Abstract
Despite decades of research on wound healing, effective biologic agents for the treatment of chronic wounds, especially diabetic wounds, are still lacking. In the present study, we report that the inert plasma protein plasminogen (plg) acts as a key regulatory molecule that potentiates wound healing in mice. Early in the healing process, plg bound to inflammatory cells is transported to the wound area, where the level of plg is increased locally, leading to the induction of cytokines and intracellular signaling events and to a potentiation of the early inflammatory response. Systemic administration of additional plg not only accelerates the healing of acute burn wounds in wild-type mice, but also improves the healing of chronic diabetic wounds in a mouse model of diabetes. Our results suggest that the administration of plg may be a novel therapeutic strategy to treat many different types of wounds, especially chronic wounds such as those caused by diabetes.
Introduction
Wound healing is a dynamic biologic process that restores damaged cellular structures and tissue layers. This tissue-interactive process is very complex and consists of 3 phases: inflammation, tissue formation, and tissue remodeling.1 Acute wounds that do not have an underlying pathologic defect normally heal readily. However, in wounds with pre-existing pathophysiologic problems such as chronic wounds, and diabetic wounds in particular, the healing processes are impaired.2 Chronic wounds not only impair the quality of life for millions of patients, but also inflict an enormous burden on the healthcare system both in terms of cost and the intensity of care required. In the last few decades, large research efforts have been undertaken to dissect the molecular mechanisms behind wound-healing processes with the aim of developing new approaches to treating chronic wounds. However, thus far, very few biologic substances have reached clinical practice, and no effective biologic substances are widely used for the treatment of chronic wounds.3
The plasminogen activator (PA) system is a general proteolytic system in which the active protease, plasmin, is generated from the conversion of the precursor, plg, by either of 2 physiological PAs: tissue-type PAs (tPAs) or urokinase-type PA (uPA).4 The PA system is widely used for the generation of extracellular proteolytic activity. It is well established that the PA system plays a pivotal role in fibrinolysis and in many tissue-remodeling processes, including wound healing.4,5 The PA system is also involved in the activation of intracellular signaling events and in the generation of inflammatory responses.6-9 With respect to the role of the PA system in wound healing, this system has mainly been studied and discussed in terms of fibrin degradation, generation of the extracellular proteolytic activity required for cell migration, and matrix degradation and tissue remodeling.5 However, whether plg plays a regulatory role in the initiation of the inflammatory phase of wound healing remains to be determined.
During the wound-healing process, tPA and uPA are expressed by migrating keratinocytes.10 Studies in plg-deficient (plg−/−) mice have shown that wound healing in the skin is largely delayed and healing of tympanic membrane (TM) perforations is completely arrested in plg−/− mice, indicating that plasmin plays an important role in the wound-healing processes.5,11 However, the concentration of the plasmin precursor plg in body fluids is as high as 2μM12 and it has been assumed that plg levels are at saturation and that this molecule does not play any major regulatory role. Rather, it has been assumed that the formation of plasmin is mainly regulated by the accessibility and activity of the PAs.4,12
In the present study, we used a standard burn injury mouse model to explore the healing potential of plg and to reveal the molecular mechanism underlying its effect in acute and diabetic wounds. Our results show that plg is a key regulatory molecule with a pronounced proinflammatory effect that potentiates the early inflammatory response during wound healing. Administration of additional plg not only accelerates the healing of acute wounds, but also initiates and improves the healing of chronic diabetic wounds. The results of the present study suggest a novel therapeutic strategy for the treatment of different types of wounds, particularly diabetic wounds.
Methods
Animals
Plg-heterozygous (plg+/−) mice13 on a C57BL/6 background were used to generate wild-type (WT), plg+/− and plg-deficient (plg−/−) mice. The mice were genotyped by a rapid chromogenic assay, as described previously, with confirmation by PCR.14 Eight- to 10-week-old mice were used for the experiments. Genetically diabetic mice (C57BLKS db/db) and control heterozygous littermates (C57BLKS db/+) were obtained from Taconic Europe. Among the db/db mice, only those that were at least 10 weeks old and with a minimal blood glucose level of 15mM were used in the experiments.15 The control littermates (db/+) were of the same age and had a maximum blood glucose level of 7.8mM. The animals were kept under standard laboratory conditions. The Regional Ethics Committee of Umeå University approved all of the experimental protocols.
Burn-wound model
The mice were anesthetized by IV injection of 100 μL of a mixture containing 5% Ketaminol Vet (Intervet) and 20% Dormitor Vet (Orion Pharma). A metal rod (25 g, 1 cm in diameter) was heated to 95-100°C by submersion in boiling water. The rod was immediately positioned vertically for 6 seconds without additional pressure on the back skin of mice that had also been depilated 3 days before wounding. After wounding, the mice were individually caged and the wounds were not dressed.
Analysis of wound healing
The mice received standardized wounds, as described in the previous section, and then 2 mg of human plg (Omnio) was administered daily by IV injection. In the control group, 2 mg of BSA (Sigma-Aldrich) was administered daily by IV injection as a placebo. In WT mice and db/db mice, the daily treatments were continued for 16 and 24 days, respectively. Digital photographs of the dorsal wounds were taken on the specified days until the end of the experiment. Photographs of the wounded areas were analyzed by tracing the wound margins and calculating the pixel areas using ImageJ Version 1.41o software. The remaining wound area was calculated as a percentage area of the original wound area.
Morphologic staining
Six-micrometer-thick sections were taken perpendicularly to the wounded skin from paraffin-fixed tissue samples and processed for H&E staining. Images were taken with a DC300F digital camera attached to a DMLB microscope (Leica).
Analysis of the plg levels in wounds
Wounded back skin and unwounded abdominal skin were sampled from each mouse. The samples were homogenized and lysed with lysis buffer (50mM Tris-HCl buffer, pH 8.0, with 120mM NaCl, 20mM NaF, 20mM β-glycerophosphate, 1mM EDTA, 6mM EGTA, 1% NP-40, and 1mM DTT). The skin lysates were then kept at −20°C until use. The total protein concentrations in the lysates were quantified using the Bio-Rad protein assay, according to the user's manual. To measure the level of plg in the tissue samples, an equal amount of total protein was used for a plg-specific chromogenic assay.14 The plg level in the unwounded abdominal skin was used as the plg basal level. The plg level in the wounded skin or unwounded matched skin was calculated as the fold increase from the basal level.
Spatial analysis of plg levels in the wound
The mice received a standardized wound as described in the “Burn-wound model” section. One day after the injury, tissue samples were collected from the wounded and unwounded skin. The samples were taken 0.5 cm (sample 1; S1) and 1.5 cm (sample 2; S2) away from the edge of the wound (see Figure 2B). The plg levels in different tissue samples were analyzed as described in the previous paragraph.
Fibrinogen depletion
The mice were defibrinogenated 3 days before the experiment, as described previously.16 On the experimental day, a burn was induced and 2 mg of human plg was injected intravenously 5 minutes after burn. Samples were collected 24 hours after injection. Plasma fibrinogen levels were semiquantified by densitometric analysis of fibrinogen by Western blotting, using rabbit anti–mouse fibrinogen serum as the primary Ab (1:1000; Nordic Immunologic Laboratories).
Macrophage depletion
One day before the experiment, the mice received an IP injection of 300 μL of clodronate liposomes, which led to a depletion of macrophages of at least 80% over the course of the experiment.17 Empty liposomes were used in the no-depletion group. On the day of the experiment, a burn was induced, followed by an IV injection of 2 mg of human plg 5 minutes after burn. Samples were collected 24 hours after injection.
Neutrophil depletion
For the 2 days before the experiment, the mice received daily 0.5 mL IP injections of saline-diluted (1:3) rabbit anti–mouse neutrophil polyclonal Ab (Accurate Chemical & Scientific), which resulted in a depletion of circulating neutrophils of at least 70% on the day of burn induction.18 Normal rabbit serum (Accurate Chemical & Scientific) was used in the no-depletion group. Burns were induced, and IV injections of 2 mg of human plg (Omnio) were given 5 minutes after burn. Samples were collected 24 hours after injection.
Western blot analysis
Twenty-four hours after wounding and injection, skin lysates were prepared and quantified as described in “Analysis of the plg levels in wounds.” Anti-STAT3 and antiphosphorylated-STAT3 (Tyr705) Abs were obtained from Cell Signaling Technology. Anti–β-actin Ab was obtained from Sigma-Aldrich. Western blotting was performed according to the manufacturer's instructions and visualized using the ECL Plus Western Blotting Detection System (Amersham Biosciences). The integrated density of the bands was quantified using ImageJ Version 1.41o software.19
ELISA
Twenty-four hours after wounding and injection, skin lysates were prepared and total protein was quantified as described in “Analysis of the plg levels in wounds.” IL-6 levels were measured using an IL-6 ELISA kit (eBiosciences). BSA levels were measured using a BSA ELISA kit (Acris Antibodies).
Statistical analysis
The results are expressed as the mean ± SD. Comparisons between 2 groups were analyzed by 2-tailed t tests. Comparisons between multiple groups were analyzed by 1-way ANOVA. P < .05 was considered significant.
Results
Plg accelerates the healing of burn wounds in WT mice
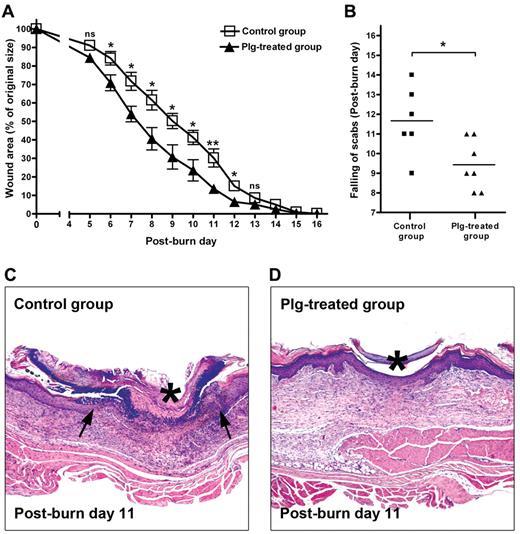
Previous studies from our group have shown that the healing of TM perforations is completely arrested in plg−/− mice, but that healing can be restored after supplementation with plg.11 To determine whether increased levels of circulating plg have any effect on the rate of wound healing, full-thickness standardized burn wounds (1 cm in diameter) were induced in WT mice. Immediately after the injury, each mouse in the plg-treated group was given 2 mg of plg by IV injection, whereas the mice in the control group received 2 mg of BSA by IV injection. This treatment was continued daily for 16 days. Quantification of the wound area at different time points showed that from day 6 after injury, healing in the plg-treated group was significantly faster compared with that in the control group (Figure 1A). As shown in Figure 1B, the time to healing (ie, the scab falling off) in the plg-treated group was also approximately 2 days earlier than in the control group. A morphologic analysis at 11 days after injury revealed that the epithelium layer in the control group remained open and was covered by a large scab (Figure 1C), whereas in the plg-treated group, the epithelium layer had fused to reepithelialize the wound completely, and only a small scab remained lightly attached above the wound (Figure 1D). These data indicate that an increased level of plg in the circulation accelerates the rate of healing in WT mice.
Effect of plg treatment on burn wound healing in WT mice. (A) Quantification of the remaining wound area at different time points after wounding in the BSA-treated control group (n = 6) and the plg-treated group (n = 7). (B) Comparison of the healing times (scab falling off) in the days after wounding. *P < .05; **P < .01. (C-D) Representative pictures of the control group and the plg-treated group on day 11 after injury. The leading edges of the epithelium layer are indicated by arrows and the scab is indicated by an asterisk. Magnification is 50×.
Effect of plg treatment on burn wound healing in WT mice. (A) Quantification of the remaining wound area at different time points after wounding in the BSA-treated control group (n = 6) and the plg-treated group (n = 7). (B) Comparison of the healing times (scab falling off) in the days after wounding. *P < .05; **P < .01. (C-D) Representative pictures of the control group and the plg-treated group on day 11 after injury. The leading edges of the epithelium layer are indicated by arrows and the scab is indicated by an asterisk. Magnification is 50×.
Plg accumulates in the wounded area during wound healing
The data presented in the previous section suggested that the level of plg in the circulation affects the rate of wound healing. Therefore, we further investigated how the level of plg is regulated at and around wound sites during the wound-healing process. A standardized burn wound was induced on the back skin of WT and plg+/− mice. The plg+/− mice were included as they have approximately half the amount of plg in their blood13 and tissue compared with WT mice (data not shown). At different time points after the injury, wounded back skin and unwounded skin from the same mouse were collected for the measurement of plg levels in wounded and unwounded skin, respectively. As shown in Figure 2A, 1 day after the injury, the level of plg increased approximately 6-fold in the wound sites in the WT mice and 4-fold in the plg+/− mice compared with the basal level of plg in control, unwounded skin. In both WT and plg+/− mice, the change in the plg levels in the wound sites coincided with inflammation activity in the wound sites. The plg levels were highest during the early inflammatory phase, became lower during the tissue-formation phase, and nearly returned to basal levels during the late tissue-remodeling phase.
Temporal and spatial regulation of plg during wound healing. (A) Kinetics of the plg levels in the wounds for 18 days after burn wounding in WT (n ≥ 5) and plg+/− mice (n ≥ 3). (B) Experimental design used to determine the spatial regulation of plg after wounding. (C) Comparison of the plg levels at different distances from the wound 24 hours after wounding in WT mice (n = 5). (D) Comparison of the plg levels at different distances from the wound 24 hours after the IV injection of plg in WT mice (n = 5).
Temporal and spatial regulation of plg during wound healing. (A) Kinetics of the plg levels in the wounds for 18 days after burn wounding in WT (n ≥ 5) and plg+/− mice (n ≥ 3). (B) Experimental design used to determine the spatial regulation of plg after wounding. (C) Comparison of the plg levels at different distances from the wound 24 hours after wounding in WT mice (n = 5). (D) Comparison of the plg levels at different distances from the wound 24 hours after the IV injection of plg in WT mice (n = 5).
We then studied the spatial distribution of plg at and around the wound sites on day 1 after injury. Tissue samples were taken from and around the wound sites, as indicated in Figure 2B, and unwounded skin from the same mouse was collected for the measurement of basal plg levels. A spatial analysis showed that the plg levels only increased in the vicinity of the wound and were at basal levels outside of the wounded area (Figure 2C). These results indicate that plg accumulates specifically at inflamed areas during the early inflammation phase of the wound-healing process. As shown in Figure 2A, the plg+/− mice had a similar pattern of plg distribution, but the increase was smaller than in the WT mice, suggesting that the increase of plg in the wound sites is correlated with the concentration of plg in the circulation. To determine whether this is also the case when the plg concentration in the circulation is higher than normal, the WT mice were injected intravenoulsy with 2 mg of plg 5 minutes after wounding. We then analyzed the level of plg in the wound sites 24 hours after plg injection. As shown in Figure 2D, there was a 22-fold increase of plg in the wound sites of the mice that had been injected intravenously with 2 mg of plg. This increase was dramatically higher than the increase seen in the normal wound group, in which there was a 6.5-fold increase (Figure 2C). Moreover, a spatial analysis of the WT mice supplemented with plg showed that the dramatic increase of plg was restricted to the wound area (Figure 2D). These findings were further confirmed by measuring plg antigen levels by ELISA and Western blot analysis (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
These data suggest that the healing effect of plg might be related to its wound-specific accumulation and that the extent of the accumulation effect can be further enhanced by supplementation with exogenous plg.
Plg is mainly transported to the wounded area by inflammatory cells
To elucidate the underlying mechanisms behind the healing potential of plg, we performed a set of experiments to investigate the mechanisms by which plg is accumulated in the wound area after systemic supplementation.
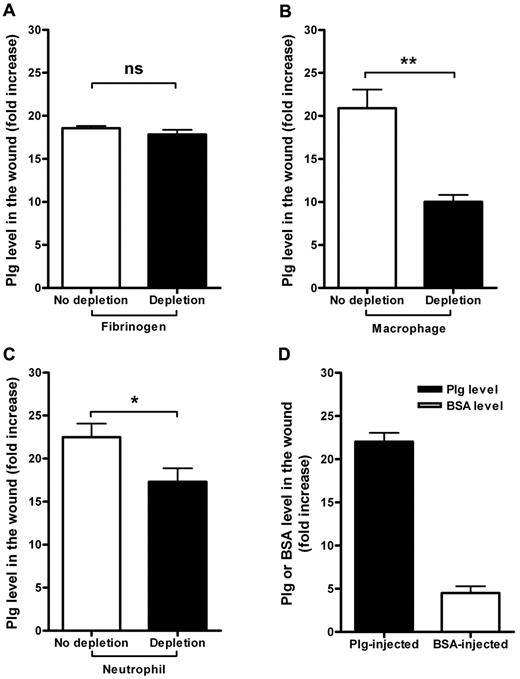
Fibrin formation followed by fibrinolysis by plasmin is one of the early hemostatic events after a wound injury.20,21 Because plg binds to fibrin surfaces with high affinity, we investigated whether the binding of plg to fibrin is responsible for the accumulation of plg in the wound. WT mice were first treated with the defibrinogenation reagent ancrod (3 U/d) for 3 days, resulting in an 80% reduction of plasma fibrinogen levels (data not shown).16 After this treatment, a standardized burn wound was induced on the back of the defibrinogenated mice and 5 minutes later, each mouse was given 2 mg of plg by IV injection and tissue samples were collected 24 hours after the injection. As shown in Figure 3A, there was no significant reduction in plg accumulation in the wounds of fibrinogen-depleted mice compared with the control group, indicating that the binding of plg to fibrin is not a major mechanism for the local accumulation of plg in the wound.
Mechanisms regulating plg accumulation in wounds after burn wounding. (A) Comparison of the plg levels in the wound sites in the control and fibrinogen-depleted mice 24 hours after the IV injection of plg (n = 5). (B) Comparison of the plg levels in the wound sites in the control and macrophage-depleted mice 24 hours after the IV injection of plg (n ≥ 4). (C) Comparison of the plg levels in the wound sites in the control and neutrophil-depleted mice 24 hours after the IV injection of plg (n ≥ 6). (D) Comparison of the plg or BSA accumulation in the wounded areas in the plg-injected (n = 12) and BSA-injected mice (n = 6). The level of plg or BSA in the abdominal skin was used as the basal level. The level of plg or BSA in the wound was determined and is displayed as the fold increase from the basal level. ns indicates not significant. *P < .05; **P < .01.
Mechanisms regulating plg accumulation in wounds after burn wounding. (A) Comparison of the plg levels in the wound sites in the control and fibrinogen-depleted mice 24 hours after the IV injection of plg (n = 5). (B) Comparison of the plg levels in the wound sites in the control and macrophage-depleted mice 24 hours after the IV injection of plg (n ≥ 4). (C) Comparison of the plg levels in the wound sites in the control and neutrophil-depleted mice 24 hours after the IV injection of plg (n ≥ 6). (D) Comparison of the plg or BSA accumulation in the wounded areas in the plg-injected (n = 12) and BSA-injected mice (n = 6). The level of plg or BSA in the abdominal skin was used as the basal level. The level of plg or BSA in the wound was determined and is displayed as the fold increase from the basal level. ns indicates not significant. *P < .05; **P < .01.
As shown in Figure 2A, the accumulation of plg in wounds is greatest during the early inflammatory phase. Immunostaining of human plg in the wound after plg treatment showed that human plg was clearly localized at the edge of the wound, where the inflammatory cells infiltrate (supplemental Figure 2). It is well known that inflammatory cells are recruited to a wounded area after injury, and several in vitro studies have shown that plg binds to inflammatory cells through different plg receptors.22-24 Consistent with this, after systemic supplementation of fluorescent plg into WT mice, exogenous fluorescent plg was bound to peripheral inflammatory cells (supplemental Figure 3). However, the in vivo significance of such binding in wound healing has not been explored. We therefore studied the contribution of 2 primary inflammatory cells, macrophages and neutrophils, to plg accumulation. In 2 sets of experiments, in vivo cell-depletion techniques were used to deplete neutrophils and macrophages before a burn wound was induced. Macrophages or neutrophils were depleted as described in “Methods” and burn wounds were induced. Five minutes after the burn induction, each mouse was given 2 mg of plg by IV injection, and tissue samples were collected 24 hours after the injection. As shown in Figure 3B, the level of plg in the wound after macrophage depletion was reduced by 52% compared with the no-depletion control group. Similarly, the plg accumulation after neutrophil depletion was reduced by 23% (Figure 3C). These data suggest that the wound-specific accumulation of plg after systemic supplementation is mainly due to the transportation of plg by macrophages and neutrophils.
Burn injuries cause vasodilation, increased blood flow, and an increased vessel permeability, which results in the increased transport of plasma proteins to the wound.25 Albumin is commonly used to measure vessel permeability.26 To investigate whether part of the wound-specific accumulation of plg is caused by these mechanisms, we used albumin to measure changes in vessel permeability during burn injury in our model. After burn induction, WT mice received an IV injection of either 2 mg of BSA or plg. As demonstrated in Figure 3D, the level of plg increased approximately 22-fold in the wound, whereas the level of BSA only increased approximately 5-fold.
These results suggest that the bulk of plg is transported to the wound site bound to inflammatory cells, and only a minor part is due to vessel leakage. However, the binding of plg to fibrin appears to play no role or only a minor role in this process.
Plg enhances the expression of IL-6 and augments the activation of STAT3 in wounded skin
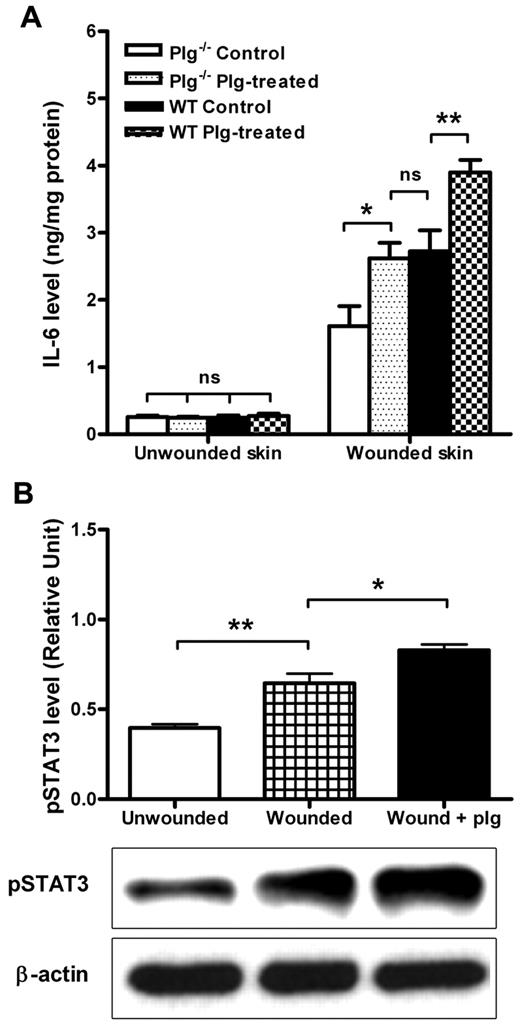
A proper inflammation response after injury is considered to be a prerequisite for wound healing, and proinflammatory cytokines are known to be involved in this process.27 IL-6 is an important mediator of host responses to tissue injury that can be induced through both TNF-α–dependent and other pathways.28 Therefore, we measured IL-6 levels in plg−/− and WT mice that were treated with BSA (control) or plg. As shown in Figure 4A, IL-6 levels in unwounded skin were low irrespective of genotype and treatment. However, the level of IL-6 in the wounded skin of the WT mice was significantly higher than that in the wounded skin of the plg−/− mice, suggesting that the inflammation response after the injury was compromised in the plg−/− mice. After plg treatment, the level of IL-6 in the wounds was enhanced in both the WT and plg−/− mice. Moreover, the level of IL-6 in the wounded skin in the plg−/− mice was restored to nearly normal WT levels. These data indicate that plg enhances the expression of IL-6 in wounds.
Effect of plg on IL-6 expression and STAT3 activation in the wound. (A) Comparison of the IL-6 levels between the WT (n ≥ 9) and plg−/− mice (n ≥ 9) treated with BSA (control) or plg. (B) Western blot analysis of the pSTAT3 levels in skin lysates from the unwounded WT mice, wounded WT mice, and wounded WT mice treated with plg (n ≥ 5). β-actin served as a loading control. The level of pSTAT3 was quantified using ImageJ Version 1.41o software. Values are expressed as the intensity of pSTAT3 divided by the intensity of total STAT3. ns indicates not significant. *P < .05; **P < .01.
Effect of plg on IL-6 expression and STAT3 activation in the wound. (A) Comparison of the IL-6 levels between the WT (n ≥ 9) and plg−/− mice (n ≥ 9) treated with BSA (control) or plg. (B) Western blot analysis of the pSTAT3 levels in skin lysates from the unwounded WT mice, wounded WT mice, and wounded WT mice treated with plg (n ≥ 5). β-actin served as a loading control. The level of pSTAT3 was quantified using ImageJ Version 1.41o software. Values are expressed as the intensity of pSTAT3 divided by the intensity of total STAT3. ns indicates not significant. *P < .05; **P < .01.
STAT3 is a key intracellular molecule that is involved in signaling during acute inflammatory responses mediated by several inflammatory cytokines, including IL-6.29 To determine how plg treatment may affect STAT3 activation, we measured the levels of phosphorylated STAT3 (pSTAT3) in wounds 24 hours after plg treatment. As shown in Figure 4B, the level of pSTAT3 was higher in wounded skin than in unwounded skin, and the pSTAT3 level was further increased after plg treatment, suggesting that plg treatment could enhance the activation of STAT3 in the wound. These data indicate that during wound healing, plg treatment enhances proinflammatory cytokines and activates intracellular signaling events.
Plg improves the healing of burn wounds in a mouse model of diabetes
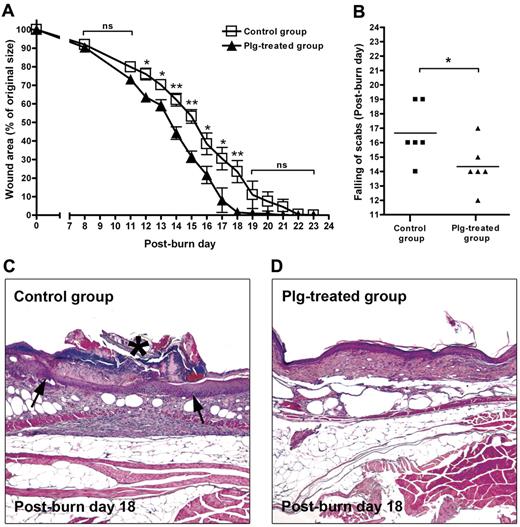
An imbalanced inflammatory response is one of the reasons for wound-healing defects in diabetic patients.2 To explore the healing potential of plg in diabetic wounds, we used db/db mice that suffer from a severe wound-healing impairment.30 A standardized burn wound was first induced on the backs of db/db mice. Immediately after the injury, the mice in the plg-treated group were administered 2 mg of plg by IV injection, whereas every mouse in the control group received 2 mg of BSA. These injections were repeated daily for 24 days. As shown in Figure 5A, the healing rate in the plg-treated group was significantly faster than that of the control group. The time to healing (ie, the scab falling off) in the plg-treated group was also significantly earlier (approximately 3 days earlier than the control group; Figure 5B). Morphologic analysis at 18 days after the injury revealed that the front of the epithelium layer in the control group had barely fused and was covered by a scab. In addition, the tissue underneath the scab was inflamed (Figure 5C). In contrast, in the plg-treated group, the injured epithelium layer and the underlying tissue had healed (Figure 5D).
Effect of plg treatment on wound healing in db/db mice. (A) Quantification of the remaining wound area at different time points after wounding in the BSA-treated control group (n = 6) and in the plg-treated group (n = 6). (B) Comparison of the healing times (scab falling off) in the days after wounding. (C-D) Representative pictures of the control group and the plg-treated group on day 18 after injury. The inflamed tissue is indicated by arrows and the scab is indicated by an asterisk. Magnification is 50×.
Effect of plg treatment on wound healing in db/db mice. (A) Quantification of the remaining wound area at different time points after wounding in the BSA-treated control group (n = 6) and in the plg-treated group (n = 6). (B) Comparison of the healing times (scab falling off) in the days after wounding. (C-D) Representative pictures of the control group and the plg-treated group on day 18 after injury. The inflamed tissue is indicated by arrows and the scab is indicated by an asterisk. Magnification is 50×.
To further explore the molecular mechanisms underlying the improved healing mediated by plg in db/db mice, we determined the plg level in wounded and unwounded skin in db/db and nondiabetic control db/+ mice. As shown in Figure 6A, the plg levels in the unwounded skin were low irrespective of genotype or treatment. After burn induction, the plg levels in the wounds of the db/db mice were only slightly enhanced compared with the control db/+ mice, in which the plg level after wounding increased approximately 5-fold. However, after receiving systemic plg treatment, the plg levels in the wounds of the db/db mice reached approximately the same level as in the control db/+ mice. Studies of the IL-6 levels 24 hours after injury in the control and diabetic wounds showed that IL-6 levels were significantly lower in the wounds of the db/db mice than in the control db/+ mice (Figure 6B). However, after plg treatment, the IL-6 levels in the wounds of the db/db mice were clearly increased (Figure 6B). We also measured the levels of pSTAT3 in skin from unwounded and wounded db/db mice and wounded db/db mice treated with plg. As shown in Figure 6C, the levels of pSTAT3 in the wound sites increased after wounding and further increased after plg treatment.
Effect of plg treatment on IL-6 expression and STAT3 activation in db/db mice. (A) Comparison of plg levels in the unwounded and wounded areas in db/db mice (n = 8), db/db mice treated with plg (n = 4), and db/+ mice (n = 4). (B) Comparison of the IL-6 levels of db/db mice (n = 8), db/db mice treated with plg (n = 8), and db/+ mice (n = 10). (C) Western blot analysis of the pSTAT3 levels in the skin lysates from unwounded db/db mice, wounded db/db mice, and wounded db/db mice treated with plg (n = 4). β-actin served as a loading control. The level of pSTAT3 was quantified using ImageJ Version 1.41o software. Values are expressed as the intensity of pSTAT3 divided by the intensity of total STAT3. ns indicates not significant. *P < .05; **P < .01.
Effect of plg treatment on IL-6 expression and STAT3 activation in db/db mice. (A) Comparison of plg levels in the unwounded and wounded areas in db/db mice (n = 8), db/db mice treated with plg (n = 4), and db/+ mice (n = 4). (B) Comparison of the IL-6 levels of db/db mice (n = 8), db/db mice treated with plg (n = 8), and db/+ mice (n = 10). (C) Western blot analysis of the pSTAT3 levels in the skin lysates from unwounded db/db mice, wounded db/db mice, and wounded db/db mice treated with plg (n = 4). β-actin served as a loading control. The level of pSTAT3 was quantified using ImageJ Version 1.41o software. Values are expressed as the intensity of pSTAT3 divided by the intensity of total STAT3. ns indicates not significant. *P < .05; **P < .01.
These data indicate that the impaired wound healing seen in diabetic db/db mice may be due in part to the dysregulation of plg. Plg supplementation appears to rebalance the early inflammatory response in these mice, which leads to improved wound healing. Therefore, plg treatment may become a novel therapeutic strategy for the treatment of diabetic wounds.
Discussion
The present study demonstrates for the first time that plg acts as a key regulatory molecule that plays a pivotal role in the control of wound healing. Plg potentiates the early inflammatory response, and systemic administration of additional plg to mice not only accelerates the healing of acute wounds in healthy WT mice, but also improves the healing of chronic diabetic wounds in db/db mice. Our results suggest that the administration of plg may be a novel therapeutic strategy to treat different types of wounds, especially diabetic wounds, for which there are no effective, widely used treatments.3
It is well established from studies of gene-deficient mice that the PA system and formed plasmin play an important role in wound healing.5,31,32 Based on these studies, it has been suggested that the activation of plg plays a role mainly in fibrin degradation, cell migration, and the degradation of the extracellular matrix during tissue-remodeling events.6,33,34 However, results of our previous study on the healing of TM perforation in plg−/− mice have revealed that plg/plasmin appears to play a profoundly different role in the healing process than what had been previously assumed based on studies of skin wounds. In particular, our studies have indicated that plasmin may not just be a protease involved in fibrin degradation and matrix remodeling, but may also act as an initiator of wound healing.11
In the present study, we have demonstrated that plg supplementation improves and speeds up skin wound healing in healthy WT mice. These mice do not have impaired fibrinolysis or a defect in extracellular matrix degradation. This suggests that plg may play further roles in wound healing in addition to acting as a protease that dissolves fibrin and tissue barriers to pave the way for cells to migrate. We have also demonstrated that plg levels increase specifically at the inflammation site and in a limited area around the wound. This accumulation of plg occurs during the early inflammation phase of wound healing and can be further enhanced after plg supplementation. Systemic plg supplementation also leads to an accelerated wound-healing process. This indicates that plg acts as a key proinflammatory factor regulating the wound-healing process by activating the early inflammatory reaction.
Inflammatory cell–depletion experiments revealed that the majority of the plg that accumulates in wound sites is connected to neutrophils and macrophages. Most likely, plg is transported to the inflammation site bound to the surface receptors present on these cells. This is consistent with previous studies showing that the capacity of leukocytes to bind plg is increased after cell activation.9,22,35,36 Proinflammatory cytokines play important roles in the activation of inflammatory responses.27 In vitro studies on monocytes have shown that active plasmin bound to the cell surface of monocytes through lysine-binding sites can activate several intracellular signaling cascades that lead to activation of proinflammatory genes.8 Consistent with this, we found that the level of IL-6 is enhanced and STAT3 is activated in the wounded area. Moreover, supplementation with additional plg not only caused additional IL-6 enhancement and a further activation of STAT3, but also improved the healing process significantly.
Based on the present study and previous results, we propose the following working model for the role of plg in wound healing. In addition to its well-known role as a protease involved in fibrinolysis, cell migration, and tissue remodeling, plg may also play an even more profound role as a key regulatory molecule that potentiates and perhaps even initiates the healing process. Under normal physiologic conditions, plg binding to the surface of inflammatory cells is low.22 However, after injury, inflammatory cytokines are released, thereby providing an “injury signal” to inflammatory cells, which results in an increased plg binding capacity9,22,35,36 and a locally increased vessel permeability.37 Plg bound to inflammatory cells is then transported to the wounded area, where it is activated to plasmin, which somehow mediates proteolytic cell activation, possibly through the proteolytic cleavage of an as-yet-unidentified receptor.8 Plasmin then activates several signaling cascades and elicits a proinflammatory reaction that potentiates the early inflammatory response during wound healing. Our data showing that plg is a key proinflammatory regulator that potentiates wound healing. Together with its previously known roles, these new findings give plg a more central role during wound healing than was thought previously.
Diabetic wounds are among the most severe types of chronic wounds. More than 20% of diabetic wounds result in amputations of the affected foot or leg.38 However, much of the available information on the biology of wound healing relates to acute and experimental wounds, and may therefore not be directly relevant to diabetic wound healing. Based on our findings in acute wounds in healthy WT mice, in the present study, we investigated whether plg was dysregulated in diabetic wound healing and if plg supplementation had any stimulatory effect on the healing of diabetic wounds. As shown in Figures 5 and 6, there was no wound-specific accumulation of plg in the wounds of diabetic db/db mice (Figure 6A), and the healing rate was also slower in these mice (Figure 5A). It has been shown that impaired healing in diabetic wounds is accompanied by decreased early infiltration of inflammatory cells.39-41 Our binding studies indicate that the binding of plg to the peripheral inflammatory cells in WT mice and db/db mice are similar (unpublished data). The impaired wound-specific accumulation of plg is therefore most likely due to a decreased infiltration of inflammatory cells. After plg administration, the level of plg in the wounds of the diabetic mice increased to the same level as that found in nondiabetic control mice. This also resulted in the activation of intracellular signaling events and a significantly increased healing rate. Therefore, our data indicate that the dysregulation of plg may be part of the pathogenesis of diabetic wound healing and that supplementation with plg results in significantly improved healing.
In conclusion, the results of the present study show for the first time that plg, in addition to its role in extracellular matrix degradation, plays a pivotal role as a key proinflammatory factor that potentiates the early inflammatory response during wound healing. Based on our findings, we propose a novel therapeutic strategy for the treatment of different types of wounds, including chronic diabetic wounds, consisting of exogenous plg administrated locally or systemically.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chun Du, Jie Zhao, Maria Ny, Jessica Nordlund, and Tina Jonsson for technical support.
This study was supported by the Swedish Research Council (grant B0322301 to T.N.) and the Medical Faculty of Umeå University.
Authorship
Contribution: Y.S., Y.G., P.M., and R.S. performed the experiments; Y.S., T.N., and J.L. conceived and designed the experiments and wrote and prepared the manuscript; and Y.S., Y.G., M.W., T.N., and J.L. analyzed the data.
Conflict-of-interest disclosure: T.N. and J.L. have patented the use of plg for the treatment of wound healing and are stockholders in a start-up company that owns the right to develop plg for therapeutic purposes. The remaining authors declare no competing financial interests.
Correspondence: Tor Ny or Jinan Li, Department of Medical Biochemistry and Biophysics, Umeå University, 90187 Umeå, Sweden; e-mail: tor.ny@mechem.umu.se or jinan.li@medchem.umu.se.
References
Author notes
T.N. and J.L. contributed equally to this study.