 cultured EC samples together, we did not find statistically significant differences in gene expression between aECs and vECs.
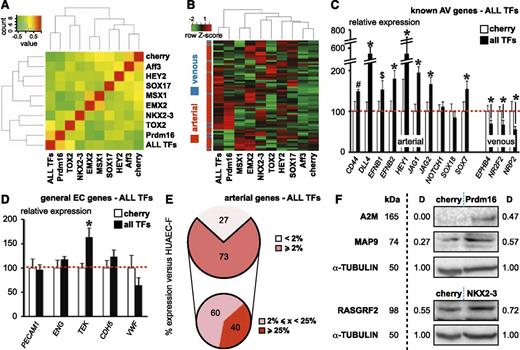
We next performed profiling analysis on the freshly isolated HUVEC/HUAEC subset using 2 statistical approximations: differential gene expression or classification analysis (Figure 1A). Both analyses detected significant differences between HUAEC-F and HUVEC-F, yielding a combined panel of 76 genes (∼102 probes; supplemental Table 3) with 64 HUAEC-F and 12 HUVEC-F genes, ∼55% of which were commonly picked up by both statistical methods (Figure 1A). From here on, we refer to this 76 gene set as the “arteriovenous fresh profile.” This profile included 16 genes with documented (differential) expression in aECs/vECs and 13 genes associated with a vascular phenotype in mice, 4 of which were described to be involved in aEC specification or development (NOTCH4, HEY2, KDR, and SOX17), revealing that our extracted gene list was reliable (Figure 1A; Table 1 and supplemental Table 4). Functional/pathway enrichment analysis produced several relevant terms, including (cardio)vascular disease, cardiovascular system development, and Notch signaling (Figure 1B-C). Literature study revealed that 10 genes (or a related family member/ortholog) had a previously documented interaction with the Notch pathway (Table 1 and supplemental Table 4). Finally, the arteriovenous fresh profile harbored 9 genes encoding a TF (8 arterial and 1 venous; Figure 1D). We validated some of the new aEC (ie, MSX1; Figure 1E-F) and vEC (ie, NR3C2; Figure 1G-H) TFs at the protein level. TFs and a random profile gene subset were validated using an independent fluorescence-activated cell sorting strategy based on a Tie2+CD31+CD45− population (supplemental Figure 1D-E).
Freshly isolated HUAECs and HUVECs have a different molecular and functional profile. An overview of the differential gene expression profile and corresponding functional signature of HUAECs and HUVECs. (A) Pie diagrams showing the number of annotated arterial (red) and venous (blue) genes emerging as differentially expressed between freshly isolated HUAECs and HUVECs from the differential gene statistical analysis (upper left) or classification analysis (upper right). The lower diagram shows the total amount of annotated genes and corresponding percentages (black lettering; arterial-enriched genes in red, venous-enriched genes in blue lettering) uniquely identified by the differential gene analysis (pink), from the classification analysis (yellow) or emerging from both analyses (orange; which included 4 genes, ie, HEY2, NOTCH4, SOX17, and KDR, previously associated with arterial specification). Other displayed names correspond to the most differentially expressed genes in each part of the diagram. (B-C) Bar diagrams showing functional terms (B) and pathways (C) significantly enriched in the 76 differential gene set. Numbers of associated genes per function/pathway are mentioned on top of the bars. Yellow bars represent subcategories of the main functional term “cardiovascular diseases.” (D) Bar diagram showing microarray probe set intensities (± standard error of the mean [SEM]; n = 4) for the 8 arterial (A) and 1 venous (V) TFs identified among the arteriovenous fresh profile, revealing their differential expression in freshly isolated HUAECs (red) and HUVECs (blue). *P < .05 vs arterial probe set intensity. (E-H) Immunofluorescence stainings on paraffin cross sections of human umbilical cord (umbilical vein is shown in E,G, umbilical artery in F,H) for αSMA (in green) and MSX1 (E-F; in red) or NR3C2 (G-H; in red). The curved bright red line in panel E represents an aspecific signal from the elastica interna. 4,6 Diamidino-2-phenylindole (blue) was used as nuclear counterstain. White arrowheads indicate positive staining in the endothelial lining. Scale bars: 20 μm. CV, cardiovascular; NO, nitric oxide; nNOS, neuronal nitric oxide synthase.
Freshly isolated HUAECs and HUVECs have a different molecular and functional profile. An overview of the differential gene expression profile and corresponding functional signature of HUAECs and HUVECs. (A) Pie diagrams showing the number of annotated arterial (red) and venous (blue) genes emerging as differentially expressed between freshly isolated HUAECs and HUVECs from the differential gene statistical analysis (upper left) or classification analysis (upper right). The lower diagram shows the total amount of annotated genes and corresponding percentages (black lettering; arterial-enriched genes in red, venous-enriched genes in blue lettering) uniquely identified by the differential gene analysis (pink), from the classification analysis (yellow) or emerging from both analyses (orange; which included 4 genes, ie, HEY2, NOTCH4, SOX17, and KDR, previously associated with arterial specification). Other displayed names correspond to the most differentially expressed genes in each part of the diagram. (B-C) Bar diagrams showing functional terms (B) and pathways (C) significantly enriched in the 76 differential gene set. Numbers of associated genes per function/pathway are mentioned on top of the bars. Yellow bars represent subcategories of the main functional term “cardiovascular diseases.” (D) Bar diagram showing microarray probe set intensities (± standard error of the mean [SEM]; n = 4) for the 8 arterial (A) and 1 venous (V) TFs identified among the arteriovenous fresh profile, revealing their differential expression in freshly isolated HUAECs (red) and HUVECs (blue). *P < .05 vs arterial probe set intensity. (E-H) Immunofluorescence stainings on paraffin cross sections of human umbilical cord (umbilical vein is shown in E,G, umbilical artery in F,H) for αSMA (in green) and MSX1 (E-F; in red) or NR3C2 (G-H; in red). The curved bright red line in panel E represents an aspecific signal from the elastica interna. 4,6 Diamidino-2-phenylindole (blue) was used as nuclear counterstain. White arrowheads indicate positive staining in the endothelial lining. Scale bars: 20 μm. CV, cardiovascular; NO, nitric oxide; nNOS, neuronal nitric oxide synthase.
The culturing process rapidly erases differential arteriovenous gene expression
Hierarchical clustering analysis for the arteriovenous fresh profile showed a perfect separation of HUAEC-F and HUVEC-F samples, confirming a high degree of difference between the 2 EC subtypes (Figure 2A). The influence of the culture process on EC gene expression has been described for brain microvascular, lymphatic, and venular ECs,23-26 but not for aECs or large vECs. Hence, we next compared freshly isolated HUVECs/HUAECs with their cultured counterparts. Global differential expression analysis showed dramatic changes in gene expression upon culturing, with 410 probesets (∼327 annotated genes) upregulated and 442 probesets (∼332 annotated genes) downregulated in HUAEC-C vs HUAEC-F and 66 probesets (∼56 annotated genes) upregulated and 105 probesets (∼69 annotated genes) downregulated in HUVEC-C vs HUVEC-F. When considering the arteriovenous fresh profile genes alone, there were no obvious differences between HUVEC-C and HUAEC-C (Figure 2B; supplemental Table 3). Expression of the 8 arterial TFs was completely lost upon culturing HUAECs (Figure 2C). This culture-induced assimilation was also underscored by incorrect clusters generated to distinguish HUVEC-C and HUAEC-C, unlike their fresh equivalents, which clustered according to their arterial/venous origin (Figure 2A). The incorrect clusters were also apparent when considering all cultured aEC/vEC types from our screen, suggesting that aECs and vECs from these different vascular beds had lost their specific expression profiles upon culturing (supplemental Figure 3).
The culturing process rapidly erases differential arteriovenous gene expression. HUAECs and HUVECs lose their differential expression profile, including that of TFs, upon culture. (A) Hierarchical clustering analysis for freshly isolated or cultured HUAECs (red) or HUVECs (blue) revealing that for fresh samples replicates of each cell type tightly cluster together and both clusters are nicely separated. For cultured samples, incorrect clustering occurred. (B) Diagram representing the probe set intensity difference between HUVECs and HUAECs for each probe of the arteriovenous fresh profile (showing arterial probes [A] on the left and venous probes [V] on the right) for cultured (filled diamonds) or freshly isolated (open triangles) cells, revealing only minor differences in cultured cells. (C) Diagram representing the expression (±SEM; n = 4) determined by qRT-PCR for arterial TFs in freshly isolated cells (open triangles) or cultured cells (filled diamonds) relative to their expression in freshly isolated cells. *P < .05 vs freshly isolated cells. (D-E) Diagrams showing probe set intensity (±SEM; n = 4) for arterial (A) and venous (V) probes of the arteriovenous fresh profile for cultured (filled diamonds) or freshly isolated (open triangles) HUAECs (D; symbols in red) or HUVECs (E; symbols in blue). (F) Diagram showing that the average probe set intensity for all arterial probes (dashed lines) or venous probes (full lines) in freshly isolated HUAECs (left; red) or HUVEC (right; blue) “bleach” to a default average expression level (gray dashed line) upon culturing (middle; pink). *P < .05 vs fresh. NS, not significant vs fresh (n = 4-5).
The culturing process rapidly erases differential arteriovenous gene expression. HUAECs and HUVECs lose their differential expression profile, including that of TFs, upon culture. (A) Hierarchical clustering analysis for freshly isolated or cultured HUAECs (red) or HUVECs (blue) revealing that for fresh samples replicates of each cell type tightly cluster together and both clusters are nicely separated. For cultured samples, incorrect clustering occurred. (B) Diagram representing the probe set intensity difference between HUVECs and HUAECs for each probe of the arteriovenous fresh profile (showing arterial probes [A] on the left and venous probes [V] on the right) for cultured (filled diamonds) or freshly isolated (open triangles) cells, revealing only minor differences in cultured cells. (C) Diagram representing the expression (±SEM; n = 4) determined by qRT-PCR for arterial TFs in freshly isolated cells (open triangles) or cultured cells (filled diamonds) relative to their expression in freshly isolated cells. *P < .05 vs freshly isolated cells. (D-E) Diagrams showing probe set intensity (±SEM; n = 4) for arterial (A) and venous (V) probes of the arteriovenous fresh profile for cultured (filled diamonds) or freshly isolated (open triangles) HUAECs (D; symbols in red) or HUVECs (E; symbols in blue). (F) Diagram showing that the average probe set intensity for all arterial probes (dashed lines) or venous probes (full lines) in freshly isolated HUAECs (left; red) or HUVEC (right; blue) “bleach” to a default average expression level (gray dashed line) upon culturing (middle; pink). *P < .05 vs fresh. NS, not significant vs fresh (n = 4-5).
Because arterial- and venous-specific genes of the arteriovenous fresh profile as well as the 2 cell types may behave differently during the assimilation process, we next analyzed the arterial and venous part of the signature separately in HUAECs and HUVECs. When HUAECs were cultured for 2 or 3 passages (∼10-15 days), there was strong silencing of most of the 64 arterial genes of the signature, while there was no obvious change in the venous arm (Figure 2D-F; supplemental Table 3). Conversely, when culturing HUVECs, venous genes were significantly downregulated, whereas arterial genes were not (Figure 2E-F; supplemental Table 3). To determine the kinetics of the downregulation, we cultured freshly isolated cells for different periods before analyzing the arteriovenous fresh profile. Twenty-four hours of culture was sufficient to induce changes for a majority of the genes (62% of arterial genes in HUAECs and 73% of venous genes in HUVECs; supplemental Figure 4A-B). Changes became more obvious after 48 hours and 6 days (supplemental Figure 4C-F), revealing that the majority of gene expression changes occurred very rapidly, independent of passaging.
Reactivation of Notch signaling only partially restores arterial gene expression in HUAEC-C
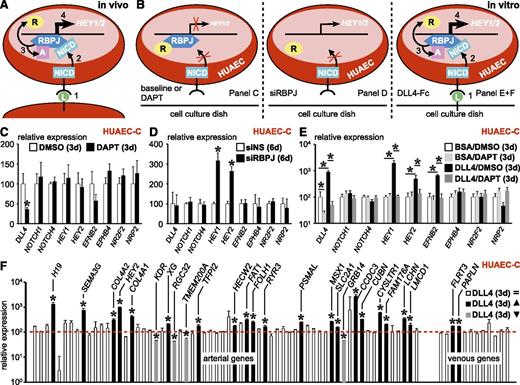
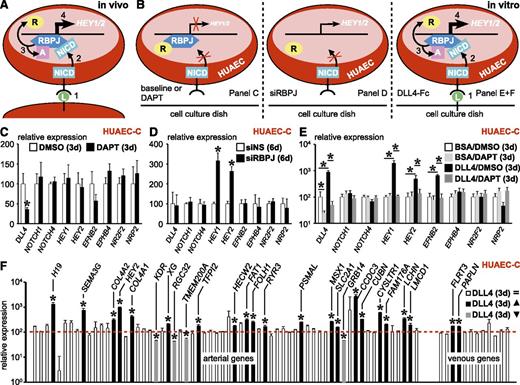
It is well established that canonical Notch signaling induces arterial specification during development by boosting expression of aEC markers like ephrinB2 and blunting expression of vEC markers like EphB4.8 Due to silencing of Notch target HEY2 in HUAEC-C compared with HUAEC-F (Figure 2C; supplemental Table 3), we hypothesized that the canonical Notch pathway might be inactive in vitro, which could explain the loss of the arterial phenotype upon culture. During canonical Notch signaling, ligand binding to the Notch receptor induces γ-secretase–mediated receptor cleavage, thereby releasing the Notch intracellular domain. The latter subsequently migrates to the nucleus and binds to recombination signal binding protein for the immunoglobulin κ J region (RBPJ). This association releases RBPJ-associated coinhibitors and makes RBPJ accessible to coactivators that induce expression of Notch-responsive genes like HEY1/2 (Figure 3A). If the canonical Notch pathway were active in HUAEC-C, then blocking it by using DAPT (a γ-secretase inhibitor) for 72 hours should induce silencing of Notch targets HEY1/2 and downstream gene EFNB2 while compensatorily upregulating vEC markers (ie, NR2F2, NRP2, and EPHB4; Figure 3B left). None of this happened, suggesting that the canonical Notch pathway is inactive in HUAEC-C (Figure 3C; 24-hour DAPT treatment revealed similar results; data not shown). Exposure of freshly isolated HUAECs for 24 hours to DAPT already showed significant loss of Notch activity, suggesting that culture-related inactivation occurs gradually and rapidly (data not shown). In this inactive state, there is no Notch intracellular domain binding to RBPJ, so the latter would be associated to coinhibitors and repress HEY1/2 expression (Figure 3B left). Accordingly, upon siRNA-mediated knockdown of RBPJ expression, this repressive mark was lost and HEY1/2 expression was induced, suggesting that RBPJ in HUAEC-C is in a repressive mode (Figure 3B middle; Figure 3D). In accordance, HEY1, HEY2, and EFNB2 expression could be reactivated in HUAEC-C by stimulation with Notch-ligand DLL4, and this could be blocked again by DAPT treatment (Figure 3B right; Figure 3E). To evaluate the overall importance of canonical Notch signaling in the arteriovenous fresh profile regulation in HUAEC-C, we exposed them to DLL4-Fc or BSA. Only a subset (20/64, ∼31%) of arterial markers were significantly upregulated upon DLL4-mediated Notch activation and 4 were even downregulated, suggesting additional pathways are involved to fully specify aECs (Figure 3F; supplemental Table 5).
Reactivation of Notch signaling only partially restores arterial gene expression in HUAEC-C. Canonical Notch signaling is lost upon culture and its reactivation only partially restores the arterial signature in HUAECs. (A-B) Schematic diagrams showing 4 numbered consecutive steps during normal canonical Notch signaling in vivo (A) or under several experimental conditions in vitro (B), ie, in the presence of DAPT (left), siRNA against RBPJ (siRBPJ; middle), or Delta-like (DLL)4-Fc anchored to the cell culture dish (right). (C) Diagram representing expression (±SEM; n = 4) of several Notch pathway members and downstream genes in cultured HUAECs treated for 72 hours with DAPT (black) or dimethlysulfoxide (DMSO) (white) relative to DMSO. *P < .05 vs DMSO. (D) Diagram representing expression (±SEM; n = 4) of the same gene panel in cultured HUAECs treated for 6 days with siRBPJ (black) or nonsilencing siRNA (siNS; white) relative to siNS. *P < .05 vs siNS. (E) Diagram representing expression (±SEM; n = 4) of the same gene panel in cultured HUAECs treated for 72 hours with BSA/DAPT (light gray), BSA/DMSO (white), DLL4-Fc/DMSO (black), or DLL4-Fc/DAPT (dark gray) relative to BSA/DMSO. *P < .05 vs BSA/DMSO or DLL4-Fc/DMSO. (F) Diagram representing expression of all arterial (left) and venous (right) genes of the arteriovenous fresh profile in cultured HUAECs exposed for 72 hours to DLL4-Fc relative to BSA (red dotted line indicates expression in BSA-treated cells). Genes with no change in expression are in white, those that are upregulated are in black, and those that are downregulated are in gray. *P < .05 vs BSA. d, day.
Reactivation of Notch signaling only partially restores arterial gene expression in HUAEC-C. Canonical Notch signaling is lost upon culture and its reactivation only partially restores the arterial signature in HUAECs. (A-B) Schematic diagrams showing 4 numbered consecutive steps during normal canonical Notch signaling in vivo (A) or under several experimental conditions in vitro (B), ie, in the presence of DAPT (left), siRNA against RBPJ (siRBPJ; middle), or Delta-like (DLL)4-Fc anchored to the cell culture dish (right). (C) Diagram representing expression (±SEM; n = 4) of several Notch pathway members and downstream genes in cultured HUAECs treated for 72 hours with DAPT (black) or dimethlysulfoxide (DMSO) (white) relative to DMSO. *P < .05 vs DMSO. (D) Diagram representing expression (±SEM; n = 4) of the same gene panel in cultured HUAECs treated for 6 days with siRBPJ (black) or nonsilencing siRNA (siNS; white) relative to siNS. *P < .05 vs siNS. (E) Diagram representing expression (±SEM; n = 4) of the same gene panel in cultured HUAECs treated for 72 hours with BSA/DAPT (light gray), BSA/DMSO (white), DLL4-Fc/DMSO (black), or DLL4-Fc/DAPT (dark gray) relative to BSA/DMSO. *P < .05 vs BSA/DMSO or DLL4-Fc/DMSO. (F) Diagram representing expression of all arterial (left) and venous (right) genes of the arteriovenous fresh profile in cultured HUAECs exposed for 72 hours to DLL4-Fc relative to BSA (red dotted line indicates expression in BSA-treated cells). Genes with no change in expression are in white, those that are upregulated are in black, and those that are downregulated are in gray. *P < .05 vs BSA. d, day.
Combined overexpression of 8 TFs robustly induces the arteriovenous fresh profile in HUVEC-C
The HUAEC-F–specific fingerprint contained 8 TFs (Figure 1D), only 2 of which were previously associated with arterial specification, ie, HEY2 and SOX17 (supplemental Table 4).4,27-29 Because canonical Notch pathway induction in HUAEC-C did not completely restore the arteriovenous fresh profile, some of the newly identified TFs might be important for arterial specification. To determine the role of the 8 TFs, we attempted to convert HUVEC-C into cells with a HUAEC-F expression profile by overexpression of them. Six days after transduction, we analyzed expression of the genes within the arteriovenous fresh profile (Figure 4A-B), of markers previously reported to be enriched in aECs or vECs (Figure 4C), and of general EC markers (Figure 4D). Figure 4A shows the heatmap analysis of the arteriovenous fresh profile for individual TFs or the combination of all 8 of them. More distant localization from the Cherry control sample indicates a stronger capacity of the TF (combination) to induce arterial specification. As can be observed from the red gene blocks in the hierarchical clustering analysis, the TFs largely complemented each other for arteriovenous fingerprint regulation, although some of these blocks also overlapped (Figure 4B). Overall, both analyses revealed that the 8 TF combination delivered the most complete restoration of the arteriovenous fresh profile, whereas the “gold standard” for arterialization, HEY2,4 did not induce strong changes in this profile (Figure 4A-B). Many established aEC markers were also significantly upregulated, whereas classical venous markers were downregulated by overexpression of the TF combination (Figure 4C). Importantly, except for TEK, we did not observe significant changes in general EC marker expression, suggesting that the TF combination specifically drives arterial specification (Figure 4D). When comparing the converted HUVEC-C to HUAEC-F, for the majority (73%) of arterial genes, the TF combination induced expression levels that were above 2%, 60% of which were in the midrange (2% to 25% of HUAEC-F levels) and 40% in the high range (>25% of HUAEC-F levels) of expression (Figure 4E). Some of the gene expression changes were validated at the protein level by western blot (Figure 4F). Additionally, analysis of a random set of genes upon constitutive TF overexpression revealed that the effects were stable up to 28 days after transduction (data not shown). Conditional overexpression experiments using a Tet-ON switch demonstrated that sustained TF overexpression is required to maintain the induced arterial expression pattern (supplemental Figure 2).
Combined overexpression of 8 TFs robustly induces the arteriovenous fresh profile in HUVEC-C. Combined overexpression of 8 TFs significantly induces an arterial expression profile in cultured HUVECs. (A) Heatmap analysis of the arteriovenous fresh profile upon transduction of cultured HUVECs with Cherry control virus, lentivirus expressing 1 TF, or a combination of all 8 TF-expressing lentiviruses (ALL). The farther the condition is removed from the Cherry control condition (or the greener the color in the Cherry column for a certain condition), the better the induction of the arteriovenous fresh profile. Note that the gold standard, HEY2, certainly is not the best-performing individual TF. (B) Hierarchical clustering analysis of the arteriovenous fresh profile upon transduction of cultured HUVECs with Cherry control virus, lentivirus expressing 1 TF, or a combination of all 8 TF-expressing lentiviruses (ALL). Red color in the bar on the left represents an arterial gene, whereas the blue color represents a venous gene. Although almost all the genes are expressed at low levels (in green) in the Cherry control condition, each of the individual TFs, except for Aff3, upregulated a subset of (mostly arterial) genes in a largely complementary but in some cases overlapping fashion. As expected from the complementarity, the 8 TFs together induce the majority of the (arterial) genes. (C-D) Diagrams representing expression for classical arterial (C, left) or venous (C, right) or general endothelial (D) markers in cultured HUVECs transduced with Cherry control virus (white) or all 8 TF-expressing lentiviruses (ALL TFs) relative to Cherry control. *P < .05 vs Cherry control. (E) Pie diagram representing the proportion of arterial genes with expression levels < 2% (light pink) or ≥ 2% (light red) in cultured HUVECs transduced with all 8 TF-expressing lentiviruses (ALL TFs) relative to those in freshly isolated HUAECs (upper). Pie diagram representing, within the subset of arterial genes with ≥ 2% values, the proportion of genes with midrange (<25%; dark pink) or high-range (≥ 25%; dark red) expression (lower). (F) Western blot showing the validation of some genes being upregulated by TF overexpression. α-TUBULIN was used as loading control. D, proportional values for the density of the protein band; kDa represents expected protein size.
Combined overexpression of 8 TFs robustly induces the arteriovenous fresh profile in HUVEC-C. Combined overexpression of 8 TFs significantly induces an arterial expression profile in cultured HUVECs. (A) Heatmap analysis of the arteriovenous fresh profile upon transduction of cultured HUVECs with Cherry control virus, lentivirus expressing 1 TF, or a combination of all 8 TF-expressing lentiviruses (ALL). The farther the condition is removed from the Cherry control condition (or the greener the color in the Cherry column for a certain condition), the better the induction of the arteriovenous fresh profile. Note that the gold standard, HEY2, certainly is not the best-performing individual TF. (B) Hierarchical clustering analysis of the arteriovenous fresh profile upon transduction of cultured HUVECs with Cherry control virus, lentivirus expressing 1 TF, or a combination of all 8 TF-expressing lentiviruses (ALL). Red color in the bar on the left represents an arterial gene, whereas the blue color represents a venous gene. Although almost all the genes are expressed at low levels (in green) in the Cherry control condition, each of the individual TFs, except for Aff3, upregulated a subset of (mostly arterial) genes in a largely complementary but in some cases overlapping fashion. As expected from the complementarity, the 8 TFs together induce the majority of the (arterial) genes. (C-D) Diagrams representing expression for classical arterial (C, left) or venous (C, right) or general endothelial (D) markers in cultured HUVECs transduced with Cherry control virus (white) or all 8 TF-expressing lentiviruses (ALL TFs) relative to Cherry control. *P < .05 vs Cherry control. (E) Pie diagram representing the proportion of arterial genes with expression levels < 2% (light pink) or ≥ 2% (light red) in cultured HUVECs transduced with all 8 TF-expressing lentiviruses (ALL TFs) relative to those in freshly isolated HUAECs (upper). Pie diagram representing, within the subset of arterial genes with ≥ 2% values, the proportion of genes with midrange (<25%; dark pink) or high-range (≥ 25%; dark red) expression (lower). (F) Western blot showing the validation of some genes being upregulated by TF overexpression. α-TUBULIN was used as loading control. D, proportional values for the density of the protein band; kDa represents expected protein size.
Individual TFs interact in a complex network to regulate the arteriovenous fresh profile
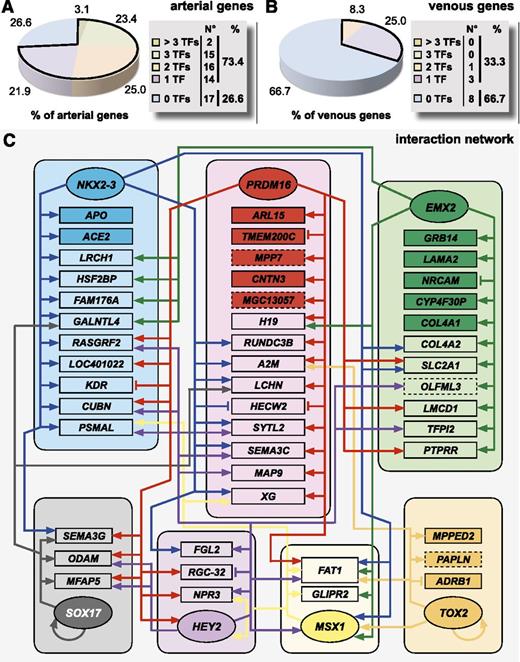
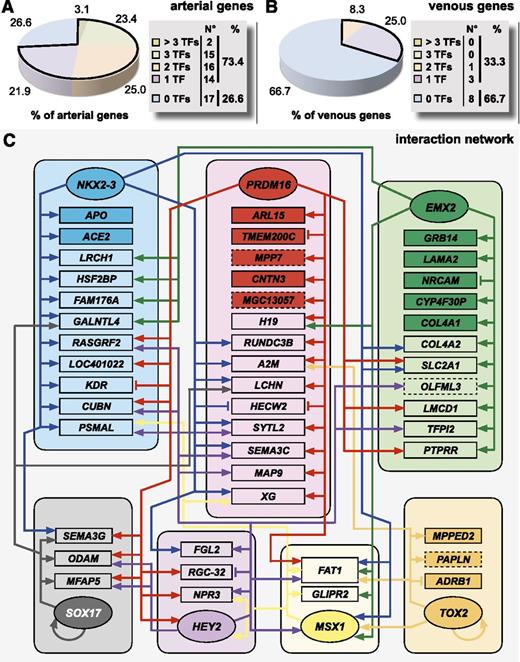
We next zoomed in on the relative contribution of individual TFs to the regulation of the arteriovenous fresh profile by evaluating their effect on a gene-by-gene basis. When looking at the arterial arm of the profile, overall, a large proportion of genes (∼73%) were regulated by at least 1 TF, with ∼51% coregulated by at least 2 TFs (Figure 5A). In contrast, only one-third of the genes were regulated at the venous side (Figure 5B). While some TFs, like Aff3, almost did not exhibit any regulatory effect, others regulated a high percentage of arterial genes from the profile (∼41% for Prdm16, ∼36% for NKX2-3, and ∼30% for EMX2; supplemental Table 6). Consistent with the heatmap and hierarchical clustering analyses, HEY2 contributed less robustly to arterial gene regulation (∼22%; supplemental Table 6). Although the hierarchical cluster analysis revealed a significant degree of complementarity between TFs, we also observed overlap, suggesting complex interactions. Therefore, we mapped these interactions in a network (Figure 5C). While some genes (eg, APO, GRB14, ARL15) were exclusively regulated by 1 TF, most genes were coregulated by >1 TF (eg, FAT1 was coregulated by 6 TFs). Furthermore, we also observed crossregulation between different TFs (for instance MSX1 being regulated by EMX2, NKX2-3, Prdm16, and TOX2 and HEY2 being regulated by Prdm16 and MSX1). Finally, some TFs (ie, TOX2 and SOX17) modulated their own expression. Thus, induction of the arterial gene signature requires a complex interaction of multiple TFs.
Individual TFs interact in a complex network to regulate the arteriovenous fresh profile. A schematic overview of the complex interaction by the TFs underlying the regulation of the arteriovenous fresh profile. (A-B) Pie diagrams representing the proportion of arterial (A) or venous (B) genes of the arteriovenous fresh profile regulated by 0 (blue), 1 (purple), 2 (orange), 3 (green), or >3 (beige) TFs. Corresponding absolute numbers are listed in the table on the right of the panel. (C) To summarize all information on the effect of TF overexpression on the arteriovenous fresh profile, we composed an interaction network of the 8 TFs and the arteriovenous fresh profile. Because Aff3 did not regulate any gene of the signature, nor was it regulated by the other TFs, it is not included in the network. Each TF hub (represented by rounded boxes) is drawn in a different color and interactions originating from each hub are shown by arrows (induction) or vertical lines (inhibition) in the corresponding color. Genes are assigned to the hubs according to the TF by which they were most strongly regulated. For those genes exclusively regulated by 1 TF, the box of that gene is drawn in dark color, whereas for genes that are regulated by >1 TF, the box is drawn in light color. TFs are in oval boxes, arterial genes are in rectangular boxes with full lines, and venous genes are in rectangular boxes with dashed lines.
Individual TFs interact in a complex network to regulate the arteriovenous fresh profile. A schematic overview of the complex interaction by the TFs underlying the regulation of the arteriovenous fresh profile. (A-B) Pie diagrams representing the proportion of arterial (A) or venous (B) genes of the arteriovenous fresh profile regulated by 0 (blue), 1 (purple), 2 (orange), 3 (green), or >3 (beige) TFs. Corresponding absolute numbers are listed in the table on the right of the panel. (C) To summarize all information on the effect of TF overexpression on the arteriovenous fresh profile, we composed an interaction network of the 8 TFs and the arteriovenous fresh profile. Because Aff3 did not regulate any gene of the signature, nor was it regulated by the other TFs, it is not included in the network. Each TF hub (represented by rounded boxes) is drawn in a different color and interactions originating from each hub are shown by arrows (induction) or vertical lines (inhibition) in the corresponding color. Genes are assigned to the hubs according to the TF by which they were most strongly regulated. For those genes exclusively regulated by 1 TF, the box of that gene is drawn in dark color, whereas for genes that are regulated by >1 TF, the box is drawn in light color. TFs are in oval boxes, arterial genes are in rectangular boxes with full lines, and venous genes are in rectangular boxes with dashed lines.
Overexpression of the 8 TFs in HUVECs conveys an arterial-like behavior in vivo
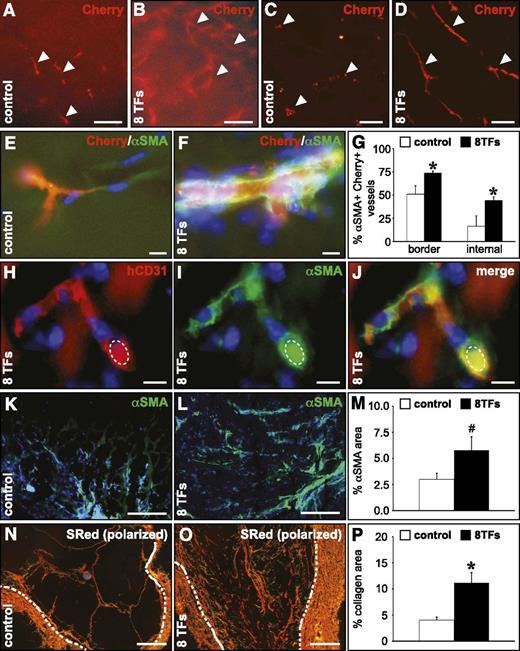
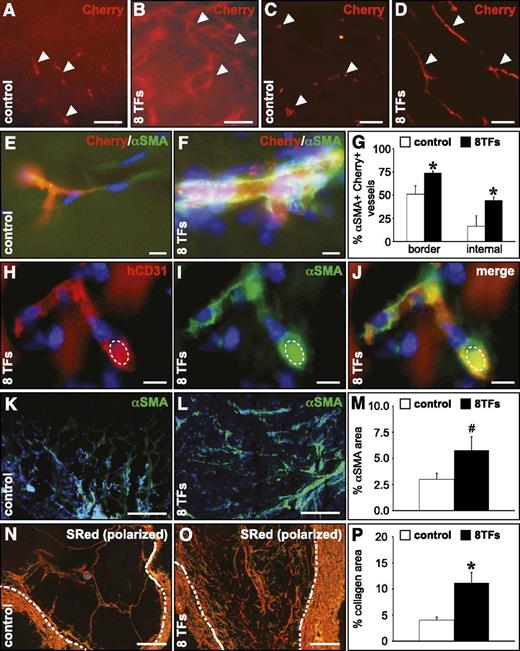
To determine whether transduction of HUVECs with the 8 arterial TF combination would enable them to participate in the formation of vascular structures with arterial-like characteristics in vivo, we performed a subcutaneous Matrigel plug assay.30 In comparison with HUVECs transduced with control virus, HUVECs infected with the 8 TFs formed more elaborate vascular networks (Figure 6A-D), which were significantly more covered by αSMA+ smooth muscle cells (SMCs) both in the outer border and in the internal core of the Matrigel (Figure 6E-G). The human endothelial identity of implanted HUVECs was confirmed by expression of human-specific CD31 and the presence of erythrocytes in these vessels showed that they were functionally connected to the host vasculature (Figure 6H-J). Moreover, the relative Matrigel area covered by SMCs (Figure 6K-M) and fibrillar collagen (Figure 6N-P) was greatly enhanced in Matrigels containing HUVECs overexpressing the 8 TF combination, both representing signs of more arterialized vessels.
Overexpression of the 8 TFs in HUVECs conveys an arterial-like behavior upon their implantation in a Matrigel plug in vivo. Overexpression of the 8 TFs in HUVECs results in a more significant arteriogenic capacity in a subcutaneous Matrigel implant. (A-D) Fluorescent micrographs of Matrigel implants containing HUVECs (revealed by the Cherry signal in red; indicated by white arrowheads) transduced with a control vector (A,C) or with the combination of the 8 TFs (B,D). Panels A-B represent the ex vivo nonsectioned Matrigel explant, and panels C-D represent frozen cross sections. (E-G) Frozen cross sections from Matrigels containing control HUVECs (E; white bars in G) or HUVECs transduced with the 8 TFs (F; black bars in G) stained with αSMA (green), and the corresponding quantification (G) showing the percentage of HUVEC-containing (revealed by the Cherry signal) vessels coated with αSMA+ cells (*P < .05 vs control). (H-J) Cross section from a Matrigel containing HUVECs transduced with 8 TFs stained with human-specific CD31 (red) and αSMA (green) and the corresponding overlay (J). An autofluorescent erythrocyte is indicated by a dashed ellipse. (K-M) Cross sections from a Matrigel containing control HUVECs (K; white bar in M) or HUVECs transduced with 8 TFs (L; black bar in M) stained with αSMA (green) and the corresponding quantification (M), showing the relative αSMA+ area (#P = .08 vs control). (N-P) Cross sections from a Matrigel containing control HUVECs (N; white bar in P) or HUVECs transduced with 8 TFs (O; black bar in P) stained with Sirius red (visualized under polarized light) and the corresponding quantification (P), showing the relative Sirius red–positive area (*P < .05 vs control). Dashed white lines indicate the inner edge of the fibrous Matrigel capsule. 4,6 Diamidino-2-phenylindole (blue) is used as nuclear counterstain in panels E, F, and H-L. Scale bars: 100 μm in A-D, 10 μm in E-F and H-J, and 200 μm in K-L and N-O.
Overexpression of the 8 TFs in HUVECs conveys an arterial-like behavior upon their implantation in a Matrigel plug in vivo. Overexpression of the 8 TFs in HUVECs results in a more significant arteriogenic capacity in a subcutaneous Matrigel implant. (A-D) Fluorescent micrographs of Matrigel implants containing HUVECs (revealed by the Cherry signal in red; indicated by white arrowheads) transduced with a control vector (A,C) or with the combination of the 8 TFs (B,D). Panels A-B represent the ex vivo nonsectioned Matrigel explant, and panels C-D represent frozen cross sections. (E-G) Frozen cross sections from Matrigels containing control HUVECs (E; white bars in G) or HUVECs transduced with the 8 TFs (F; black bars in G) stained with αSMA (green), and the corresponding quantification (G) showing the percentage of HUVEC-containing (revealed by the Cherry signal) vessels coated with αSMA+ cells (*P < .05 vs control). (H-J) Cross section from a Matrigel containing HUVECs transduced with 8 TFs stained with human-specific CD31 (red) and αSMA (green) and the corresponding overlay (J). An autofluorescent erythrocyte is indicated by a dashed ellipse. (K-M) Cross sections from a Matrigel containing control HUVECs (K; white bar in M) or HUVECs transduced with 8 TFs (L; black bar in M) stained with αSMA (green) and the corresponding quantification (M), showing the relative αSMA+ area (#P = .08 vs control). (N-P) Cross sections from a Matrigel containing control HUVECs (N; white bar in P) or HUVECs transduced with 8 TFs (O; black bar in P) stained with Sirius red (visualized under polarized light) and the corresponding quantification (P), showing the relative Sirius red–positive area (*P < .05 vs control). Dashed white lines indicate the inner edge of the fibrous Matrigel capsule. 4,6 Diamidino-2-phenylindole (blue) is used as nuclear counterstain in panels E, F, and H-L. Scale bars: 100 μm in A-D, 10 μm in E-F and H-J, and 200 μm in K-L and N-O.
Discussion
Multiple studies addressed how the specific identity of aECs is established, each highlighting a particular pathway. None took a genome-wide approach, except for one study reporting a global differential gene screen between several aEC and vEC types.4 However, this study only included cultured cells. Here, we demonstrate that the culturing process rapidly and largely “erased” the specific expression profile of HUVECs/HUAECs. Interestingly, this dramatic loss was not due to the presence of serum, which may induce endothelial-to-mesenchymal transition upon culture (data not shown). To get insight into the in vivo expression pattern of aECs, we undertook a genome-wide differential screen on freshly isolated HUVECs/HUAECs. In this way, we established a specific arteriovenous fresh profile of 76 genes. This gene signature was not determined simply by Notch signaling (currently considered as the gold standard for arterial-fate decision making), but rather was coregulated by additional TFs, many of which not being previously associated with aEC specification. Overexpressing them in HUVECs revealed that they regulate arterial identity in a complex interactive fashion and that this overexpression can convey an arterial-like behavior in vivo. Finally, maintenance of the arterial expression profile was dependent on sustained TF overexpression.
The biological relevance of our profile is supported by several observations. First, for 16 (21%) of the genes, expression has been described in aECs and/or vECs, 4 of which have been documented to play a role in arteriovenous specification. Second, mining the literature and gene databases revealed that genes within this profile encode proteins related to functions of central importance in the cardiovascular system and its development. Third, for about one-third of the genes for which a transgenic mouse has been reported, vascular phenotypes were observed. Quite possibly, this number is underestimated because not all of the studies focused on vascular biology. Fourth, 7 genes have been associated with cardiovascular disease or risk factors (eg, hypertension) in humans. Finally, our screen also extracted the Notch pathway in the form of its downstream effector HEY2, which has been advocated as a critical denominator of arterial identity.4,27,31 Nevertheless, certain arteriovenous specification pathways (eg, BMP17 or Wnt16 ) known to be involved during development, or factors related to SMC recruitment (eg, platelet-derived growth factor-B or transforming growth factor-β), did not seem to be represented in our arteriovenous fresh profile. This could be due to the stringency we applied to our data set or to the fact that their differential involvement in EC specification is rather apparent at the posttranscriptional level, eg, by differential phosphorylation of certain pathway members. Furthermore, some of the genes from our profile (eg, MSX1, EMX2) were linked to these pathways according to an ingenuity pathway analysis, although this link has not necessarily been demonstrated in ECs. A fourth possibility is that our arterial profile originating from ECs from established vessels may rather include a gene set important for maintenance of the arterial phenotype instead of genes involved during arteriovenous specification of emerging vessels during development. This set may for instance contain alternative SMC “retention” signals, such as SEMA3G and SEMA3C, previously reported to be involved in SMC (progenitor) recruitment.32,33 Our in vivo results showing increased SMC coating of host-derived vessels upon TF overexpression in HUVECs indeed suggest induction of such signals.
The lack of differential expression in cultured ECs in our study is at variance with the study by Chi et al, who conducted a differential screen on 53 cultured EC samples, including ECs from 5 artery and 2 vein types.4 Unlike for our cultured samples, their samples grouped according to their sites of origin upon hierarchical clustering, suggesting that the different sample subtypes had a characteristic expression profile that persisted in culture.4 A plausible explanation for the discrepancies could be the inclusion of different types of samples in the 2 studies combined with a difference in statistical stringency. Chi et al did not perform a multiple testing correction because of the heterogeneity of the samples, whereas we applied such a correction to limit the false-positive discovery rate. Most likely, however, although deprivation of environmental in vivo signals induces a dramatic loss of differential gene expression in ECs, the existence of specific aEC and vEC signatures during development before the exposure to certain environmental influences (such as flow) suggests that some intrinsic expression differences should remain in cultured aECs vs vECs. Nevertheless, our findings underscore that studies in cultured ECs should be interpreted with caution, because they certainly do not represent the in vivo situation.
Our study is the first to report an unbiased genome-wide screen on freshly isolated aECs and vECs, which enabled us to unveil new transcriptional regulators of arteriovenous specification. In addition, we reveal for the first time, by inhibiting Notch, that canonical Notch signaling is inactive in cultured aECs, which together with the loss of TF expression is in large part responsible for the culture-induced assimilation between aECs and vECs. Although DAPT is generally used as a canonical Notch signaling inhibitor downstream of the Notch receptor/ligand level, we found that it was downregulating DLL4. Although others have speculated this could be due to a positive feedback loop of Notch signaling on DLL4 expression,34 it is also possible that γ-secretase, the DAPT target, has a direct effect on DLL4 expression, independent of Notch signaling. Despite its commonly accepted role in arteriovenous specification, the importance of Notch signaling in general and of HEY2 in particular was not greater than that of some other TFs identified in our profiling study, emphasizing the critical role of these novel TFs. Interestingly, some of the latter (eg, Prdm16, EMX2, NKX2-3) have been associated with Notch signaling in various biological contexts and may thus regulate arteriovenous identity partially through Notch signaling.35-37 Also, others have shown that Notch activation is essential, but not sufficient, to induce arterial specification, such as during the differentiation of aECs from progenitors.18 Furthermore, recent studies have shown that Notch acts downstream of or in parallel with additional pathways during arteriovenous specification.20,21 The need of Hey2 to cooperate with Hey1 to manifest its effect27 might be responsible for the limited effect upon HEY2 overexpression. Also, the limited overlap of regulated genes upon HEY2 overexpression and canonical DLL4-induced activation suggests that Notch acts through additional effectors. In addition to identifying new transcriptional regulators of aEC specification, we also sought to determine how these factors interact to establish the aEC signature. Our network did not include Aff3 because it did not have any effect as a single factor on gene expression. Nevertheless, this does not rule out that Aff3 plays an important role in combination with the other TFs or that Aff3 needs certain cofactors to exert its effect on (arterial) gene transcription. Furthermore, such interactions may explain why a vascular phenotype has not been detected for mice lacking the expression of a certain TF or regulated arterial genes (supplemental Table 4). The only partial overlap in regulated genes together with the synergy among the TFs further suggests that each of them plays an indispensible role in the specification process. Of note, even though Notch activation, which was one of the effects accomplished by the 8 TF combination, has generally been associated with lower vascular density,38 we observed a more elaborate vascular network in the 8 TF condition. The latter could be due to Notch-independent effects of certain TFs or to a reduced regression owing to improved stabilization with SMCs.
Although the TFs collectively regulated ∼75% of the genes in the arteriovenous fresh profile and achieved high-range expression levels for many of them upon overexpression, additional mechanisms likely exist that drive the remaining 25% of unregulated genes or boost the expression levels of still lowly expressed genes. For instance, our studies were performed under static conditions, whereas others documented the importance of flow in arteriovenous plasticity.15 Interestingly, our arterial gene list included H19, a mir675 precursor,39 suggesting that posttranscriptional regulation adds another level of complexity. Our overexpression experiments were performed in the absence of SMCs or a 3-dimensional microenvironment, which all may codetermine arteriovenous gene expression. Therefore, re-creating the proper microenvironment in culture should help to maintain EC identity. Furthermore, we need to take into account that oxygen levels may also influence arterial specification.40 In this context, a limitation of our study is the fact that our ex vivo profile was established based on arteries and veins from the umbilical cord, which differ in several aspects from most arteries and veins: the oxygenation level of the blood is reversed, they are of fetal origin, and they are exposed to different flow dynamics. Nevertheless, our studies revealed that, with the exception of 2 genes (SLC2A1 and SLC2A3) representing the HIF1α pathway, the oxygenation level was not a major determinant of our arteriovenous fresh profile (data not shown), and hence this did not represent a bias toward the establishment of a more generalized arteriovenous fingerprint.
In conclusion, our pioneering profiling study on freshly isolated ECs unveiled a combinatorial transcriptional code that induced an arterial fingerprint more proficiently than the current gold standard, HEY2, and this code conveyed an in vivo arterial-like behavior upon venous ECs. This more profound insight into arterial specification can lead to important applications. First, atherosclerosis, resulting in ischemia, only affects arteries. Restoration of the perfusional defect mostly requires expansion of arterial blood supply. Current “general” revascularization strategies have not taken into account this specific need for arterial supply, and this may in part explain their limited clinical success.41 Arterial prespecification before cell transfer may significantly improve arterial engraftment. In addition, increased insight in aEC specification may provide new targets for specific growth factor therapy. Overall, the increased understanding of the in vivo gene signature of aEC might enable us to provide more adequate treatments.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
The authors thank P. Vandervoort, T. Vervoort, B. Hoekman, and T. Koninckx for technical assistance. Nanostring nCounter analyses were performed by the VIB Nucleomics Core Facility (http://www.nucleomics.be).
This work was supported by grants from the European Commission (FP7-StG-IMAGINED 203291) (A.L.) (STREP-FP6-StrokeMAP) (A.L., F.P.) (FP7-INELPY) (F.P.), a Fund for Scientific Research (FWO) postdoctoral fellowship (X.L.A.), an Agentschap voor Innovatie door Wetenschap en Technologie (IWT) predoctoral fellowship (M. Beerens), an FWO predoctoral fellowship (I.V.), a KU Leuven Geconcerteerde Onderzoeksacties grant (GOA11/012), a KU Leuven Center of Excellence grant (EF/05/013) (A.L.), a KU Leuven Program Financing grant (PF/10/014) (A.L.), four FWO research grants (FWO KAN2007 1.5219.08, KAN2012 1.5102.12, G.0393.12, and G.0B09.13) (A.L.), an Interuniversitaire Attractiepolen (IUAP) grant (DevRepair) (A.L.), and the UTE project CIMA (F.P.).
Authorship
Contribution: X.L.A. designed and performed research, analyzed data, and assisted in writing the manuscript. X.A., M. Beerens, G.C., M.U., and I.V. designed and performed research and analyzed data. M. Benkheil, J.P., A. P-M., and V.S. analyzed data. N.A. performed research. F.P. assisted in writing the manuscript. A.L. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aernout Luttun, Molecular and Vascular Biology Research Unit, KU Leuven, Campus Gasthuisberg, Onderwijs & Navorsing 1, Herestraat 49, B-3000 Leuven, Belgium; e-mail: aernout.luttun@med.kuleuven.be; or Felipe Prósper, Hematology and Cell Therapy Area, Clinica Universidad de Navarra and Division of Oncology, Center for Applied Medical Research, University of Navarra, Avenida Pio XII 36, 31008 Pamplona, Spain; e-mail: fprosper@unav.es.

![Figure 1. Freshly isolated HUAECs and HUVECs have a different molecular and functional profile. An overview of the differential gene expression profile and corresponding functional signature of HUAECs and HUVECs. (A) Pie diagrams showing the number of annotated arterial (red) and venous (blue) genes emerging as differentially expressed between freshly isolated HUAECs and HUVECs from the differential gene statistical analysis (upper left) or classification analysis (upper right). The lower diagram shows the total amount of annotated genes and corresponding percentages (black lettering; arterial-enriched genes in red, venous-enriched genes in blue lettering) uniquely identified by the differential gene analysis (pink), from the classification analysis (yellow) or emerging from both analyses (orange; which included 4 genes, ie, HEY2, NOTCH4, SOX17, and KDR, previously associated with arterial specification). Other displayed names correspond to the most differentially expressed genes in each part of the diagram. (B-C) Bar diagrams showing functional terms (B) and pathways (C) significantly enriched in the 76 differential gene set. Numbers of associated genes per function/pathway are mentioned on top of the bars. Yellow bars represent subcategories of the main functional term “cardiovascular diseases.” (D) Bar diagram showing microarray probe set intensities (± standard error of the mean [SEM]; n = 4) for the 8 arterial (A) and 1 venous (V) TFs identified among the arteriovenous fresh profile, revealing their differential expression in freshly isolated HUAECs (red) and HUVECs (blue). *P < .05 vs arterial probe set intensity. (E-H) Immunofluorescence stainings on paraffin cross sections of human umbilical cord (umbilical vein is shown in E,G, umbilical artery in F,H) for αSMA (in green) and MSX1 (E-F; in red) or NR3C2 (G-H; in red). The curved bright red line in panel E represents an aspecific signal from the elastica interna. 4,6 Diamidino-2-phenylindole (blue) was used as nuclear counterstain. White arrowheads indicate positive staining in the endothelial lining. Scale bars: 20 μm. CV, cardiovascular; NO, nitric oxide; nNOS, neuronal nitric oxide synthase.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/24/10.1182_blood-2013-02-483255/4/m_3982f1.jpeg?Expires=1769099507&Signature=QDQMbYWugxRtiYQw5Cj7E~TH4GWwKTssqrSUnDBM2vYR~DCk~ByWykszEG6146yeUBT8CshAPcPKI8CJoHn1rIJBN5c5HIF4d63~hWLFruYNFPdHsu95x4U9dKq6BwpiD9lUy5rqE4GkSc6EpUZbw-2Tdp0GYeXrgPW7oECfwDcdKlW3v~t~axuxyGPvgVMNsPWEurR--DCtE3Giz~lOy~L00T0afNtNpAeAbfBEnyArxsxD8hIPPESjQJmPIeEbO2WtiNoj3dJdGP9SLmVgtzSDZz7oZqyx1sMwRy23c-2euUeUi1S6uv6CEhEQ0C60Fy~fRmbrTXjuXDLotM9Tbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. The culturing process rapidly erases differential arteriovenous gene expression. HUAECs and HUVECs lose their differential expression profile, including that of TFs, upon culture. (A) Hierarchical clustering analysis for freshly isolated or cultured HUAECs (red) or HUVECs (blue) revealing that for fresh samples replicates of each cell type tightly cluster together and both clusters are nicely separated. For cultured samples, incorrect clustering occurred. (B) Diagram representing the probe set intensity difference between HUVECs and HUAECs for each probe of the arteriovenous fresh profile (showing arterial probes [A] on the left and venous probes [V] on the right) for cultured (filled diamonds) or freshly isolated (open triangles) cells, revealing only minor differences in cultured cells. (C) Diagram representing the expression (±SEM; n = 4) determined by qRT-PCR for arterial TFs in freshly isolated cells (open triangles) or cultured cells (filled diamonds) relative to their expression in freshly isolated cells. *P < .05 vs freshly isolated cells. (D-E) Diagrams showing probe set intensity (±SEM; n = 4) for arterial (A) and venous (V) probes of the arteriovenous fresh profile for cultured (filled diamonds) or freshly isolated (open triangles) HUAECs (D; symbols in red) or HUVECs (E; symbols in blue). (F) Diagram showing that the average probe set intensity for all arterial probes (dashed lines) or venous probes (full lines) in freshly isolated HUAECs (left; red) or HUVEC (right; blue) “bleach” to a default average expression level (gray dashed line) upon culturing (middle; pink). *P < .05 vs fresh. NS, not significant vs fresh (n = 4-5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/24/10.1182_blood-2013-02-483255/4/m_3982f2.jpeg?Expires=1769099507&Signature=IiqtLBZqoSTnOjnCmRkuCc5N91aUC~lIKQO1~bf386enSbf9LWw40BXIemea7eLElBVd0M5H7okkWB-5xek569UaKXSRlnfL1YRghEkxWQeJNchY72CIExCLFL3E7-jGOT6F-BcfDsUQYnFMcTGuE2ho082sVoOWOvNPhz6df90PUrydLizScosJxg3ndkYXLS6Xiygxk2sKJ5irtYk4L4hUBQEjsCy3ZmBWlv31TO8v1aqi86Mre0BemVWi9TLbe4NsceVgEjzjUh3TP-9t6RDhSlvwAC-jnglr5xwIiGKKv8-KrDBvEYEOKwub03fqxoNBxzCLAIGyhzGvU~uaYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. Freshly isolated HUAECs and HUVECs have a different molecular and functional profile. An overview of the differential gene expression profile and corresponding functional signature of HUAECs and HUVECs. (A) Pie diagrams showing the number of annotated arterial (red) and venous (blue) genes emerging as differentially expressed between freshly isolated HUAECs and HUVECs from the differential gene statistical analysis (upper left) or classification analysis (upper right). The lower diagram shows the total amount of annotated genes and corresponding percentages (black lettering; arterial-enriched genes in red, venous-enriched genes in blue lettering) uniquely identified by the differential gene analysis (pink), from the classification analysis (yellow) or emerging from both analyses (orange; which included 4 genes, ie, HEY2, NOTCH4, SOX17, and KDR, previously associated with arterial specification). Other displayed names correspond to the most differentially expressed genes in each part of the diagram. (B-C) Bar diagrams showing functional terms (B) and pathways (C) significantly enriched in the 76 differential gene set. Numbers of associated genes per function/pathway are mentioned on top of the bars. Yellow bars represent subcategories of the main functional term “cardiovascular diseases.” (D) Bar diagram showing microarray probe set intensities (± standard error of the mean [SEM]; n = 4) for the 8 arterial (A) and 1 venous (V) TFs identified among the arteriovenous fresh profile, revealing their differential expression in freshly isolated HUAECs (red) and HUVECs (blue). *P < .05 vs arterial probe set intensity. (E-H) Immunofluorescence stainings on paraffin cross sections of human umbilical cord (umbilical vein is shown in E,G, umbilical artery in F,H) for αSMA (in green) and MSX1 (E-F; in red) or NR3C2 (G-H; in red). The curved bright red line in panel E represents an aspecific signal from the elastica interna. 4,6 Diamidino-2-phenylindole (blue) was used as nuclear counterstain. White arrowheads indicate positive staining in the endothelial lining. Scale bars: 20 μm. CV, cardiovascular; NO, nitric oxide; nNOS, neuronal nitric oxide synthase.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/24/10.1182_blood-2013-02-483255/4/m_3982f1.jpeg?Expires=1769183501&Signature=26vxMn55TmXHORlz~tjn6~iI6o4Uw381CoaO7e557OfCLdzKSRtSZgOrFHYKo7vvXLxletRyOxq~3iQkPV63IrQwXsJfN1hiRh~nYtprU~GdseWurSvUdfSOf1XTxPIvkiF8kEwTg78F~XC8kPPWe1sDJnhLKEffm2lLi5GgoXaRRyDSZ8-dEr7r0DDzxVXnTxJWVA6KbK~naI6UtBd-hkKMnufphtLW-QYBD~tJfD1mrIUEQbdbOz6JHAR2Fzw5i3JhqojacQ8hvDMONQCCuMwFY2zjEMs7sD3ANo6pI2s8w311cNFx115r73Z6XXTcpmxAn2KVtrroIcUektzQgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. The culturing process rapidly erases differential arteriovenous gene expression. HUAECs and HUVECs lose their differential expression profile, including that of TFs, upon culture. (A) Hierarchical clustering analysis for freshly isolated or cultured HUAECs (red) or HUVECs (blue) revealing that for fresh samples replicates of each cell type tightly cluster together and both clusters are nicely separated. For cultured samples, incorrect clustering occurred. (B) Diagram representing the probe set intensity difference between HUVECs and HUAECs for each probe of the arteriovenous fresh profile (showing arterial probes [A] on the left and venous probes [V] on the right) for cultured (filled diamonds) or freshly isolated (open triangles) cells, revealing only minor differences in cultured cells. (C) Diagram representing the expression (±SEM; n = 4) determined by qRT-PCR for arterial TFs in freshly isolated cells (open triangles) or cultured cells (filled diamonds) relative to their expression in freshly isolated cells. *P < .05 vs freshly isolated cells. (D-E) Diagrams showing probe set intensity (±SEM; n = 4) for arterial (A) and venous (V) probes of the arteriovenous fresh profile for cultured (filled diamonds) or freshly isolated (open triangles) HUAECs (D; symbols in red) or HUVECs (E; symbols in blue). (F) Diagram showing that the average probe set intensity for all arterial probes (dashed lines) or venous probes (full lines) in freshly isolated HUAECs (left; red) or HUVEC (right; blue) “bleach” to a default average expression level (gray dashed line) upon culturing (middle; pink). *P < .05 vs fresh. NS, not significant vs fresh (n = 4-5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/24/10.1182_blood-2013-02-483255/4/m_3982f2.jpeg?Expires=1769183501&Signature=CWkSgk7phLD5TSiChAPFhbuP0jwXKUlspuiF2m2u58NYC-N1HQyHuUM3TggRvojb2A2yglOffyOG~5ivMbHbX4YnaRgDU3p99KstGTQWCxcm-fvTXTRATOFjwwupB4itep2nMkudI7An6a107XELeQaHuXY7rHV2THpkkIVGSgbUsE4eCkHXiSlS6JuKySZ0m2WmV5Lv2imPh2d2~hT3xDomZyRynirA3UNZgVjMCMed2bVre-ZqPdJ-RB3sEHXAzkxhY-JvPvjgPaaJLvS2kldCoHwFg-st7v9w5ukIAblNRgkVpKwa0ZHrmsHJVey83a3M3q4to27j0wuZe~wGFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)