Abstract
Several studies have found that high levels of reactive oxidative species (ROS) are associated with stem cell dysfunction. In the present study, we investigated the role of nuclear factor erythroid-2–related factor 2 (Nrf2), a master regulator of the antioxidant response, and found that it is required for hematopoietic stem progenitor cell (HSPC) survival and myeloid development. Although the loss of Nrf2 leads to increased ROS in most tissues, basal ROS levels in Nrf2-deficient (Nrf2−/−) BM were not elevated compared with wild-type. Nrf2−/− HSPCs, however, had increased rates of spontaneous apoptosis and showed decreased survival when exposed to oxidative stress. Nrf2−/− BM demonstrated defective stem cell function, as evidenced by reduced chimerism after transplantation that was not rescued by treatment with the antioxidant N-acetyl cysteine. Gene-expression profiling revealed that the levels of prosurvival cytokines were reduced in Nrf2−/− HSPCs. Treatment with the cytokine G-CSF improved HSPC survival after exposure to oxidative stress and rescued the transplantation defect in Nrf2−/− cells despite increases in ROS induced by cytokine signaling. These findings demonstrate a critical role for Nrf2 in hematopoiesis and stem cell survival that is independent of ROS levels.
Introduction
Reactive oxygen species (ROS) are required for normal cellular homeostasis because they serve as critical mediators of cytokine signaling and antimicrobial host defenses. However, excessive ROS levels can induce cellular damage that compromises cellular survival and generates genetic mutations that eventually lead to cancer.1 ROS levels influence normal hematopoiesis; therefore, identifying the cellular processes that regulate responses to oxidative stress may improve our understanding of BM failure states and hematopoietic malignancies. In mature blood cells, ROS regulates the production and life span of erythrocytes,2 the proliferation of leukocytes in response to cytokines,3-6 and the maturation of megakaryocytes.6 Hematopoietic stem progenitor cells (HSPCs) have been found to have relatively low ROS levels compared with their mature progeny.7 Furthermore, HSPCs may be particularly sensitive to oxidative stress, because transgenic mouse models have demonstrated that the functional loss of various genes (ATM, FOXO3a, p38 MAPK, Prdm16, and Mdm2) results in defective HSPC self-renewal, apoptosis, and differentiation associated with increased ROS.8-13 However, the precise mechanisms by which ROS coordinate signals critical to HSPC survival and function are not clear.
Nuclear factor erythroid-2–related factor 2 (Nrf2), a bZIP transcription factor, acts as a master regulator of the cellular response to increased oxidative states.14 Nrf2 establishes a reduced intracellular redox state by inducing the expression of a myriad of cytoprotective genes, including those involved in the glutathione and thioredoxin systems, xenobiotic metabolism, and the members of the glutathione-S-transferase family.14-21 Nrf2-deficient (Nrf2−/−) mice are born in expected Mendelian ratios and develop normally, but succumb prematurely to multiorgan autoimmune inflammation. Moreover, responses to chemical and biologically induced oxidative stress are impaired, as evidenced by increased disease severity in models of emphysema,17,22 asthma,16 sepsis,23 rheumatoid arthritis,24,25 gastritis,26 and colitis.27 Nrf2 has been recently implicated as key regulator of Drosophila intestinal stem cells, because its loss is associated with increased intracellular ROS and stem cell dysfunction.28 Therefore, Nrf2 signaling plays an essential role in attenuating oxidative damage and may play such a role in stem cells as well. Given the critical requirement for appropriate levels of ROS during hematopoiesis and the essential role of Nrf2 in regulating ROS, we hypothesized that Nrf2 would be required for normal hematopoiesis and HSPC function.
In the present study, we characterized the HSPC compartment in Nrf2−/− mice and demonstrated that the loss Nrf2 results in defective differentiation, decreased survival, and impaired engraftment of HSPCs after BM transplantation. Unexpectedly, Nrf2−/− HSPCs did not display elevated basal levels of ROS, although they were more sensitive to apoptosis by induced oxidative stress. Gene-expression profiling suggested the loss of Nrf2 leads to global defects in cytokine signaling, and the administration of the exogenous G-CSF improved HSPC survival despite increasing intracellular ROS. Although increased ROS levels have been generally associated with impaired HSPC function, our findings indicate that increased levels of ROS are not always deleterious. Furthermore, our data suggest an essential role for Nrf2 in regulating HSPC survival independently of its role in regulating ROS.
Methods
Animals and care
Nrf2-deficient C57BL6 mice (SLC Japan) were generated as described previously.29 Mice were fed an AIN-76A diet and water ad libitum and housed under controlled conditions (23 ± 2°C; 12-hour light/dark periods). All experimental protocols conducted on the mice were performed in accordance with the standards established by the US Animal Welfare Acts as set forth in National Institutes of Health (NIH) guidelines and the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee.
Peripheral blood analysis
Mice used for analysis were between 8 and 12 weeks old. Peripheral blood was obtained by retroorbital puncture in heparinized capillaries, and complete blood counts were measured on a Hemavet hematology analyzer (Drew Scientific).
Analysis of BM fractions and cell sorting
Mice used for analysis were between 8 and 12 weeks old. BM was flushed from femurs and tibia with staining medium (RPMI with 2% FBS), filtered, and resuspended at a concentration of 108 cells/mL in staining medium. The following Abs were used for staining the BM cells: CD34 (clone: RAM34)–allophycocyanin (APC) or FITC, FcRγ (clone: 93)–PE or Flt3 (clone: A2F10)-PE, c-Kit (clone: 2B8)–APC-Alexa Fluor 750, Sca1 (clone: D7)–PE-cyanin 7, CD150 (clone: TC15-12F12.2)–APC (BioLegend), CD48 (clone: HM48-1)–PE (all BD Biosciences) and biotin streptavidin-peridinin-chlorophyll-protein complex-cyanin 5.5 (PerCP-Cy5.5) or eFlour450-labeled lineage cocktail (CD3e, Gr1, B220, and Ter119, all eBioscience). Apoptotic cells were identified using the FITC Annexin V Apoptosis Kit (BD Biosciences). Fractions were analyzed on a FACSCalibur, 4 laser LSRII and/or sorted on a 2-laser FACSAria flow cytometer (BD Biosciences).
Quantitative PCR
Cell populations were isolated by FACS as described in “Analysis of BM fractions and cell sorting.” RNA was isolated using the RNeasy Microkit (QIAGEN), and cDNA was made using the Sensiscript cDNA synthesis kit (QIAGEN) primed with oligo dT primers as per the manufacturer's protocol. Quantitative RT-PCR for selected genes was carried out using predesigned Taqman real-time primer/probes from Applied Biosystems. Expression levels were normalized to β-actin and GAPDH RNA and compared using the threshold cycle method.
BM transplantation
CD45.2 whole BM or sorted c-Kit+Sca1+Lin− (KSL) cells were obtained and transplanted into lethally irradiated (1100 rads in 2 divided doses at least 4 hours apart) CD45.1 hosts. Peripheral blood was obtained at the time points indicated and analyzed for WBC chimerism (CD45.2) and multilineage engraftment. Myeloid engraftment assessed by expression of Mac-1 and Gr1, B-cell engraftment by B220, and T-cell engraftment by CD3 on a FACSCalibur flow cytometer (BD Biosciences).
Analysis of in vivo proliferation by BrdU incorporation
Mice were treated with single dose (100 mg/kg) of bromodeoxyuridine (5-bromo-2-deoxyuridine [BrdU]; BD Biosciences) by IP injection.11 Three to 14 hours after injection, BM was isolated, filtered, labeled with Abs, fixed, and labeled with an FITC-conjugated anti–BrdU Ab using the BrdU Flow Kit (BD Biosciences) according to the manufacturer's protocol.
NAC administration in vivo
Five- to 6-week-old donor Nrf2+/+ and Nrf2−/− mice were treated with N-acetyl-L-cysteine (NAC; 100 mg/kg/d; Sigma-Aldrich) or saline solution by subcutaneous administration for 3 weeks. At the end of 3 weeks, mice were killed and the BM was isolated, analyzed, and transplanted in the recipient mice. The recipient mice were treated with NAC for 1 week after BM transplantation.9
G-CSF treatment
Mice were treated with 100 μg/kg of recombinant G-CSF (Filgrastim; Amgen) by daily IP injection.
Colony-forming assays
Whole BM or KSL cells were plated in methylcellulose medium supplemented with cytokines (Methocult M3434; StemCell Technologies) according to the manufacturer's directions, and colonies were scored at 12-14 days.
Statistics
Statistical significance was calculated using a Student t test, and P < .05 was considered significant.
Results
Nrf2 is highly expressed in HSPCs and is required for stem cell function
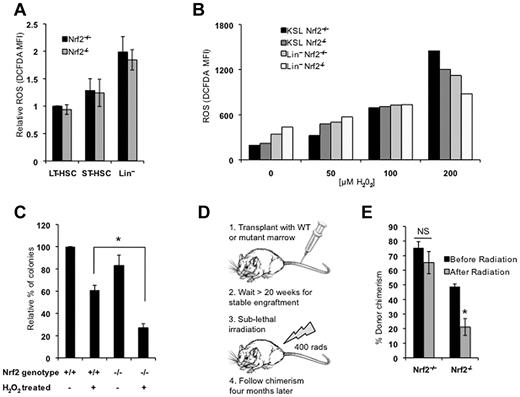
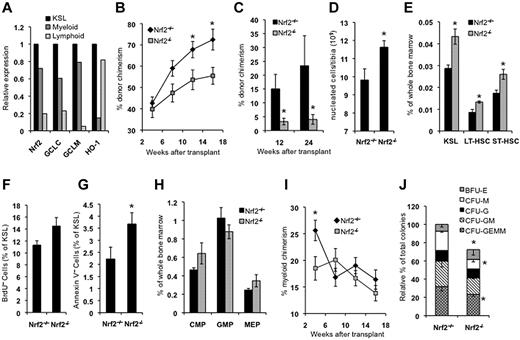
We initially analyzed the peripheral blood counts of Nrf2+/+ and Nrf2−/− mice and observed no differences in the total leukocyte count (7.1 vs 8.3 × 104/μL, P = .41), absolute neutrophil count (1.6 vs 2.0 × 104/μL, P = .30), or absolute lymphocyte count (5.3 vs 6.0 × 104/μL, P = .44) between Nrf2+/+ and Nrf2−/− mice. However, consistent with previous reports,6,30 Nrf2−/− mice had reductions in RBC counts (9.40 vs 7.67 × 106/μL, P = .005), hemoglobin concentration (14.2 vs 11.9 g/dL, P = .002) and platelet count (845 vs 550 × 104/μL, P = .018). We studied the expression of Nrf2 and select target genes in HSPCs isolated from Nrf2+/+ mice using quantitative RT-PCR. The KSL compartment is highly enriched for HSPCs and demonstrated the highest expression of Nrf2 and target genes, followed by committed myeloid (c-Kit+Sca1−Lin−) and lymphoid (c-KitlowSca1+Lin−) progenitors (Figure 1A). To determine whether Nrf2 is required for HSPC function, we performed competitive transplantation of BM from CD45.2 Nrf2+/+ and Nrf2−/− mice into CD45.1 congenic wild-type recipients. Mice receiving Nrf2−/− BM displayed significantly lower levels of long-term (> 12 weeks) CD45.2 peripheral blood chimerism compared with mice receiving Nrf2+/+ BM (Figure 1B). To confirm that Nrf2−/− cells are defective during transplantation, we repeated these studies using an HSPC-enriched population and transplanted equal numbers of Nrf2+/+ or Nrf2−/− CD45.2 KSL cells into wild-type CD45.1 recipients. Mice transplanted with Nrf2−/− KSL cells showed a 5-fold decrease in long-term peripheral blood chimerism, confirming that the loss of Nrf2 leads to HSPC dysfunction (Figure 1C).
Nrf2−/− mice have abnormalities in BM and HSPC function. (A) Nrf2 and target gene expression is highest in HSPCs. Expression of the Nrf2 target genes glutamate-cysteine ligase catalytic subunit (GCLC), glutamate-cysteine ligase modifier subunit (GCLM), and heme oxygenase 1 (HO-1) were quantified in pooled BM from 5 Nrf2+/+ mice. Cells from the KSL, myeloid (c-Kit+Sca1−Lin−), and lymphoid (c-KitdimSca1+Lin−) progenitor compartments were isolated by FACS and quantitative RT-PCR performed in triplicate. Gene expression was normalized to β-actin and quantified relative to KSL cells. (B) Whole BM from Nrf2−/− mice has decreased stem cell activity. Whole BM cells (666 000) from Nrf2+/+ and Nrf2−/− were mixed with 333 000 CD45.1 competitor normal BM cells (2:1 ratio) and injected into lethality irradiated CD45.1 hosts. Peripheral blood was analyzed for donor chimerism at the indicated time points. Data are the combined averages from 6 total mice per genotype from 2 separate experiments. *P < .05. (C) Phenotypic HSPCs from Nrf2−/− show defective transplantation. Five hundred KSL cells from Nrf2+/+ and Nrf2−/− mice were mixed with 250 000 CD45.1 competitor normal BM cells and injected into lethally irradiated CD45.1 hosts. Peripheral blood was analyzed for donor chimerism at the indicated time points. Data represent the average peripheral blood engraftment from 10 total recipient mice per genotype in 2 separate experiments.*P < .05. (D) BM cellularity in Nrf2+/+ and Nrf2−/− mice. Average number of cells per tibia. n = 6 mice each group. *P = .019. (E) Progenitor and stem cell numbers are increased in Nrf2−/− mice. Relative frequency of KSL, LT-HSC (CD34− c-Kit+Sca1+Lin−), and ST-HSC (CD34+ c-Kit+Sca1+Lin−) cells. (F) HSPCs in Nrf2−/− mice are more proliferative as shown by BrdU uptake in vivo. Mice were injected with 100 mg/kg of BrdU to mark actively proliferating cells, and BM was harvested 14 hours later and assessed for BrdU labeling by flow cytometry. Data represent the average number of BrdU-positive KSL cells for each genotype. P = .06. (G) Greater rates of spontaneous apoptosis in Nrf2−/− HSPCs. Freshly isolated BM cells were analyzed by flow cytometry for annexin V positivity to identify apoptotic cells. Data are average values of annexin V+ cells as a percentage of the KSL compartment. *P < .01. (H) Myeloid progenitor frequency. Nrf2−/− BM cells were examined by flow cytometry. Increased common myeloid progenitors (FcRγlowCD34+c-Kit+Sca1−Lin−) and decreased granulocyte-monocyte progenitors (FcRγhighCD34+c-Kit+Sca1−Lin−) were observed, with a trend (P < .1) toward statistical significance. (I) Early myeloid engraftment is impaired in mice transplanted with Nrf2−/− BM. Percentage of CD45.2+ cells in peripheral blood expressing myeloid lineage markers Gr1+/Mac1+ from mice transplanted with Nrf2+/+ and Nrf2−/− BM as in Figure 1C. *P = .007. (J) In vitro myeloid colony formation is impaired in Nrf2−/− mice. One hundred KSL cells were plated in methylcellulose medium supplemented with cytokines, and the colonies were scored at 14 days. *P < .05
Nrf2−/− mice have abnormalities in BM and HSPC function. (A) Nrf2 and target gene expression is highest in HSPCs. Expression of the Nrf2 target genes glutamate-cysteine ligase catalytic subunit (GCLC), glutamate-cysteine ligase modifier subunit (GCLM), and heme oxygenase 1 (HO-1) were quantified in pooled BM from 5 Nrf2+/+ mice. Cells from the KSL, myeloid (c-Kit+Sca1−Lin−), and lymphoid (c-KitdimSca1+Lin−) progenitor compartments were isolated by FACS and quantitative RT-PCR performed in triplicate. Gene expression was normalized to β-actin and quantified relative to KSL cells. (B) Whole BM from Nrf2−/− mice has decreased stem cell activity. Whole BM cells (666 000) from Nrf2+/+ and Nrf2−/− were mixed with 333 000 CD45.1 competitor normal BM cells (2:1 ratio) and injected into lethality irradiated CD45.1 hosts. Peripheral blood was analyzed for donor chimerism at the indicated time points. Data are the combined averages from 6 total mice per genotype from 2 separate experiments. *P < .05. (C) Phenotypic HSPCs from Nrf2−/− show defective transplantation. Five hundred KSL cells from Nrf2+/+ and Nrf2−/− mice were mixed with 250 000 CD45.1 competitor normal BM cells and injected into lethally irradiated CD45.1 hosts. Peripheral blood was analyzed for donor chimerism at the indicated time points. Data represent the average peripheral blood engraftment from 10 total recipient mice per genotype in 2 separate experiments.*P < .05. (D) BM cellularity in Nrf2+/+ and Nrf2−/− mice. Average number of cells per tibia. n = 6 mice each group. *P = .019. (E) Progenitor and stem cell numbers are increased in Nrf2−/− mice. Relative frequency of KSL, LT-HSC (CD34− c-Kit+Sca1+Lin−), and ST-HSC (CD34+ c-Kit+Sca1+Lin−) cells. (F) HSPCs in Nrf2−/− mice are more proliferative as shown by BrdU uptake in vivo. Mice were injected with 100 mg/kg of BrdU to mark actively proliferating cells, and BM was harvested 14 hours later and assessed for BrdU labeling by flow cytometry. Data represent the average number of BrdU-positive KSL cells for each genotype. P = .06. (G) Greater rates of spontaneous apoptosis in Nrf2−/− HSPCs. Freshly isolated BM cells were analyzed by flow cytometry for annexin V positivity to identify apoptotic cells. Data are average values of annexin V+ cells as a percentage of the KSL compartment. *P < .01. (H) Myeloid progenitor frequency. Nrf2−/− BM cells were examined by flow cytometry. Increased common myeloid progenitors (FcRγlowCD34+c-Kit+Sca1−Lin−) and decreased granulocyte-monocyte progenitors (FcRγhighCD34+c-Kit+Sca1−Lin−) were observed, with a trend (P < .1) toward statistical significance. (I) Early myeloid engraftment is impaired in mice transplanted with Nrf2−/− BM. Percentage of CD45.2+ cells in peripheral blood expressing myeloid lineage markers Gr1+/Mac1+ from mice transplanted with Nrf2+/+ and Nrf2−/− BM as in Figure 1C. *P = .007. (J) In vitro myeloid colony formation is impaired in Nrf2−/− mice. One hundred KSL cells were plated in methylcellulose medium supplemented with cytokines, and the colonies were scored at 14 days. *P < .05
Loss of Nrf2 causes increased apoptosis and BM compensation
To better characterize the hematopoietic defect in Nrf2−/− mice, we examined the BM cellularity and distribution of cells in the stem and progenitor compartments. Total cellularity was increased by approximately 15% in Nrf2−/− BM (Figure 1D), as was the frequencies of hematopoietic stem cells (HSCs)/progenitors (KSL; Figure 1E). Identification of HSCs using SLAM markers (CD150+CD48−), however, revealed a decrease in the numbers of HSCs (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Analysis of the most primitive long-term HSC (CD150+CD34−KSL) compartment showed an approximately 20% reduction in Nrf2−/− BM (supplemental Figure 1B).31 This modest reduction was not statistically significant and is unlikely to account for the 5-fold difference in engraftment seen during KSL transplantation (Figure 1C). Therefore, the loss of Nrf2 leads to both qualitative and quantitative defects in HSPCs. Interestingly, there appeared to be a bias toward CD150low lymphoid primed HSCs and away from CD150high myeloid primed HSCs, which is consistent with the defect in myeloid development we observed (supplemental Figure 1B).32,33
To determine whether the changes in the BM compartment were due to increased proliferation, we compared in vivo BrdU incorporation by Nrf2+/+ and Nrf2−/− KSL cells (Figure 1F). There was a trend toward increased KSL proliferation in the Nrf2−/− mice (P = .06). To determine whether the increased BM cellularity and proliferation were associated with increased cell turnover, we measured basal rates of apoptosis in the KSL compartment of freshly isolated BM from Nrf2+/+ and Nrf2−/− mice. Although absolute levels of apoptosis were low in both groups, there was a 65% relative increase in apoptosis in Nrf2−/− KSL cells (Figure 1G). Therefore, the combination of normal to reduced peripheral blood counts, increased BM cellularity, and increased BM proliferation all suggest BM compensation in response to decreased HSPC survival in Nrf2−/− mice.
Loss of Nrf2 impairs normal myeloid differentiation
Nrf2 and target gene expression has been shown to increase with myeloid maturation.34 Therefore, we examined the effects of Nrf2 loss on the frequency of myeloid progenitors. In Nrf2−/− mice, we observed a trend (P < .1) toward increased common myeloid progenitors (FcRγlowCD34+c-Kit+Sca1−Lin−) and decreased granulocyte-monocyte progenitors (FcRγhighCD34+c-Kit+Sca1−Lin−) (Figure 1H), suggesting impaired differentiation. This was correlated with a significant decrease in early myeloid engraftment (at 4 weeks) in mice transplanted with Nrf2−/− BM (Figure 1I). Furthermore, total in vitro colony formation was also impaired, with decreased macrophage, granulocyte-macrophage, and granulocyte-erythrocyte-macrophage-megakaryocyte colonies (Figure 1J).
Nrf2−/− BM has normal basal levels of ROS but increased susceptibility to oxidative stress
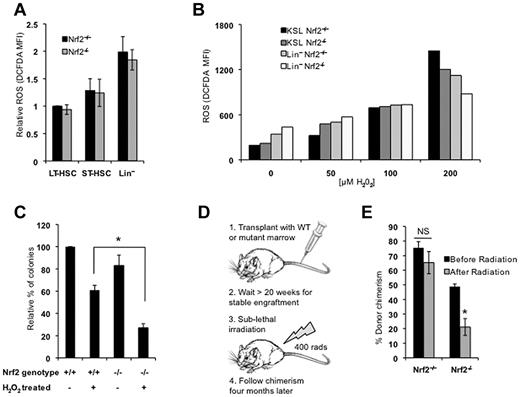
Nrf2 loss results in increased ROS levels in most tissues. Therefore, we hypothesized that HSPC dysfunction in Nrf2−/− cells was because of increased oxidative stress. We quantified ROS by flow cytometry using H2-CM-DCFDA, and found that ROS levels were lowest in the long-term HSC compartment of Nrf2+/+ mice and increased with differentiation, as described previously (Figure 2A).7 Unexpectedly, Nrf2−/− BM cells displayed ROS levels similar to those of Nrf2+/+ cells, suggesting that the functional defect in Nrf2−/− HSPCs is not because of increased oxidative stress (Figure 2A). When we examined the expression of redox regulatory genes in KSL cells from Nrf2+/+ and Nrf2−/− BM, we observed that the levels of the Nrf2 targets GCLM and GCLC were, as expected, decreased and the HO-1 levels were unchanged, whereas Trx1 levels were increased (supplemental Figure 2). We suspect that the increased Trx1 expression may represent a compensatory mechanism in HSCs and may explain why ROS levels are not increased at baseline in Nrf2−/− HSCs. Although baseline levels of ROS did not change with the loss of Nrf2 expression, we speculated that Nrf2 could be important for protecting HSCs from induced oxidative stress. To determine whether Nrf2−/− cells handle ROS differently, we treated Nrf2+/+ and Nrf2−/− cells with increasing concentrations of H2O2. At low doses of H2O2, Nrf2−/− KSL cells displayed higher levels of ROS, which is consistent with the role of Nrf2 in attenuating oxidative stress (Figure 2B). However, at the highest doses of H2O2, higher levels of ROS were induced in the Nrf2+/+ KSL compartment, suggesting that the ability of Nrf2−/− cells to tolerate high levels of induced ROS was impaired. We plated Nrf2+/+ and Nrf2−/− BM cells in methylcellulose following treatment with H2O2, and found that colony formation was significantly reduced in Nrf2−/− cells (70% vs 40%, Figure 2C). These results demonstrated that the loss of Nrf2 increases the sensitivity of myeloid progenitors to induced oxidative stress in vitro. To examine whether Nrf2 similarly protects HSPCs from induced ROS in vivo, we exposed wild-type CD45.1 mice stably engrafted (> 20 weeks after transplantation) with CD45.2 BM from Nrf2+/+ or Nrf2−/− mice to sublethal radiation and quantified peripheral blood chimerism (Figure 2D). Radiation exposure is known to generate oxidative stress, and treatment with antioxidants can prevent radiation-induced injury.35 Four months after irradiation, the percentage of CD45.2 peripheral blood cells was significantly decreased in mice transplanted with Nrf2−/− BM compared with Nrf2+/+ BM (Figure 2E). Therefore, the survival of Nrf2−/− HSPCs is reduced after the induction of oxidative stress both in vitro and in vivo.
Nrf2−/− HSPCs have no increase in basal ROS but are more susceptible to oxidative stress. (A) ROS levels in Nrf2+/+ and Nrf2−/− BM cells. BM isolated from Nrf2+/+ and Nrf2−/− mice was incubated with H2-CM-DCFDA to detect intracellular ROS by flow cytometry. Mean fluorescence intensity was measured in LT-HSC (CD34− c-Kit+Sca1+Lin−), ST-HSC (CD34+ c-Kit+Sca1+Lin−), and Lin+ cells. Relative MFIs are normalized to wild-type LT-HSCs. Data represent mean values from 13 mice of each genotype in 4 different experiments. (B) Induction of intracellular ROS in Nrf2+/+ and Nrf2−/− BM cells by increasing doses of H2O2. BM cultured in normoxia for 30 minutes with H2O2 (50-200μM) to induce oxidative stress. BM from 3 mice per genotype was pooled for each concentration of H2O2. Data are mean fluorescence intensity values of H2-CM-DCFDA (arbitrary units). (C) Nrf2−/− progenitors show increased sensitivity to oxidative stress in vitro. Whole BM cells were treated with 50μM H2O2 for 30 minutes, plated in methylcellulose medium supplemented with cytokines, and colonies were scored at 14 days. Data are mean values from 3 individual mice plated in duplicated. *P = .012. (D) Schema for testing in vivo sensitivity of Nrf2−/− LT-HSCs to radiation injury. Mice were transplanted with Nrf2+/+ or Nrf2−/− and allowed to form stable chimeras. Mice were then treated with a sublethal dose of radiation (400 rads) and their chimerism compared 4 months later to assess the effect of radiation on LT-HSCs. (E) Nrf2−/− LT-HSCs show increased radiosensitivity. Four months after radiation, peripheral blood chimerism returned to baseline in mice transplanted with Nrf2+/+ BM, but remained much lower in Nrf2−/− chimeras, suggesting a selective loss of Nrf2−/− LT-HSCs. *P = .018
Nrf2−/− HSPCs have no increase in basal ROS but are more susceptible to oxidative stress. (A) ROS levels in Nrf2+/+ and Nrf2−/− BM cells. BM isolated from Nrf2+/+ and Nrf2−/− mice was incubated with H2-CM-DCFDA to detect intracellular ROS by flow cytometry. Mean fluorescence intensity was measured in LT-HSC (CD34− c-Kit+Sca1+Lin−), ST-HSC (CD34+ c-Kit+Sca1+Lin−), and Lin+ cells. Relative MFIs are normalized to wild-type LT-HSCs. Data represent mean values from 13 mice of each genotype in 4 different experiments. (B) Induction of intracellular ROS in Nrf2+/+ and Nrf2−/− BM cells by increasing doses of H2O2. BM cultured in normoxia for 30 minutes with H2O2 (50-200μM) to induce oxidative stress. BM from 3 mice per genotype was pooled for each concentration of H2O2. Data are mean fluorescence intensity values of H2-CM-DCFDA (arbitrary units). (C) Nrf2−/− progenitors show increased sensitivity to oxidative stress in vitro. Whole BM cells were treated with 50μM H2O2 for 30 minutes, plated in methylcellulose medium supplemented with cytokines, and colonies were scored at 14 days. Data are mean values from 3 individual mice plated in duplicated. *P = .012. (D) Schema for testing in vivo sensitivity of Nrf2−/− LT-HSCs to radiation injury. Mice were transplanted with Nrf2+/+ or Nrf2−/− and allowed to form stable chimeras. Mice were then treated with a sublethal dose of radiation (400 rads) and their chimerism compared 4 months later to assess the effect of radiation on LT-HSCs. (E) Nrf2−/− LT-HSCs show increased radiosensitivity. Four months after radiation, peripheral blood chimerism returned to baseline in mice transplanted with Nrf2+/+ BM, but remained much lower in Nrf2−/− chimeras, suggesting a selective loss of Nrf2−/− LT-HSCs. *P = .018
Treatment with NAC does not rescue transplantation defect in Nrf2−/− HSPCs
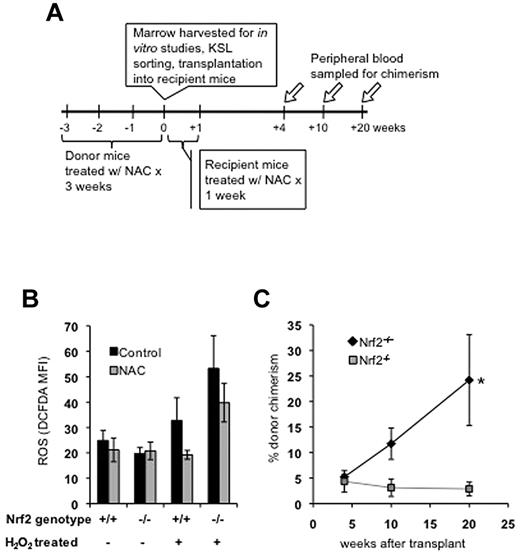
Because Nrf2 appears to protect HSPCs from induced oxidative stress, increased ROS during the peritransplantation period could potentially explain the decreased engraftment of Nrf2−/− cells. Therefore, we treated donor mice with the free radical scavenger NAC for 3 weeks before BM harvesting and recipient mice for 1 week after transplantation (Figure 3A). NAC, a thiol antioxidant and GSH precursor, has been shown to rescue the loss of HSPC function caused by increased ROS,8 and NAC treatment can partially suppress oxidative stress in Nrf2-deficient mice.16,23,36 NAC treatment for 3 weeks did not change basal ROS levels in HSPCs from Nrf2+/+ or Nrf2−/− mice, but limited the induction of ROS after ex vivo treatment with H2O2 (Figure 3B). We transplanted 500 KSL cells isolated from NAC- or vehicle-treated donors and subsequently treated recipient mice with NAC for 1 week after transplantation. NAC treatment failed to rescue the impaired long-term chimerism of Nrf2−/− cells, demonstrating that their transplantation defect is not because of ROS induced during the peritransplantation period (Figure 3C).
NAC protects HSPCs from exogenous ROS, but does not rescue transplantation defect in Nrf2−/− cells. (A) Experimental schema for NAC experiments. (B) Treatment with NAC protects Nrf2+/+ and Nrf2−/− HPSCs from induction of ROS. Mice were treated with daily IP injection of NAC (100 μg/kg/d) for 3 weeks and their BM harvested. Cells were labeled with Abs, treated with 50μM H2O2 for 30 minutes, and then stained with H2-CM-DCFDA to measure intracellular ROS. Data are average MFI values in the KSL population (arbitrary units). Three mice were analyzed in each group. (C) Treatment with NAC does not rescue transplantation defect in Nrf2−/− HPSCs. Mice were treated with NAC for 3 weeks, BM was harvested, and KSL cells were isolated by FACS. KSL cells (500/mouse) were transplanted, along with 250 000 CD45.1 competitor normal BM cells, and injected into lethally irradiated CD45.1 hosts. Peripheral blood was analyzed for donor chimerism at the indicated time points. Data are average peripheral blood engraftments from 5 total recipient mice per genotype. *P = .03 at 20 weeks.
NAC protects HSPCs from exogenous ROS, but does not rescue transplantation defect in Nrf2−/− cells. (A) Experimental schema for NAC experiments. (B) Treatment with NAC protects Nrf2+/+ and Nrf2−/− HPSCs from induction of ROS. Mice were treated with daily IP injection of NAC (100 μg/kg/d) for 3 weeks and their BM harvested. Cells were labeled with Abs, treated with 50μM H2O2 for 30 minutes, and then stained with H2-CM-DCFDA to measure intracellular ROS. Data are average MFI values in the KSL population (arbitrary units). Three mice were analyzed in each group. (C) Treatment with NAC does not rescue transplantation defect in Nrf2−/− HPSCs. Mice were treated with NAC for 3 weeks, BM was harvested, and KSL cells were isolated by FACS. KSL cells (500/mouse) were transplanted, along with 250 000 CD45.1 competitor normal BM cells, and injected into lethally irradiated CD45.1 hosts. Peripheral blood was analyzed for donor chimerism at the indicated time points. Data are average peripheral blood engraftments from 5 total recipient mice per genotype. *P = .03 at 20 weeks.
Cytokine signaling is reduced in Nrf2−/− HSPCs
To better understand the differentiation and survival defect of Nrf2−/− HSPCs, we performed gene-expression studies on KSL cells isolated by FACS from Nrf2+/+ and Nrf2−/− mice. BM cells from 5 mice per genotype were pooled and FACS sorted in 3 replicate experiments (total of 15 mice). RNA isolated from the first sort was profiled using Affymetrix GeneChip Mouse Gene ST 1.0 chips, and changes in gene expression were verified by quantitative RT-PCR on RNA from the second and third sorts. The microarray data were analyzed using the Partek Genomics suite, and differentially expressed genes were subjected to pathway analysis using Ingenuity pathway analysis. All microarray data are available in the Gene Expression Omnibus (GEO) under accession number GSE33139. The differentially expressed genes were primarily involved in the development of the hematologic system and immune cell trafficking (Table 1). The expression of genes mediating the inflammatory response (CCL2, CCL5, CXCL10, IL1B, IL1R, IL10, and Cox-2) and myeloid development (CSF1, CSF1R, and TREM1) were reduced in Nrf2−/− HSPCs (Figure 4A). These findings were unexpected, because previous studies showed that Nrf2 suppresses the inflammatory response and that Nrf2 deficiency results in amplified inflammatory signaling and myeloid cell activation.16,17,23 However, these differences may be explained by our examination of HSPCs rather than mature myeloid cells.
G-CSF restores cytokine signaling and promotes survival of Nrf2−/− HSPCs. (A) Gene expression of inflammatory cytokines, cytokine receptors, and prosurvival molecules is down-regulated in Nrf2−/− KSL cells. BM was harvested from 5 mice/group and KSL cells were isolated by FACS. RNA was isolated from sorted cells and quantitative RT-PCR performed for specific genes. Data are average values from 2 separate experiments and are normalized to expression levels in Nrf2+/+ KSL cells (represented by the dotted line). BCL2A1 inidicates BCL2-related protein A1; CCL2, chemokine (C-C motif) ligand 2; CCL5, chemokine (C-C motif) ligand 5; CSF1, colony-stimulating factor 1 (macrophage); CSF1R, colony-stimulating factor 1 receptor; CXCL10, chemokine (C-X-C motif) ligand 10; IL1B, interleukin 1 beta; IL1R, interleukin 1 receptor type II; IL10, interleukin 10; and TREM1, triggering receptor expressed on myeloid cells 1. (B) G-CSF treatment leads to expansion of KSL, LT-HSC, and ST-HSC compartments in both Nrf2+/+ and Nrf2−/− mice. Mice were treated with 100 μg/kg of G-CSF daily or vehicle control for 1 week, and BM was isolated and labeled with Abs and analyzed by flow cytometry. Data are averages from 3 mice per group. (C) G-CSF treatment enhances survival of Nrf2+/+ and Nrf2−/− HSPCs. Mice were treated with 100 μg/kg of G-CSF daily or vehicle control for 1 week. BM from 3 mice in each group was isolated, pooled, and treated with H2O2 (50μm for 30 minutes) or control and cultured in serum-free medium at an atmospheric O2 concentration for 6 hours. Viable cells were annexin V−/propidium iodide−. Data are averages from 3 replicates. (D) G-CSF treatment corrects many of the gene-expression differences seen between Nrf2+/+ and Nrf2−/− KSL cells. Mice were treated with 100 μg/kg of G-CSF daily or control for 1 week, and KSL cells were isolated from BM. Quantitative RT-PCR was performed and analyzed as described in panel A.
G-CSF restores cytokine signaling and promotes survival of Nrf2−/− HSPCs. (A) Gene expression of inflammatory cytokines, cytokine receptors, and prosurvival molecules is down-regulated in Nrf2−/− KSL cells. BM was harvested from 5 mice/group and KSL cells were isolated by FACS. RNA was isolated from sorted cells and quantitative RT-PCR performed for specific genes. Data are average values from 2 separate experiments and are normalized to expression levels in Nrf2+/+ KSL cells (represented by the dotted line). BCL2A1 inidicates BCL2-related protein A1; CCL2, chemokine (C-C motif) ligand 2; CCL5, chemokine (C-C motif) ligand 5; CSF1, colony-stimulating factor 1 (macrophage); CSF1R, colony-stimulating factor 1 receptor; CXCL10, chemokine (C-X-C motif) ligand 10; IL1B, interleukin 1 beta; IL1R, interleukin 1 receptor type II; IL10, interleukin 10; and TREM1, triggering receptor expressed on myeloid cells 1. (B) G-CSF treatment leads to expansion of KSL, LT-HSC, and ST-HSC compartments in both Nrf2+/+ and Nrf2−/− mice. Mice were treated with 100 μg/kg of G-CSF daily or vehicle control for 1 week, and BM was isolated and labeled with Abs and analyzed by flow cytometry. Data are averages from 3 mice per group. (C) G-CSF treatment enhances survival of Nrf2+/+ and Nrf2−/− HSPCs. Mice were treated with 100 μg/kg of G-CSF daily or vehicle control for 1 week. BM from 3 mice in each group was isolated, pooled, and treated with H2O2 (50μm for 30 minutes) or control and cultured in serum-free medium at an atmospheric O2 concentration for 6 hours. Viable cells were annexin V−/propidium iodide−. Data are averages from 3 replicates. (D) G-CSF treatment corrects many of the gene-expression differences seen between Nrf2+/+ and Nrf2−/− KSL cells. Mice were treated with 100 μg/kg of G-CSF daily or control for 1 week, and KSL cells were isolated from BM. Quantitative RT-PCR was performed and analyzed as described in panel A.
G-CSF promotes survival of HSPCs
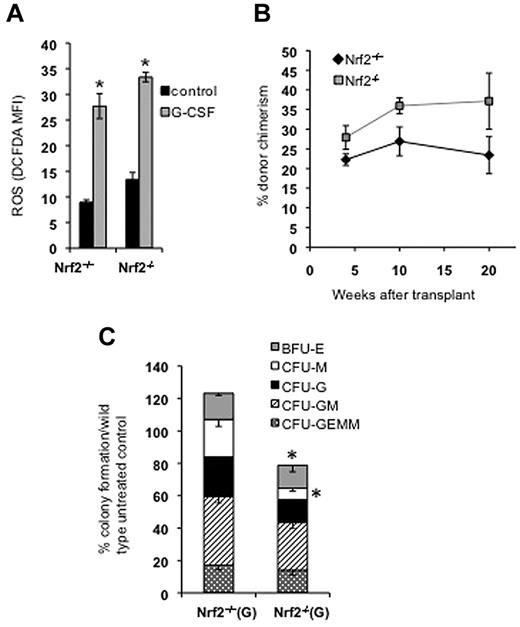
Cytokines are known to have an important prosurvival effect on BM cells; therefore, we hypothesized that the loss of Nrf2 leads to decreased cytokine activity and cell survival. To test this hypothesis, we treated Nrf2+/+ and Nrf2−/− mice with G-CSF for 1 week before harvesting BM for study. G-CSF treatment resulted in a marked increase in the numbers of phenotypic HSPCs in both Nrf2+/+ and Nrf2−/− mice (Figure 4B). BrdU uptake by CD34−Flt3−KSL LT-HSCs was not significantly affected by G-CSF in Nrf2+/+ and Nrf2−/− mice (supplemental Figure 3). In contrast, the proliferation of CD34+Flt3−KSL shortening-HSCs and CD34+Flt3+KSL multipotent progenitors was significantly increased by G-CSF in Nrf2+/+ mice, but not in Nrf2−/− mice. These findings suggest that the increase in HSPC frequency after G-CSF is because of enhanced survival rather than changes in proliferation. To test this hypothesis, we cultured G-CSF–treated and control BM in cytokine-free medium and measured cell viability (annexin V−/propidium iodide−) in control and H2O2-treated cells (Figure 4C). The numbers of viable cells were equal in untreated Nrf2+/+ and Nrf2−/− cells; however, after exposure to H2O2, Nrf2−/− HSPCs showed reduced survival that could be rescued with G-CSF. In accordance with these findings, we also found that G-CSF restored the expression of multiple genes involved in cytokine signaling and cell survival (including the antiapoptotic gene BCL2A1) to wild-type levels (Figure 4D and supplemental Figure 4).
G-CSF rescues HSPC function in Nrf2−/− cells despite inducing ROS
G-CSF enhances cell survival, but also generates ROS,3 therefore, we examined Nrf2−/−HSPC function after cytokine treatment. As expected, ROS levels increased 3-fold in the KSL compartment after G-CSF treatment, but this increase was similar to that of Nrf2+/+ cells (Figure 5A). This degree of increased ROS was similar to the levels reported in the HSPCs of FOXO-knockout mice, which are thought to be the cause of the marked HSPC dysfunction observed in that model.9 However, we found that G-CSF–treated cells from Nrf2−/− mice competed equally well during transplantation with G-CSF–treated Nrf2+/+ HSPCs despite the dramatic increase in intracellular ROS (Figure 5B). In contrast, G-CSF treatment failed to rescue defective in vitro colony formation (Figure 5C), suggesting that Nrf2 function may be different between HSPCs and more differentiated myeloid cells.
G-CSF treatment induces ROS in HSPCs and rescues Nrf2−/− HSPC transplantation. (A) G-CSF treatment induces ROS in KSL cells. Nrf2+/+ and Nrf2−/− mice were treated with 100 μg/kg of G-CSF daily for 1 week and their BM was examined for ROS using H2-CM-DCFDA. G-CSF induces a 3-fold increase in ROS in the KSL compartment. Data are mean values from 3 mice in each group. *P < .05. (B) G-CSF treatment rescued the hematopoietic transplantation defect in the Nrf2−/− HSPCs. Nrf2+/+ and Nrf2−/− mice were treated with 100 μg/kg of G-CSF daily for 1 week. CD45.2 BM cells (250 000) from each genotype were transplanted with 250 000 CD45.1 competitor cells, and peripheral blood was examined for chimerism at the indicated time points. Peripheral blood chimerism was higher mice transplanted with Nrf2−/− cells, but this was not statistically significant (P = .19), at 20 weeks after transplantation (n = 5 in each group). (C) G-CSF treatment does not improve in vitro myeloid colony formation of Nrf2−/− BM. Nrf2+/+ and Nrf2−/− mice were treated with 100 μg/kg of G-CSF daily for 1 week, 20 000 unsorted BM cells were plated in methylcellulose medium supplemented with cytokines, and colonies were scored at 14 days. Data represent mean colony number from 3 separate mice each plated in duplicate (normalized to untreated wild-type). *P < .03.
G-CSF treatment induces ROS in HSPCs and rescues Nrf2−/− HSPC transplantation. (A) G-CSF treatment induces ROS in KSL cells. Nrf2+/+ and Nrf2−/− mice were treated with 100 μg/kg of G-CSF daily for 1 week and their BM was examined for ROS using H2-CM-DCFDA. G-CSF induces a 3-fold increase in ROS in the KSL compartment. Data are mean values from 3 mice in each group. *P < .05. (B) G-CSF treatment rescued the hematopoietic transplantation defect in the Nrf2−/− HSPCs. Nrf2+/+ and Nrf2−/− mice were treated with 100 μg/kg of G-CSF daily for 1 week. CD45.2 BM cells (250 000) from each genotype were transplanted with 250 000 CD45.1 competitor cells, and peripheral blood was examined for chimerism at the indicated time points. Peripheral blood chimerism was higher mice transplanted with Nrf2−/− cells, but this was not statistically significant (P = .19), at 20 weeks after transplantation (n = 5 in each group). (C) G-CSF treatment does not improve in vitro myeloid colony formation of Nrf2−/− BM. Nrf2+/+ and Nrf2−/− mice were treated with 100 μg/kg of G-CSF daily for 1 week, 20 000 unsorted BM cells were plated in methylcellulose medium supplemented with cytokines, and colonies were scored at 14 days. Data represent mean colony number from 3 separate mice each plated in duplicate (normalized to untreated wild-type). *P < .03.
Discussion
Our findings are the first to implicate a role for Nrf2 in HSPC function and myeloid development. Surprisingly, Nrf2 does not control basal levels of ROS in BM cells; however, Nrf2−/− BM cells are more sensitive to oxidative stress. To delineate the antioxidative function of Nrf2 in ROS-mediated HSPC dysfunction, we used an in vitro model of ROS induction with H2O2 and an in vivo model of ROS injury using ionizing radiation treatment (Figure 6). H2O2, a potent oxidizing agent, is naturally produced as a by-product of oxidative metabolism and generates ROS that play an important role in cellular signaling. Exposure of primary cells to sublethal doses of H2O2 has been commonly used as a model of oxidative injury. Conversely, exposure to ionizing radiation induces hydrolysis of water and thus triggers the formation of free radicals that can further damage cellular DNA and oxidize protein thiols and lipids. A study by Wang et al demonstrated that total body irradiation induces a persistent and prolonged increase in ROS in the HSC compartment.37 Using both of these models, we show herein that Nrf2−/− HSPCs are more susceptible to oxidative stress. However, the failure of NAC to rescue the engraftment of Nrf2−/− HSPCs suggests that induced ROS is not the cause the transplantation defect we observed. It is possible that ROS detection performed using H2-CM-DCFDA is relatively insensitive to superoxide anion- and nitrogen-containing ROS, but similar methods have been used previously to study HSPCs.7
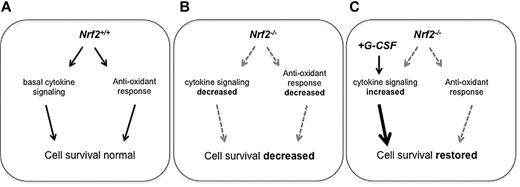
Model demonstrating role of Nrf2 in HSPCs. (A) In Nrf2+/+ BM, normal levels of Nrf2 are associated with low levels of ROS in HSPCs and intact cytokine signaling. (B) In Nrf2−/− BM, basal levels ROS are not increased in HSPCs, but HSPC survival is decreased due to reduced cytokine signaling levels. Nrf2−/− stem/progenitor cells show increased sensitivity to induced ROS. (C) G-CSF induces ROS in both Nrf2+/+ and Nrf2−/− HSPCs. Although Nrf2 loss is associated with increased sensitivity to ROS, G-CSF is able to restore HSPC survival despite an increase in ROS.
Model demonstrating role of Nrf2 in HSPCs. (A) In Nrf2+/+ BM, normal levels of Nrf2 are associated with low levels of ROS in HSPCs and intact cytokine signaling. (B) In Nrf2−/− BM, basal levels ROS are not increased in HSPCs, but HSPC survival is decreased due to reduced cytokine signaling levels. Nrf2−/− stem/progenitor cells show increased sensitivity to induced ROS. (C) G-CSF induces ROS in both Nrf2+/+ and Nrf2−/− HSPCs. Although Nrf2 loss is associated with increased sensitivity to ROS, G-CSF is able to restore HSPC survival despite an increase in ROS.
Our findings that G-CSF treatment could enhance HSPC survival after H2O2 treatment and during BM transplantation despite causing a 3-fold increase in ROS suggest that the role of Nrf2 in promoting survival is more important in HSPCs than its role in reducing levels of intracellular ROS. The linkage of ROS generation (via cytokines), the detoxification of electrophiles, and the expression of antiapoptotic genes (eg, IL-10 and BCL2A1)—all via Nrf2 function—suggests an interesting fail-safe mechanism whereby HSPCs would be well equipped to survive large increases in ROS associated with sudden bursts of cytokine-driven hematopoiesis. Our finding that H2O2 induced the highest levels of intracellular ROS in Nrf2+/+ KSL cells supports this idea. Therefore, optimal survival during oxidative stress requires both detoxification of ROS and activation of prosurvival pathways, and Nrf2 participates in both functions to a greater or lesser degree in a tissue-specific manner.
In the myeloid compartment, loss of Nrf2 impairs the terminal differentiation and growth of mature cells. This was demonstrated in both colony-forming assays and in vivo during early myeloid engraftment after BM transplantation. Whereas the administration of G-CSF rescued HSPC function in Nrf2-deficient mice, it failed to restore myeloid progenitor function. Interestingly, G-CSF failed to restore levels of CSF1 and TREM1, both critical factors for myeloid differentiation, to wild-type levels in Nrf2−/− mice.34,38,39 Because the differentiation of BM cells is associated with increases in intracellular ROS, and becaise mature myeloid cells generate ROS as part of their host defense function, we speculate that coupling of Nrf2 signaling with myeloid differentiation programs protect developing myeloid cells from ROS-induced toxicity. Further evidence for a link between myeloid differentiation in Nrf2 was recently reported in a myeloid leukemia model.40 A precise analysis of the transcriptional targets of Nrf2 at various stages of hematopoietic development is needed to understand how Nrf2 regulates myeloid differentiation.
Nrf2 has been shown to confer chemoresistance in several malignancies,41-44 and its role in regulating HSPC survival and myeloid differentiation suggest that it may also play an important role in myeloid neoplasms. In particular, Nrf2 may contribute to the chemoresistance of myeloid leukemia stem cells, making it an attractive therapeutic target. Conversely, Nrf2 agonists may have utility in cases of ineffective hematopoiesis and impaired differentiation, such as myelodysplastic syndrome and refractory anemia.
Finally, our findings demonstrate that increased ROS levels are not necessarily correlated with HSPC dysfunction, because we observed decreased HSPC function in Nrf2−/− HSPCs with normal ROS levels and improved HSPC function in Nrf2−/− BM after treatment with G-CSF despite increased ROS levels. Similarly, a recent study demonstrated that HSPC dysfunction resulting from the loss of AKT1/AKT2 function is associated with reduced ROS levels, and in that study, HSPC function could be restored by the pharmacologic induction of ROS.45 Undoubtedly, ROS can be deleterious to cells, but given the diversity of cellular functions that generate—and in some cases require—ROS, simply detecting elevated levels of ROS in HSPCs can no longer be accepted as an adequate explanation for HSPC dysfunction. Future work is needed to correlate these findings with measures of oxidative stress and, ideally, markers of cellular damage.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Thomas Kensler (Johns Hopkins University, Baltimore, MD) and Dr M. Yamamoto (Tohoku University Graduate School of Medicine, Sendai, Japan) for providing the Nrf2−/− mice used in this study.
This work was supported by the National Institutes of Health (grants RO1 CA140492 to S.B., P50 CA058184 to S.B., R33 AI080541 to S.B., R01CA127574 to W.M., and P01CA015396 to W.M.), the Leukemia & Lymphoma Society (to W.M.), the Gabrielle's Angel Foundation for Cancer Research (to W.M.), the Sidney Kimmel Foundation for Cancer Research (to W.M.), an American Society of Hematology Scholar Award (to A.A.M.), an American Society for Clinical Oncology Young Investigator Award (to A.A.M.), an American Association for Cancer Research-Astellas USA Fellowship (to A.A.M.), the Maryland Stem Cell Research Fund (to S.B. and W.M., and the Flight Attendant Medical Research Institute (to S.B. and A.S.).
National Institutes of Health
Authorship
Contribution: A.M. and A.S. performed the experiments and analyzed the data, and all authors designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shyam Biswal, PhD, Department of Environmental Health Sciences, Johns Hopkins School of Public Health, 615 N Wolfe St, Baltimore, MD 21205; e-mail: sbiswal@jhsph.edu; or William Matsui, MD, The Sidney Kimmel Comprehensive Cancer Center, Department of Oncology, Johns Hopkins University School of Medicine 1650 Orleans St, Baltimore, MD 21287; e-mail: matsuwi@jhmi.edu.
References
Author notes
A.A.M. and A.S. contributed equally to this study.