Key Points
ESL-1 and PSGL-1 cooperate to mediate E-selectin binding, myeloid homeostasis, and inflammatory cell recruitment.
ESL-1 dominates E-selectin binding and homing of hematopoietic progenitors.
Abstract
Beyond its well-established roles in mediating leukocyte rolling, E-selectin is emerging as a multifunctional receptor capable of inducing integrin activation in neutrophils, and of regulating various biological processes in hematopoietic precursors. Although these effects suggest important homeostatic contributions of this selectin in the immune and hematologic systems, the ligands responsible for transducing these effects in different leukocyte lineages are not well defined. We have characterized mice deficient in E-selectin ligand-1 (ESL-1), or in both P-selectin glycoprotein-1 (PSGL-1) and ESL-1, to explore and compare the contributions of these glycoproteins in immune and hematopoietic cell trafficking. In the steady state, ESL-1 deficiency resulted in a moderate myeloid expansion that became more prominent when both glycoproteins were eliminated. During inflammation, PSGL-1 dominated E-selectin binding, rolling, integrin activation, and extravasation of mature neutrophils, but only the combined deficiency in PSGL-1 and ESL-1 completely abrogated leukocyte recruitment. Surprisingly, we find that the levels of ESL-1 were strongly elevated in hematopoietic progenitor cells. These elevations correlated with a prominent function of ESL-1 for E-selectin binding and for migration of hematopoietic progenitor cells into the bone marrow. Our results uncover dominant roles for ESL-1 in the immature compartment, and a functional shift toward PSGL-1 dependence in mature neutrophils.
Introduction
To perform their immune functions, leukocytes traffic actively from the blood into immune organs or inflamed tissues. In the particular case of neutrophils, inflammatory recruitment critically relies on the expression of the 2 endothelial selectins by the activated endothelium.1 P- and E-selectins recognize highly glycosylated proteins decorated with fucosylated and sialylated moieties present on neutrophils, monocytes, and some lymphocyte subsets.2,3 Although P-selectin glycoprotein-1 (PSGL-1) was found in early studies to almost exclusively mediate binding to P-selectin,4,5 the identification of leukocyte ligands for E-selectin has proven to be more challenging. This has been in part because of the highly glycosylated structure and poor immunogenicity of its ligands, which have prevented generation of function-blocking antibodies.6 Another reason has been that many different leukocyte glycoconjugates are capable of mediating binding to E-selectin, at least in vitro.
Through the use of gene-deficient mice, several surface glycoproteins, including PSGL-1, CD44, and CD43, have been shown to function as physiological ligands for E-selectin in different leukocyte subsets.3 PSGL-1 and CD44 were shown in these mice to cooperate for E-selectin–mediated recruitment of neutrophils and inflammatory T cells in vivo7,8 ; however, the significant selectin-binding activity remaining in double-deficient mice argued for the presence of 1 or more additional ligands. Although the unavailability of mice deficient in E-selectin ligand-1 (ESL-1; encoded by Glg1) has prevented analysis of the contribution of this glycoprotein to leukocyte trafficking, studies that used short hairpin RNA–mediated silencing of Glg1 suggested that this glycoprotein cooperated with PSGL-1 and CD44 for E-selectin binding and recruitment of transduced neutrophils during inflammation.9 However, the reduced fraction of transduced leukocytes in these studies precluded analysis of global hematologic or immune alterations because of the absence of the glycoprotein during inflammation or in the steady state. These limitations also prevented dissection of the roles of ESL-1 in rare populations of hematopoietic cells. Particularly relevant among these are hematopoietic progenitor cells (HPCs), which rely on E-selectin for efficient migration to the bone marrow (BM).10-12 Remarkably, however, the ligands that control E-selectin binding on HPCs in vivo have not been defined.
Beyond its contributions to rolling, recent studies have uncovered important additional functions for E-selectin. Engagement of E-selectin on rolling neutrophils can initiate intracellular signals through the canonical ligands PSGL-1 and CD44. These signals, in turn, promote Gαi-independent activation of the β2 integrin lymphocyte function antigen-1 (LFA-1), resulting in slow rolling, enhanced adhesion, and recruitment into inflamed tissues.13,14 More recently, an elegant study demonstrated that E-selectin expressed by vascular cells in the BM also controls the quiescence, self-renewal, and chemoresistance of hematopoietic stem cells.15 Notably, this study also revealed that E-selectin–binding activity in murine HPCs is largely independent of PSGL-1 and CD44, implying that a different ligand mediates E-selectin binding in the immature compartment.
In this study, we have analyzed mice deficient in ESL-1 and PSGL-1, and generated double-deficient mutants to dissect their physiological contributions within the hematopoietic compartment. We report that PSGL-1 and ESL-1 cooperate to maintain myeloid homeostasis in the steady state and allow neutrophil recruitment during inflammation, but only PSGL-1 controls integrin activation and slow rolling. In contrast, ESL-1 was the dominant ESL in, and controlled homing of, immature hematopoietic progenitors to the BM. Our results thus reveal a functional shift of selectin ligand use, from ESL-1 to PSGL-1, during myeloid maturation.
Materials and methods
Mice
Six- to twelve-week-old C57BL/6 male and female mice were used for the transplantation studies. DsRed-transgenic mice (under a β-actin promoter) were used as donors in some transplantation studies. ESL-1–deficient (Glg1−/−) mice were generated as reported previously.16 PSGL-1–deficient (Selplg−/−) and E-selectin–deficient (Sele−/−) mice have already been described.5,9,11 Selplg−/−Glg1−/+ mice were bred to generate ESL-1/PSGL-1 double-deficient mice. The genotypes were determined by polymerase chain reaction. Chow and water were available ad libitum. All mice were in a pure C57BL/6 background. Mice were housed in a specific pathogen-free facility at Centro Nacional de Investigaciones Cardiovasculares (CNIC). Experimental procedures were approved by the Animal Care and Ethics Committee of the CNIC.
Generation of BM chimeras by transplantation
We harvested donor BM cells from the appropriate genotype (wild-type [WT], WT DsRed, PSGL-1−/−, ESL-1−/−, or ESL-1/PSGL-1−/− double-knockout [DKO]), which were mixed with equal numbers of WT-Dsred BM cells for transplantation. For some experiments, only BM cells from the 4 groups without DsRed+ competitors were used. Recipient WT C57BL/6 mice were lethally irradiated (2 doses of 6.5 Gy at 3 hours apart; total, 13 Gy) before receiving 2 million BM cells intravenously. We assessed engraftment of recipient animals 6 weeks after transplantation by flow cytometry.
Flow cytometry and E-selectin–binding assay
Primary blood leukocytes were incubated with DyLight650-conjugated anti-Ly6G antibody (clone 1A8, BioXcell) to detect the neutrophil population. Fluid-phase binding of the E-selectin-IgM chimera to blood leukocytes was performed as described previously.7 Neutrophils were gated on the basis of Ly6G+ expression, and E-selectin binding was compared between DsRed-negative and DsRed-positive populations within the same sample. For hematopoietic progenitors, 5 × 106 BM cells were first stained to exclude lineage (CD11b, Gr-1, CD3ε, B220, and TER119)–positive cells and then were stained for Sca-1 and c-Kit. LSK cells were defined as LineageNEG Sca1+ c-kitHI cells and myeloid progenitors as LineageNEG Sca1NEG c-kitHI cells. In other experiments, blood or BM cells were stained with DyLight649-conjugated anti-Gr1 antibody (Clone RB6, eBioscience) and phycoerythrin (PE)-conjugated or biotin-conjugated anti-CD115 antibody (BioXcell), followed by Streptavidin-eFluor 450 (eBioscience) to identify neutrophil and monocytes (supplemental Figure 3). These samples were additionally stained with PerCP/Cy 5.5-conjugated anti-CXCR2 antibody (Biolegend), PE-conjugated anti-CXCR4 antibody (eBioscience), or isotype controls. For annexin V binding, blood leukocytes stained for Gr-1/CD115 were stained with PE-conjugated annexin V following the manufacturer's instructions (BD Biosciences). Samples were acquired using a fluorescence-activated cell sorter Canto flow cytometer equipped with DIVA software (BD Biosciences). Data were analyzed with the DIVA or FlowJo (TreeStar Inc.; Ashland, OR) software. All experiments were conducted at the CNIC-Cellomics Unit.
Intravital microscopy
Intravital microscopy of the cremaster muscle after tumor necrosis factor-alpha (TNF-α) injection (0.5 μg, intrascrotal injection) was performed exactly as reported.17 The intravital microscopy system was built by 3i (Intelligent Imaging Innovations, Denver, CO) on an Axio Examiner Z.1 workstation (Zeiss, Oberkochen, Germany) mounted on a 3-Dimensional Motorized Stage (Sutter Instrument, Novato, CA), allowing precise computer-controlled lateral movement between the X-Y positions and a Z-focusing drive to allow the focal plane to be rapidly changed. The microscope is equipped with a CoolLED pE widefield fluorescence LED light source system (CoolLED Ltd, UK). A quad pass filter cube was used with a Semrock Di01-R405/488/561/635 dichroic and FF01-446/523/600/677 emitter. We used a plan-APOCHROMAT 40× NA 1.0 ∞/0 water-immersion objective (Zeiss). Images were collected with a CoolSnap HQ2 camera (6.45 × 6.45-µm pixels, 1392 × 1040 pixel format; Photometrics, Tucson, AZ). The SlideBook software (Intelligent Imaging Innovations), run on a Dell Precision T7500 computer system (Dell Inc., Round Rock, TX), coordinated image acquisition and facilitated offline data analysis. Six to ten venules per mouse were analyzed 150 to 210 minutes after TNF-α treatment by acquisition of fluorescence (Cy3 channel for dsRed) and bright-field images with 2 × 2 or 4 × 4 binning for 2 minutes. For obtaining centerline blood velocities, a 7- to 10-second movie was acquired for each venule at 40 Hz with the Cy3 channel, 4 × 4 binning, to allow velocity measurement of free-flowing cells.
Thioglycollate-induced peritonitis
To assess recruitment efficiency of mutant neutrophils, we generated cohorts of hematopoietic chimeras by transplantation of BM cells from WT-DsRed mice mixed with BM cells from the 4 experimental genotypes (WT, PSGL-1−/−, ESL-1−/−, or DKO). After a 6-week recovery period, the mice were injected intraperitoneally with 1 mL of 3% thioglycollate. At 8 hours after thioglycollate injection, venous blood and peritoneal exudates were collected and aliquots stained with DyLight650-conjugated anti-Ly6G antibody. Samples were analyzed by flow cytometry as indicated above. For estimation of the efficiency of recruitment into the peritoneum, the ratio of knockout vs WT-DsRed neutrophils was determined in blood and peritoneal exudates.
Analysis of leukocyte rolling in autoperfused flow chambers
Autoperfused flow chamber experiments were performed as described previously.14,18 In brief, rectangular glass capillaries were coated with 2.5 µg/mL of E-selectin alone or in combination with 2 µg/mL of intercellular adhesion molecule (ICAM)-1 (R&D Systems) for 2 hours and then blocked for 1 hour using casein (Thermo Fisher Scientific). To control the wall shear stress in the capillary, a PE-50 tubing device (BD) was connected to 1 side of the capillary. The other side of the chamber was connected to a PE-10 tubing device and inserted into a mouse carotid artery. Leukocyte rolling was recorded for 1 minute using an SW40/NA0.75 objective and a digital camera (Sensicam QE; Cooke Corporation).
Progenitor homing assays
BM cells were extracted from WT, PSGL-1−/−, ESL-1−/−, or ESL-1/PSGL-1−/− DKO mice and mixed in a 1:1 ratio with BM cells from a WT-DsRed donor mouse. Donor BM cells at 107 (5 × 106 from each donor) were injected into lethally irradiated recipient mice. Irradiated mice that did not receive a transplant were used as controls. At 3 hours after injection, we harvested femurs, and 50% of the total volume was subjected to 60% Percoll gradient purification. One half of the mononuclear fraction was washed and used for colony-forming units in culture (CFU-C) assay, which were scored on days 7 to 10 using an epifluorescence inverted microscope. The presence or absence of DsRed fluorescence was used to determine whether the colonies were from WT (fluorescent) or mutant (nonfluorescent) donors, and the ratio of CFU-C from experimental donors relative to control WT-DsRed CFU-C was evaluated. In each experiment, we plated 0.5% of the injected mix to assess the input WT:mutant ratios and to calculate the homing efficiencies relative to the WT-DsRed reference. The average mutant:WT-DsRed ratios for the WT, ESL-1−/−, PSGL-1−/−, and DKO groups were 1.03, 1.58, 1.15, and 1.03, respectively. CFU-C counts in the BM of nontransplanted mice were 0 in all experiments.
Statistical analysis
Data are represented as mean ± standard error of the mean (SEM) and were analyzed using Prism software (GraphPad, Inc.). Data consisting of only 2 data sets were analyzed using 2-tailed Student t test unless stated otherwise. For data with more than 2 data sets, we used 1-way or 2-way analysis of variance (ANOVA) for statistical analysis. Tukey multiple comparisons posttests and Bonferroni posttests were used. We deemed P values < .05 as significant. * P < .05, ** P < .01, *** P < .001.
Results
ESL-1 cooperates with PSGL-1 to maintain myeloid homeostasis
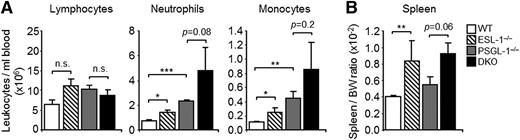
ESL-1 presents robust E-selectin–binding activity in vitro,19 but Glg1-silenced leukocytes display only minor alterations in E-selectin binding in vitro and of rolling dynamics in vivo.9 To understand how this glycoprotein may contribute to global immune and hematologic homeostasis, we set out to analyze mice deficient in ESL-1. These mice display major skeletal defects and reduced body weight16,20 but are fertile and live for at least 40 weeks. However, the frequency of live ESL-1–deficient mice born from heterozygous parents was very low (1.7%; 7/412 live births), suggesting a high rate of embryonic lethality. Hematologic characterization of the mice at ages 7 to 12 weeks revealed mild elevations in total blood leukocytes, monocytosis, and neutrophilia (Figure 1A), as well as splenomegaly (Figure 1B), but no difference in lymphocyte counts. All other hematologic values were normal, and ESL-1 heterozygous littermates were indistinguishable from WT controls (supplemental Table 1). These results suggest a more prominent role for ESL-1 in hematologic homeostasis than anticipated.
ESL-1-deficiency is characterized by a myeloid expansion in the steady state. Hematologic characterization of WT, ESL-1−/−, PSGL-1−/−, and DKO mice. (A) Lymphocyte, neutrophil, and monocyte counts in the blood of WT and mutant mice. (B) Spleen-to-body-weight (BW) ratios in WT and mutant mice. There were 3 to 9 mice per group. Data are shown as mean ± SEM and was analyzed by the unpaired Student t test.
ESL-1-deficiency is characterized by a myeloid expansion in the steady state. Hematologic characterization of WT, ESL-1−/−, PSGL-1−/−, and DKO mice. (A) Lymphocyte, neutrophil, and monocyte counts in the blood of WT and mutant mice. (B) Spleen-to-body-weight (BW) ratios in WT and mutant mice. There were 3 to 9 mice per group. Data are shown as mean ± SEM and was analyzed by the unpaired Student t test.
PSGL-1 is a prominent ligand for E-selectin and the main ligand for P-selectin in myeloid leukocytes,21 and mice deficient in PSGL-1 consequently display elevations in neutrophil counts (Figure 1A).5,21 To determine whether ESL-1 cooperates with PSGL-1 to allow myeloid cell trafficking, we generated mice doubly deficient in both glycoproteins (herein referred to as DKO mice). Approximately 2% of double-deficient mice were born from PSGL-1−/− ESL-1+/− parents (5/251 live births), and displayed morphologic defects and splenomegaly (Figure 1B) similar to single ESL-1–deficient mice (supplemental Table 1). Compared with the PSGL-1−/− or ESL-1−/− parental lines, however, DKO mice had further elevations in blood neutrophil and monocyte counts, but not in lymphocyte numbers (Figure 1A). Analysis of BM chimeras generated by transplantation of BM cells from the different mutant mice into WT recipients (supplemental Figure 1A) confirmed that the myeloid expansion in the mutant mice was intrinsic to the hematopoietic compartment. This peripheral expansion in DKO mice correlated with mild elevations in G-CSF levels in plasma (supplemental Figure 2A), but no changes were observed in the absolute number of total cells or myeloid subsets in the BM of any group (supplemental Figure 2B). Because extravasation and clearance of neutrophils are required for the homeostatic control of granulopoiesis through the production of G-CSF,22 these data support a role for both glycoproteins in myeloid trafficking in the steady state. We also measured the levels of CXCR2 and CXCR4, which are 2 receptors that are modulated during neutrophil aging.23,24 Although the levels of CXCR2 were not altered in mutant mice, those of CXCR4 were moderately elevated in both neutrophils and monocytes that lacked ESL-1 (supplemental Figure 3). In addition, the low frequency of annexin V–binding cells in all groups ruled out major effects in cell viability in the absence of ESL-1 or PSGL-1 (supplemental Figure 3). Altogether, these findings provide strong evidence for an important combined role for ESL-1 and PSGL-1 during the homeostatic trafficking and clearance of myeloid leukocytes.
Contributions of PSGL-1 and ESL-1 to neutrophil rolling and recruitment during inflammation
Because the phenotypes of mice deficient in ESL-1 were consistent with a role for this glycoprotein in neutrophil trafficking, we analyzed the adhesive behavior of ESL-1–deficient leukocytes in vitro and in vivo. Because of the limited availability of ESL-1−/− and DKO mice, we generated mixed hematopoietic chimeras by BM transplantation for our analyses. We transplanted lethally irradiated recipient mice with a 1:1 mixture of BM cells obtained from WT-DsRed transgenic mice together with BM from nonfluorescent WT, ESL-1−/−, PSGL-1−/−, or DKO mice. This approach afforded 2 additional advantages: first, it provided an internal WT reference within each mouse; and second, it eliminated potential environmental alterations originating from ESL-1 deficiency in nonhematopoietic cells. Interestingly, the frequency of ESL-1 and DKO neutrophils was elevated relative to WT-DsRed control cells (not shown), further confirming that both glycoproteins are important for neutrophil homeostasis.
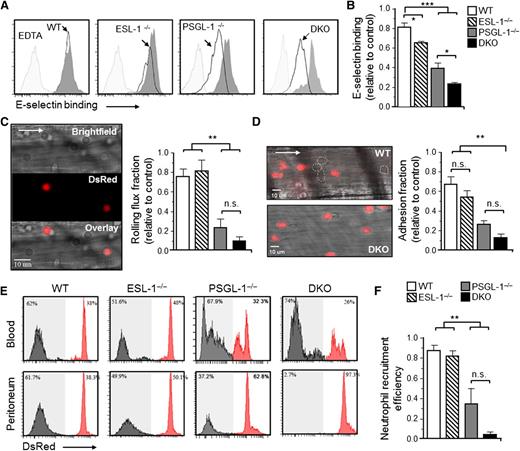
Next, we assessed the capacity of blood Ly6GHI neutrophils from the different chimeric mice to bind an E-selectin/IgM soluble protein by flow cytometry. Binding was mildly but reproducibly reduced in the absence of ESL-1 compared with cocirculating WT neutrophils, and the reduction was stronger in the absence of PSGL-1 (Figure 2A-B).9,25 Notably, binding was reduced by ∼80% in the absence of both glycoproteins (Figure 2A-B). These data confirmed that ESL-1 displays high-affinity ESL activity and, together with PSGL-1, accounts for the majority of E-selectin binding on neutrophils.
ESL-1 cooperates with PSGL-1 in all stages of neutrophil recruitment during inflammation. Analyses were performed in mice transplanted with BM from WT, ESL-1−/−, PSGL-1−/−, or DKO mice together with competing WT-DsRed BM cells. (A) Flow cytometric analyses of soluble E-selectin binding to Ly6GHI blood neutrophils. Histograms show overlays of E-selectin binding to experimental neutrophils (empty histograms) and WT-DsRed competitors (dark gray histograms). Binding in the presence of EDTA was used as a negative control (light gray histograms). (B) Quantification of E-selectin binding, as measured by the mean fluorescence intensities, in all groups. Values are represented as ratios relative to internal WT-DsRed competitor cells. There were 7 to 9 mice per group from 3 independent experiments. (C) Intravital microscopy analysis of neutrophil rolling within inflamed cremasteric venules. Near-simultaneous acquisition in 2 channels discriminates experimental (bright field) and WT-DsRed (red) neutrophils. The bar graph shows rolling flux fractions represented as ratios relative to internal WT-DsRed competitor cells. There were 22 to 58 venules in 5 to 7 mice per group. (D) Representative micrographs of adherent neutrophils from the same groups shown in (C). White dotted lines demarcate adherent nonfluorescent cells. Nonfluorescent structures in the bottom panel are erythrocytes bound to WT-DsRed cells. Bar graph shows ratios of adherent fractions relative to WT-DsRed competitors. There were 26 to 58 venules in 5 to 8 mice per group. (E) Neutrophil extravasation after 8 hours of thioglycollate-induced peritonitis. Histograms show percentages of mutant (gray histograms) and WT-DsRed (red histograms) neutrophils in blood before extravasation (top panels), and in peritoneal exudates (bottom panels). (F) Ratios of mutant relative to WT-DsRed neutrophils in blood vs the peritoneum, thereby representing extravasation efficiencies. There were 5 to 14 mice per group from 2 independent experiments. Data are shown as mean ± SEM and was analyzed by 1-way ANOVA and the Tukey multiple comparison test.
ESL-1 cooperates with PSGL-1 in all stages of neutrophil recruitment during inflammation. Analyses were performed in mice transplanted with BM from WT, ESL-1−/−, PSGL-1−/−, or DKO mice together with competing WT-DsRed BM cells. (A) Flow cytometric analyses of soluble E-selectin binding to Ly6GHI blood neutrophils. Histograms show overlays of E-selectin binding to experimental neutrophils (empty histograms) and WT-DsRed competitors (dark gray histograms). Binding in the presence of EDTA was used as a negative control (light gray histograms). (B) Quantification of E-selectin binding, as measured by the mean fluorescence intensities, in all groups. Values are represented as ratios relative to internal WT-DsRed competitor cells. There were 7 to 9 mice per group from 3 independent experiments. (C) Intravital microscopy analysis of neutrophil rolling within inflamed cremasteric venules. Near-simultaneous acquisition in 2 channels discriminates experimental (bright field) and WT-DsRed (red) neutrophils. The bar graph shows rolling flux fractions represented as ratios relative to internal WT-DsRed competitor cells. There were 22 to 58 venules in 5 to 7 mice per group. (D) Representative micrographs of adherent neutrophils from the same groups shown in (C). White dotted lines demarcate adherent nonfluorescent cells. Nonfluorescent structures in the bottom panel are erythrocytes bound to WT-DsRed cells. Bar graph shows ratios of adherent fractions relative to WT-DsRed competitors. There were 26 to 58 venules in 5 to 8 mice per group. (E) Neutrophil extravasation after 8 hours of thioglycollate-induced peritonitis. Histograms show percentages of mutant (gray histograms) and WT-DsRed (red histograms) neutrophils in blood before extravasation (top panels), and in peritoneal exudates (bottom panels). (F) Ratios of mutant relative to WT-DsRed neutrophils in blood vs the peritoneum, thereby representing extravasation efficiencies. There were 5 to 14 mice per group from 2 independent experiments. Data are shown as mean ± SEM and was analyzed by 1-way ANOVA and the Tukey multiple comparison test.
To simultaneously track the intravascular behavior of neutrophils from each mutant relative to WT-DsRed controls, we next used high-speed multichannel intravital microscopy of the cremasteric microcirculation after TNF-α treatment (Figure 2C; supplemental Table 2; supplemental Video), at times during which neutrophils constitute the majority of recruited cells.26 Compared with the marked reductions in rolling flux fractions seen in the PSGL-1−/− group (69% reduction), we found no alterations in the absence of ESL-1 alone (Figure 2C). In contrast, and consistent with the soluble E-selectin–binding experiments, the rolling fractions were reduced by 88% in the DKO group, although the differences did not reach significance compared with the PSGL-1−/− group (Figure 2C). We observed a similar trend for the fraction of neutrophils that adhered to the endothelium, with a marked decrease in the PSGL-1−/− group that was further reduced in the absence of both ligands (Figure 2D).
Next, we used a model of thioglycollate-induced peritonitis in our chimeric mice to assess the roles of ESL-1 and PSGL-1 during neutrophil recruitment to inflamed sites. In keeping with the intravital imaging analyses, ESL-1−/− neutrophils were recruited with efficiencies similar to those of WT-DsRed control cells, whereas the number of recruited PSGL-1−/− neutrophils was reduced by 60% (Figure 2E-F). Notably, DKO leukocytes were almost undetectable in the inflamed peritoneum, with 95% reduction compared with control WT cells (Figure 2E-F), indicating that the presence of both glycoproteins is essential for the recruitment of neutrophils to inflamed tissues. Consistent with these results, we found that the absolute number of DKO neutrophils and monocytes remained strongly elevated in the blood of mice treated with thioglycollate (supplemental Figure 1B).
Because the differences between the PSGL-1−/− and DKO groups did not reach significance for several parameters and this was important to conclusively define a role for ESL-1 in neutrophil recruitment, we also compared the behavior of PSGL-1−/−and DKO neutrophils within the same animals. To this end, we generated PSGL-1–DsRed+ mice whose BM was cotransplanted together with nonfluorescent DKO BM into lethally irradiated WT recipient mice. Cytometric, intravital microscopy, and migration analyses in these mice revealed significant reductions in E-selectin binding, rolling, adhesion, and recruitment of neutrophils that lacked both glycoproteins compared with those deficient only in PSGL-1 (Figure 3A-D). Together, these results indicate that ESL-1 cooperates with PSGL-1 in all aspects of E-selectin–mediated recruitment during inflammation, from tethering and rolling to extravasation; the results also showed that the absence of ESL-1 on neutrophils is almost completely compensated by PSGL-1.
ESL-1 mediates neutrophil recruitment in the absence of PSGL-1, but is dispensable for integrin-mediated slow rolling. Analyses in panels A-D were performed in WT mice transplanted with BM cells from PSGL-1−/−, DsRed+, and DKO donor mice. (A) Flow cytometric analyses of soluble E-selectin binding to Ly6GHI blood neutrophils. Histograms show overlays of E-selectin binding to DKO vs PSGL-1−/− DsRed+ neutrophils present in the blood of the same mice. Binding in the presence of EDTA was used as a negative control. E-selectin binding of WT neutrophils is included as a reference. Bar graphs at right show quantification of E-selectin–binding intensities of DKO and PSGL-1−/− DsRed+ neutrophils. Data are from 4 mice and was analyzed using the paired t test. (B) Rolling-flux fractions of DKO and PSGL-1−/− DsRed+ neutrophils in inflamed cremastric venules. There were 27 venules from 5 mice. (C) Representative micrograph of adherent neutrophils in an inflamed venule during the adhesion phase. Dotted lines demarcate a nonfluorescent DKO cell. Bar graph at right shows quantification of the adherent fractions. There were 27 venules from 5 mice. (D) Neutrophil extravasation in thioglycollate-induced peritonitis. Bar graph represents the relative frequencies of DKO and PSGL-1−/− DsRed+ neutrophils in blood vs the peritoneum, thereby representing extravasation efficiencies. There were 4 mice from 2 independent experiments. Data were compared using a paired t test. (E) Cumulative frequency histograms of rolling velocities of mutant neutrophils obtained from analyses of chimeric mice reconstituted with BM cells from WT, ESL-1−/−, PSGL-1−/−, or DKO mice. Dotted lines indicate velocities of the median. The bar graph represents mean rolling velocities. There were 37 to 82 cells per group from 5 to 7 mice per group, from 2 independent sets of experiments. Data were analyzed using a 1-way ANOVA with the Tukey multiple comparison test. (F) Rolling velocities of leukocytes in autoperfused flow chambers coated with E-selectin alone or in combination with ICAM-1. Data are from 3 to 4 individual mice per group and was analyzed using the 2-tailed Student t test. Data are shown as mean ± SEM.
ESL-1 mediates neutrophil recruitment in the absence of PSGL-1, but is dispensable for integrin-mediated slow rolling. Analyses in panels A-D were performed in WT mice transplanted with BM cells from PSGL-1−/−, DsRed+, and DKO donor mice. (A) Flow cytometric analyses of soluble E-selectin binding to Ly6GHI blood neutrophils. Histograms show overlays of E-selectin binding to DKO vs PSGL-1−/− DsRed+ neutrophils present in the blood of the same mice. Binding in the presence of EDTA was used as a negative control. E-selectin binding of WT neutrophils is included as a reference. Bar graphs at right show quantification of E-selectin–binding intensities of DKO and PSGL-1−/− DsRed+ neutrophils. Data are from 4 mice and was analyzed using the paired t test. (B) Rolling-flux fractions of DKO and PSGL-1−/− DsRed+ neutrophils in inflamed cremastric venules. There were 27 venules from 5 mice. (C) Representative micrograph of adherent neutrophils in an inflamed venule during the adhesion phase. Dotted lines demarcate a nonfluorescent DKO cell. Bar graph at right shows quantification of the adherent fractions. There were 27 venules from 5 mice. (D) Neutrophil extravasation in thioglycollate-induced peritonitis. Bar graph represents the relative frequencies of DKO and PSGL-1−/− DsRed+ neutrophils in blood vs the peritoneum, thereby representing extravasation efficiencies. There were 4 mice from 2 independent experiments. Data were compared using a paired t test. (E) Cumulative frequency histograms of rolling velocities of mutant neutrophils obtained from analyses of chimeric mice reconstituted with BM cells from WT, ESL-1−/−, PSGL-1−/−, or DKO mice. Dotted lines indicate velocities of the median. The bar graph represents mean rolling velocities. There were 37 to 82 cells per group from 5 to 7 mice per group, from 2 independent sets of experiments. Data were analyzed using a 1-way ANOVA with the Tukey multiple comparison test. (F) Rolling velocities of leukocytes in autoperfused flow chambers coated with E-selectin alone or in combination with ICAM-1. Data are from 3 to 4 individual mice per group and was analyzed using the 2-tailed Student t test. Data are shown as mean ± SEM.
ESL-1 does not participate in E-selectin–induced slow rolling
Neutrophil rolling velocities are controlled both by direct engagement of selectins as well as by partial activation of the integrin LFA-1, which can, in turn, be initiated by signaling events following the engagement of E-selectin.13,14,18 To test whether ESL-1 was capable of transducing LFA-1–activating signals in neutrophils that promoted slow rolling, we next performed in vivo and ex vivo experiments. Intravital microscopy experiments in chimeric mice revealed that ESL-1−/− leukocytes rolled at velocities that were comparable to those of WT controls in the same vessels, whereas PSGL-1−/− leukocytes rolled significantly faster (Figure 3E). These data suggested that ESL-1 is not involved in controlling rolling velocities and does not trigger LFA-1–activating signals in circulating neutrophils. However, these findings contradicted our previous results using silencing of the gene encoding ESL-1,9 possibly because off-target effects of the short hairpin RNA or biased lentiviral transduction of progenitor subsets caused experimental artifacts. We therefore decided to confirm this observation in a more controlled setting in which we measured leukocyte rolling velocities on E-selectin coimmobilized with the β2-integrin ligand ICAM-1 in an autoperfused flow chamber system.18 Consistent with the data in the microcirculation, PSGL-1−/− neutrophils rolled faster than WT cells, whereas the absence of ESL-1 did not alter rolling velocities (Figure 3F). These data indicate that, although ESL-1 is a bona fide ligand for E-selectin on mature neutrophils, it is not capable of inducing LFA-1 activation and slow rolling on ICAM-1.
ESL-1 dominates E-selectin binding and homing of hematopoietic progenitors to the BM
Because ESL-1−/− mice displayed hematologic abnormalities in the steady state, we speculated that ESL-1 might also be a functional ESL in noninflammatory leukocytes. Indeed, we found that the levels of HPCs were moderately elevated in the blood of ESL-1−/− mice in the steady state (Figure 4A), which suggested a role for this glycoprotein in controlling HPC trafficking. Because the functional ESLs that participate in homing to the BM are not well characterized,11,15 we decided to study the functions of ESL-1 in hematopoietic precursors. We first analyzed the binding of soluble E-selectin to myeloid progenitors (LineageNEG Sca-1NEG cKitHI) and to the more primitive population of LineageNEG Sca-1+ cKitHI (LSK) precursor cells, together with immature Ly6G+ neutrophils located within the same BM. To increase the sensitivity of this assay, we again used chimeric mice reconstituted with WT-DsRed+ BM together with the nonfluorescent experimental groups, and compared binding relative to the internal WT competitor cells. Although E-selectin binding to Ly6G+ BM neutrophils in the PSGL-1−/− group was strongly reduced (Figure 4B-C), binding to myeloid progenitors and LSK cells lacking PSGL-1 was only partially reduced compared with control WT cells (Figure 4B-C). In contrast, P-selectin binding was completely abrogated in PSGL-1−/− LSK cells (Figure 4D), indicating that PSGL-1 was otherwise functional in HPCs. Interestingly, when we tested myeloid progenitors and LSK cells from ESL-1−/− mice, we noted that binding of soluble E-selectin was reduced by 41% and 56%, respectively, and binding was not further reduced in LSK cells from DKO mice (Figure 4B-C). Although the reduced ligand activity of PSGL-1 in HPCs agrees with a recent report,15 the marked contribution of ESL-1 represents, to our knowledge, the first identification of a glycoprotein with prominent E-selectin–binding activity on hematopoietic progenitors. Thus, in contrast to the dominant ligand activity of PSGL-1 in mature neutrophils, ESL-1 dominates E-selectin binding in immature progenitors.
ESL-1 dominates E-selectin binding in hematopoietic progenitors. (A) Number of progenitors in the blood of mice transplanted with WT or ESL-1−/− BM cells, measured as CFU-C. (B) Representative dot plot at left shows the gates used for myeloid progenitors and LSK cells among LineageNEG BM cells. Panels at right show representative histograms of E-selectin binding to experimental (gray) and WT-DsRed (red) BM-derived neutrophils (top panel), myeloid progenitors (middle), and LSK cells (bottom). Dashed lines show levels of binding in the presence of EDTA. (C) Quantification of E-selectin-binding to BM neutrophils, myeloid progenitors, and LSK cells. Values represent fluorescence intensity ratios relative to internal WT-DsRed competitor cells. There were 4 to 6 mice per group from 3 independent experiments. (D) Quantification of P-selectin binding in LSK cells from all groups represented as in (C). There were 4 to 5 mice per group from 3 independent experiments. Bars represent mean ± SEM. Data were analyzed by 1-way ANOVA using the Tukey multiple comparison test.
ESL-1 dominates E-selectin binding in hematopoietic progenitors. (A) Number of progenitors in the blood of mice transplanted with WT or ESL-1−/− BM cells, measured as CFU-C. (B) Representative dot plot at left shows the gates used for myeloid progenitors and LSK cells among LineageNEG BM cells. Panels at right show representative histograms of E-selectin binding to experimental (gray) and WT-DsRed (red) BM-derived neutrophils (top panel), myeloid progenitors (middle), and LSK cells (bottom). Dashed lines show levels of binding in the presence of EDTA. (C) Quantification of E-selectin-binding to BM neutrophils, myeloid progenitors, and LSK cells. Values represent fluorescence intensity ratios relative to internal WT-DsRed competitor cells. There were 4 to 6 mice per group from 3 independent experiments. (D) Quantification of P-selectin binding in LSK cells from all groups represented as in (C). There were 4 to 5 mice per group from 3 independent experiments. Bars represent mean ± SEM. Data were analyzed by 1-way ANOVA using the Tukey multiple comparison test.
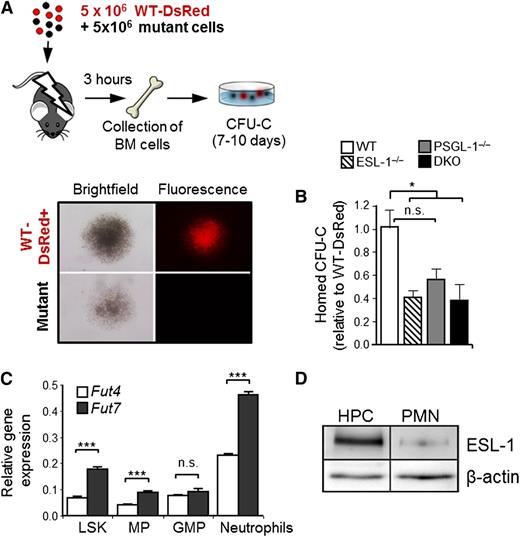
To functionally confirm these findings, we next assessed the differential requirement for each ligand during HPC homing to the BM. Lethally irradiated recipient mice were injected with a mixture of BM cells obtained from WT, ESL-1−/−, PSGL-1−/−, or DKO mice, together with cells from WT-DsRed+ mice, and the homing of fluorescent and nonfluorescent colonies in the BM was scored 3 hours later using semisolid clonogenic cultures (Figure 5A). In agreement with the in vitro E-selectin–binding data, the homing of ESL-1−/− and DKO progenitors (measured as CFU-C) was reduced by ∼60%, whereas that of PSGL-1−/− CFU-C was reduced by ∼45% relative to the internal WT-DsRed controls (Figure 5B). The reduced homing seen for PSGL-1−/− progenitors is comparable to that reported previously using genetically deficient mice or using an antibody that exclusively interferes with P-selectin binding,11 suggesting that most of the impaired homing of PSGL-1−/− progenitors may be the result of an inability to bind P-selectin. Together, these data demonstrate that ESL-1 is a major ligand for E-selectin on HPCs that contributes to their efficient homing to the BM.
ESL-1 mediates progenitor homing to the BM. (A) Design of the BM homing assays. Lethally irradiated mice were injected with experimental and DsRed+ competitor BM cells, which were allowed to home for 3 hours. Homed CFU-C were scored 7 to 10 days later and were differentiated on the basis of red fluorescence (WT-DsRed+) or no fluorescence, as illustrated in the micrographs. (B) Homing efficiencies of progenitors from each group, calculated as the ratios of homed CFU-C from each mutant donor relative to competing WT-DsRed+ progenitors, and corrected by the ratio of CFU-C injected. There were 6 to 8 mice per group from 3 independent experiments. Bars represent mean ± SEM. Data were analyzed by 1-way ANOVA using the Tukey multiple comparison test. (C) Relative expression of Fut4 and Fut7 in purified LineageNEG Sca-1+ cKitHI cells (LSK), myeloid progenitors (MP), granulocyte-monocyte progenitors (GMP), and circulating neutrophils. Data show the mean ± SEM from 3 independent samples and was analyzed by an unpaired Student t test. (D) Western blot analysis of total ESL-1 protein and β-actin (load control) present in sorted LineageNEG cKitHI progenitors (HPCs) and blood neutrophils (PMNs). Data are representative of 2 independent experiments, with increases of 10.0-fold and 10.3-fold in ESL-1 protein levels in HPCs relative to PMNs.
ESL-1 mediates progenitor homing to the BM. (A) Design of the BM homing assays. Lethally irradiated mice were injected with experimental and DsRed+ competitor BM cells, which were allowed to home for 3 hours. Homed CFU-C were scored 7 to 10 days later and were differentiated on the basis of red fluorescence (WT-DsRed+) or no fluorescence, as illustrated in the micrographs. (B) Homing efficiencies of progenitors from each group, calculated as the ratios of homed CFU-C from each mutant donor relative to competing WT-DsRed+ progenitors, and corrected by the ratio of CFU-C injected. There were 6 to 8 mice per group from 3 independent experiments. Bars represent mean ± SEM. Data were analyzed by 1-way ANOVA using the Tukey multiple comparison test. (C) Relative expression of Fut4 and Fut7 in purified LineageNEG Sca-1+ cKitHI cells (LSK), myeloid progenitors (MP), granulocyte-monocyte progenitors (GMP), and circulating neutrophils. Data show the mean ± SEM from 3 independent samples and was analyzed by an unpaired Student t test. (D) Western blot analysis of total ESL-1 protein and β-actin (load control) present in sorted LineageNEG cKitHI progenitors (HPCs) and blood neutrophils (PMNs). Data are representative of 2 independent experiments, with increases of 10.0-fold and 10.3-fold in ESL-1 protein levels in HPCs relative to PMNs.
Because these data revealed that ESL-1 was the dominant ESL in HPCs, we searched for possible mechanisms underlying this lineage-restricted function. Transcriptional regulation of fucosyltransferase genes (Fut) is the critical regulator of selectin ligand synthesis in leukocytes. Because Fut4 and Fut7 differentially fucosylate and mediate maturation of ESL-1 and PSGL-1, respectively,27 we explored whether expression of Fut4 was elevated in HPCs. We found, however, that the expression of both Fut genes was higher in neutrophils than in any of the hematopoietic precursors analyzed (LSK, myeloid progenitors, and granulocyte-monocyte progenitors; Figure 5C), thus ruling out that differential expression of Fut genes was responsible for the preferential use of ESL-1 in HPCs. In contrast, we found that the levels of ESL-1 protein in HPCs were 10.1 times higher than those present in circulating neutrophils (Figure 5D), whereas the levels of PSGL-1 were similar in the 2 cell types (supplemental Figure 4). Therefore, the higher levels of ESL-1 on immature progenitors may account for its predominant function in these cells.
Discussion
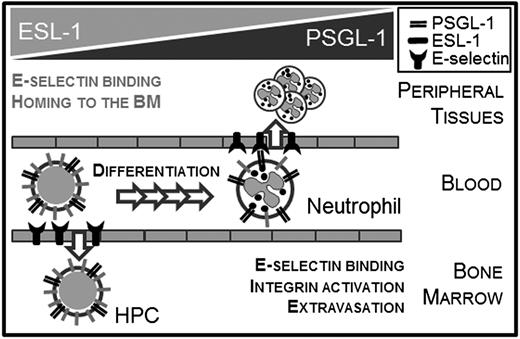
In this study, we have examined the functions of ESL-1 in the hematopoietic system, which allowed us to uncover distinct signaling and migratory functions for this glycoprotein in the mature and immature hematopoietic compartments (Figure 6). We report that, in contrast to the preferred use of PSGL-1 on mature neutrophils, ESL-1 dominates E-selectin binding and migration of immature hematopoietic progenitors. In neutrophils, our results reveal a complex specialization of each ligand in specific signaling events; whereas PSGL-1 and CD44 regulate activation of the β2 integrin LFA-1 and promote slow rolling on E-selectin engagement,13,14 ESL-1 is devoid of this function. Interestingly, ESL-1 has been reported to regulate activation of the other major myeloid β2-integrin, Mac-1,17 suggesting that E-selectin can deliver different signals depending on the ligand involved even within the same leukocyte.
Shared and unique functions of ESL-1 during mature and immature leukocyte trafficking. Schematic representation of the ESLs preferentially used by immature (HPCs) and mature (neutrophils) hematopoietic cells to migrate. The elevated levels of ESL-1 in HPCs correlate with its predominant function for E-selectin binding and migration in these cells. In neutrophils, both functions rely predominantly on PSGL-1 despite similar expression of the glycoprotein in mature and immature leukocytes.
Shared and unique functions of ESL-1 during mature and immature leukocyte trafficking. Schematic representation of the ESLs preferentially used by immature (HPCs) and mature (neutrophils) hematopoietic cells to migrate. The elevated levels of ESL-1 in HPCs correlate with its predominant function for E-selectin binding and migration in these cells. In neutrophils, both functions rely predominantly on PSGL-1 despite similar expression of the glycoprotein in mature and immature leukocytes.
By analyzing mice deficient in PSGL-1 and ESL-1, we demonstrated that neutrophil recruitment is severely impaired in the absence of both receptors. This impairment correlates with global alterations in myeloid homeostasis both during inflammation and in the steady state, which partially recapitulate those reported in mice deficient in P- and E-selectins.22,28 Although our results show that the ligands are important for neutrophil clearance from the circulation, at present we cannot rule out a role in myeloid retention or release from the BM.
An important conclusion of our study is that each ligand dominates E-selectin binding in different hematopoietic compartments, with a dominant role of PSGL-1 in mature neutrophils, and an unexpected prominent function of ESL-1 in immature progenitors. Besides neutrophils, monocytes and inflammatory T lymphocytes have been shown to rely heavily on PSGL-1 for E-selectin binding and migration,29,30 suggesting a general use of this glycoprotein in fully differentiated leukocytes. It is puzzling that HPCs are largely independent of PSGL-1 for binding to E-selectin. We speculate that this may be related to the additional roles that E-selectin plays in the hematopoietic niche, functions that may require a more specialized ligand endowed with additional signaling properties.15 ESL-1, which shares functional and structural homology with receptors for the fibroblast growth factor,19,31 may thus represent a more suitable ligand in the immature compartment.
Interestingly, we find that deficiency in ESL-1 results in major HPC homing defects not previously seen in mice deficient in E-selectin.11 This is likely because of the competitive nature of the assays used in this study, which yields more robust quantitative results compared with assays used to investigate E-selectin–deficient mice. It is also possible that the stronger effect of ESL-1 deletion arises from its capacity to activate additional homing receptors through both selectin-dependent (as shown for β2-integrins in neutrophils3 ) and selectin-independent mechanisms. Although in the present study we have focused on the migratory functions of ESL-1, its identification as the major glycoprotein ligand for E-selectin in HPCs raises the possibility that it mediates other important functions of this selectin in the immature compartment, including the recently reported regulation of hematopoietic stem/progenitor cell dormancy and chemoresistance.15 Our current findings thus pave the way for future work on the control of HPC biology by ESL-1.
We present evidence that the preferential use of ESL-1 may be caused by the elevated levels of the glycoprotein in progenitors, rather than by the expression of glycosyltransferases involved in its functional maturation. It is interesting that the increased levels of ESL-1 are not transcriptionally regulated (not shown), suggesting that complex posttranslational processing of this glycoprotein regulates its cellular functions. It will be important to understand in more detail how ESL-1 is processed in different hematopoietic lineages, or in tumor cells that display tropism to the BM.32
In summary, the pleiotropic and specialized functions of ESL-1 in the hematopoietic system identify this poorly characterized glycoprotein as a potential target for both inflammatory and hematopoietic disorders. The differential use of selectin ligands on mature and immature hematopoietic cells suggests the feasibility of targeting the migration of specific leukocyte subsets for therapeutic purposes.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank G. Crainiciuc, M. Nácher, V. Zorita, J.M. Ligos, and the Cellomics and Comparative Medicine Units at the CNIC for technical support; and L. Weiss and D. Lucas for reviewing the manuscript.
This study was supported by National Institutes of Health P01-HD070394 (B.L.); German Research Foundation ZA428/3-1, ZA428/6-1 and INST211/604-1 (A.Z.); and Ramón y Cajal Fellowship (RYC-2007-00697) and SAF2009-11037 from MINECO, S2010/BMD-2314 from Comunidad de Madrid, and FP7-People-IRG Program (246655) (A.H.). M.K.W. is supported by the Max Planck Society. The CNIC is supported by the Spanish Ministry of Economy and Competitivity and the Pro-CNIC Foundation.
Authorship
Contribution: V.S. performed experiments and wrote the manuscript; M.L., A.S., and C.P. performed experiments; I.O.-R. maintained the animal colonies; B.L. and M.K.W. contributed reagents; A.Z. designed experiments and edited the manuscript; and A.H. conceived the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrés Hidalgo, Department of Epidemiology, Atherothrombosis and Imaging, CNIC, Melchor Fernández Almagro 3, 28029 Madrid, Spain; e-mail: ahidalgo@cnic.es.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal