Abstract
One of the greatest challenges in cell therapy is to minimally invasively deliver a large quantity of viable cells to a tissue of interest with high engraftment efficiency. Low and inefficient homing of systemically delivered mesenchymal stem cells (MSCs), for example, is thought to be a major limitation of existing MSC-based therapeutic approaches, caused predominantly by inadequate expression of cell surface adhesion receptors. Using a platform approach that preserves the MSC phenotype and does not require genetic manipulation, we modified the surface of MSCs with a nanometer-scale polymer construct containing sialyl Lewisx (sLex) that is found on the surface of leukocytes and mediates cell rolling within inflamed tissue. The sLex engineered MSCs exhibited a robust rolling response on inflamed endothelium in vivo and homed to inflamed tissue with higher efficiency compared with native MSCs. The modular approach described herein offers a simple method to potentially target any cell type to specific tissues via the circulation.
Introduction
Cell therapy offers enormous hope for solving some of the most tragic illnesses, diseases, and tissue defects; however, a significant barrier to the effective implementation of cell therapies is the inability to target a large quantity of viable cells with high efficiency to tissues of interest. Systemic infusion is desired because it minimizes the invasiveness of cell therapy and maximizes practical aspects of repeated doses. Systemic infusion also permits the cells to mimic natural cell trafficking processes and helps to ensure that cells remain in close proximity to oxygen and nutrient-rich blood vessels. Mesenchymal stem cells (MSCs) represent a potent source of immunoprivileged postnatal cells that are conveniently isolated autologously or used from an allogeneic source without the addition of an immunosuppressive regimen, and are currently being investigated in more than 100 clinical trials,1 the majority of which use a systemic route of delivery. Although they exhibit favorable therapeutic properties, including the capacity for multilineage differentiation followed by production of a specific extracellular matrix (eg, bone, cartilage, or fat)2,3 and they exhibit immunomodulatory potential to reduce inflammation through secretion of soluble paracrine or endocrine factors,4 typically less than 1% of the infused MSCs reach the target tissue.5,6 The inefficient MSC homing is the result of a variety of factors but is typically attributed to an absence of relevant cell surface homing ligands.7,8 Specifically, culture expanded MSCs develop heterogeneous receptor expression and lose key homing ligands during cell culture,9 which contributes to the inefficiency of in vivo MSC homing. This represents a major challenge for minimally invasive MSC-based therapies that require a high efficiency of engraftment within specific tissues.10 Thus, it can be rationalized that engineering the surface of cells, such as MSCs, with adhesion ligands can enhance the homing of cells to specific tissues after systemic infusion.
The first step of leukocyte extravasation involves capture of leukocytes flowing freely in the bloodstream, mediated by glycoproteins known as selectins. P- and E-selectins are highly expressed by the vascular endothelium locally within inflamed tissue and are the principal mediators for initial rolling response for the homing of leukocytes to sites of inflammation.11,12 Selectins also mediate hematopoietic stem cell rolling within the bone marrow.13 These interactions are transient in nature, being characterized by rapid on rates and force-sensitive off rates, which results in a slow rolling motion of the leukocytes along the vascular endothelium and are typically mediated by selectins that recognize ligands containing carbohydrate moieties of the sialyl Lewisx (sLex) family.12,14 sLex is the active site of P-selectin glycoprotein ligand 1 (PSGL-1), which is expressed by hematopoietic stem cells and leukocytes. This rolling response is critical for enabling chemokine signaling and arrest by integrins, which eventually results in extravasation; indeed, in vitro and in vivo studies have demonstrated that cell rolling is prerequisite for firm adhesion of leukocytes, and abrogation of the rolling response leads to decreased firm adhesion.11,12,15,16 This indicates the importance of cell rolling as a crucial step for cell homing. Thus, inducing an MSC rolling response may be expected to enhance their homing ability and increase the engraftment efficiency after systemic delivery. The proof of principle for this hypothesis is provided by approaches that have involved enzymatic and genetic modification of MSCs to alter the repertoire of cell surface markers.7,17 Although these strategies can improve the delivery of MSCs to sites of inflammation, the broad applicability of these technologies is limited. Enzymatic modification is complex and limited to modification of existing cell surface receptors, whereas genetic manipulation of cells may not be practical for altering the expression of more than a single receptor, and presents potential safety concerns. Recently, we demonstrated simple, platform strategies to conjugate sLex, a ligand that interacts with selectins to promote cell rolling.18,19 However, in vitro the sLex-modified MSCs were not able to roll on a P-selectin–coated surface beyond approximately 0.7 dyne/cm2 shear stress, which represents a challenge to target these modified MSCs in vivo.
Here we present a strategy to promote a robust MSC rolling response that offers a simple method to potentially target any cell type to specific tissues via the circulation. Functionalization of MSCs with a high ligand density was achieved through systematic optimization of a new protocol to modify the cells in suspension with a nanometer-scale polymer construct containing sLex, which induced a robust rolling response both in vitro and in vivo. We demonstrate that this method enhances the ability of MSCs to home to inflamed tissues without compromising MSC proliferation, multilineage differentiation potential, and secretion of pertinent paracrine factors.
Methods
Materials
Primary human MSCs, isolated from human marrow of healthy consenting donors, were obtained from the Center for Gene Therapy at Tulane University (which has a grant from National Center for Research Resources of the National Institutes of Health, grant P40RR017447). P-selectin was purchased from R&D Systems, and multivalent biotinylated sialyl Lewis(x)–poly(acrylamide) (sialyl-LewisX-PAA-biotin [BsLex]) was purchased from Glycotech where sialyl-LewisX-PAA-biotin contains 4 sLex units. α-MEM, L-glutamine, and Penn-Strep were purchased from Invitrogen. Sulfonated biotinyl-N-hydroxy-succinimide (BNHS) was purchased from Thermo Fisher Scientific, and FBS was purchased from Atlanta Biologicals. Biotin-4-fluorescein was purchased from Invitrogen. Anti–human cutaneous lymphocyte antigen antibody (HECA-452), the secondary antibody (FITC mouse anti–rat IgM), FITC CD90, and PE-Cy5 antibody were purchased from BD Biosciences. FACS buffer is PBS with 1% FBS. All other chemicals and reagents were purchased from Sigma-Aldrich and were used without further purification unless specified.
Surface modification of MSCs with a rolling ligand
The conjugation of BsLex to the MSC surface through biotin-streptavidin was performed under optimized conditions (supplemental Figure 1, see the Supplemental Materials link at the top of the article) in PBS (pH 7.4, without Ca/Mg) at room temperature. Typically, media was aspirated from 80% to 90% confluent T75 flasks, and cells were trypsinized using 1 times trypsin-EDTA solution, centrifuged into a pellet, and washed with PBS twice. The resulting cell pellet was dispersed in sulfonated biotinyl-N-hydroxy-succinimide, BNHS solution (1mM, 1 mL), which was allowed to incubate for 10 minutes at room temperature (Reaction Step 1, Figure 1A). The cells were then pelleted and washed with PBS twice by centrifugation to remove unattached and/or physically adsorbed BNHS from the cell surface. Streptavidin solution (50 μg/mL in PBS, 1 mL) was then used to treat the cells for 1 minute at room temperature (Reaction Step 2, Figure 1A). The cells were then pelleted and washed with PBS. To the streptavidin-conjugated cells, BsLex solution (5 μg/mL in PBS, 1 mL) was added, and the suspension was allowed to incubate for 5 minutes at room temperature (Reaction Step 3, Figure 1A). Finally, the cells were pelleted and washed with PBS.
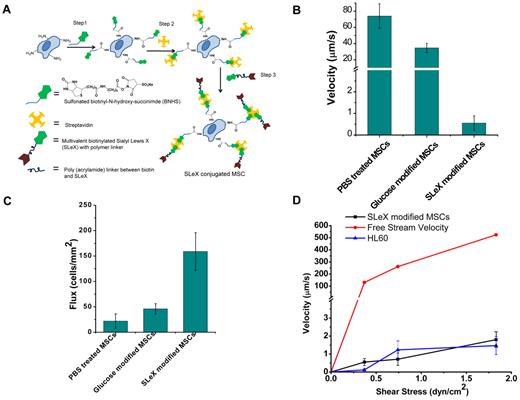
Cell surface engineered MSCs display enhanced rolling intactions in vitro. (A) Conjugation of sLex on the surface of the MSCs through covalent biotinylation and a streptavidin-biotin bridge. (B) Velocity of sLex-modified cells compared with PBS-treated cells and glucose-modified cells at 0.36 dyne/cm2 on P-selectin–coated substrates. (C) Number of interacting sLex-modified cells compared with PBS-treated cells and glucose-modified cells per unit area at 0.36 dyne/cm2 on P-selectin–coated substrate over 10 seconds with 0.45 mm2 area. (D) Velocity of sLex-modified MSCs, HL60, and free stream velocity (theoretically calculated from flow chamber geometry and fluid flow rate) at increasing shear stress.
Cell surface engineered MSCs display enhanced rolling intactions in vitro. (A) Conjugation of sLex on the surface of the MSCs through covalent biotinylation and a streptavidin-biotin bridge. (B) Velocity of sLex-modified cells compared with PBS-treated cells and glucose-modified cells at 0.36 dyne/cm2 on P-selectin–coated substrates. (C) Number of interacting sLex-modified cells compared with PBS-treated cells and glucose-modified cells per unit area at 0.36 dyne/cm2 on P-selectin–coated substrate over 10 seconds with 0.45 mm2 area. (D) Velocity of sLex-modified MSCs, HL60, and free stream velocity (theoretically calculated from flow chamber geometry and fluid flow rate) at increasing shear stress.
Preparation of P-selectin surfaces
The well surfaces within a 6-well plate were coated with P-selectin solution (5 μg/mL in PBS, 1 mL) for 18 hours on a plate shaker at room temperature. All P-selectin surfaces were freshly prepared before the flow chamber assay.
Flow chamber assay
For the analysis of cell velocities through the flow chamber, the cells were suspended in MSC expansion media (∼ 1 × 105 cells/mL) for the flow chamber assay. A circular parallel plate flow chamber (Glycotech) with 127-μm gasket thickness and a width of 2.5 mm was used. To monitor cell rolling, phase-contrast microscopy (TE2000-U Inverted Nikon Microscope with a DS-Qi1 Monochrome Cooled Digital Camera) was used, and images were recorded in a 10 times field at 10-second intervals. The velocity of the cells was calculated by measuring the distance cells traveled within a 10-second interval. A cell was classified as rolling if it rolled for 10 seconds while remaining in the field of view and if it traveled at an average velocity less than 50% of the calculated free stream velocity of a noninteracting cell. The flux was calculated manually based on number of cells interacting with the substrate and remaining in the field view for 10 seconds. Both the firmly adhered cells and rolling cells were considered for the flux calculation. To assess the effect of shear rate, the rolling velocity and the flux were measured at shear stresses, including 0.36, 0.72, and 1.89 dyne/cm2.
In vivo animal experiment
Dynamic real-time intravital confocal microscopy.
Homing of unmodified and sLex-modified MSCs to the skin was imaged noninvasively (in real time) using a custom-built video-rate laser-scanning confocal microscope designed specifically for live animal imaging.20 To image the vasculature and surrounding tissue, we positioned the mouse's ear on a coverslip (with index matching gels) and obtained high-resolution images with cellular details through the intact mouse skin at depths of up to 250 μm. The laser beams were focused onto the sample (mouse ear skin) using a 60×, 1.2NA water immersion objective lens (Olympus). DiD- and DiR-labeled MSCs were excited with a 635-nm continuous wave laser (Coherent) and detected through a 695-nm ± 27.5-nm band pass filter (Omega Optical) for DiD (red channel) and through a 770-nm-long pass filter (Omega Optical) for DiR (green channel). Because a large portion of DiD fluorescence leaks into the DiR channel and some DiR fluorescence leaks into the DiD channel, DiD+ cells were identified as having a red-to-green ratio more than 1 (orange-red hue), whereas DiR+ cells were identified as having a red-to-green ratio less than 1 (yellow-green hue). FITC-dextran (blue channel) was excited with a 491-nm continuous wave laser (Cobalt) and detected through a 520-nm ± 20-nm bandpass filter (Semrock). Video-rate movies were recorded for analysis of cell rolling. Because this system operates at 30 frames per second, the live video was recorded to measure the velocities of rolling cells. In the case of static images, 15 to 30 frames were averaged from the live video mode to improve the signal-to-noise ratio. However, instead of recording single images, we recorded z-stacks to quantify the number of cells that transmigrated the blood vessels. We computed the average rolling velocity (using ImageJ Version 1.45a software, National Institutes of Health) as the displacement of the centroid of the cell divided by the time interval between observations. The total number of “homed” cells from each MSC population and the percentage of MSCs in each population that had transmigrated the blood vessel endothelium within the mouse ear were quantified from the z-stacks acquired. For publication purposes, the contrast and brightness of the images were changed using ImageJ Version 1.45a software.
Expression of P-selectin and E-selectin.
Anti–P-selectin (CD62P), anti–E-selectin (CD62E), and IgG1λ were purchased from BD Biosciences and labeled with Cy5 or Cy3 monoreactive dyes according to the manufacturer's protocol (GE Healthcare). Antibody conjugates were diluted into sterile saline to a final concentration of 0.1 mg/mL. A total of 100 μL of antibody solution was administered via tail vein injection 20 hours after lipopolysaccharide (LPS) injection. Cy5 was excited using 633 nm, and emission was detected between 667 nm and 722 nm. Cy3 was excited using 532 nm, and emission was detected between 573 nm and 613 nm. Adjacent z-stack images with 10-μm step size were acquired along a vein 24 hours after LPS injection. Maximum intensity projections of the z-stacks were created using ImageJ Version 1.45a software and then manually stitched together in Photoshop CS2 to create a map of the vessel.
In vivo blocking of P-selectin.
A total of 100 μg/20 g unlabeled anti–P-selectin (BD Biosciences PharMingen, NA/LE rat anti–mouse 553741) or IgG1λ isotype (BD Biosciences PharMingen, NA/LE rat anti– mouse 559157) was mixed with MSC and injected retro-orbitally 24 hours after LPS injection. Homing was assessed 24 hours after MSC injections as described previously.
Calculation of in vivo cell velocities and hemodynamic parameters.
The velocity of the fastest moving cells was used as an estimate of the maximal cell velocity (Vmax), and the mean blood flow velocity (Vmean) was calculated, assuming Newtonian flow, from the maximal cell velocity and the ratio of cell diameter to vessel diameter, as Vmax/(2 − ϵ2), where ϵ = cell diameter/vessel diameter. Cells with velocity greater than the mean blood flow velocity were not considered for analysis of rolling interactions. Critical velocity (Vcrit), which represents the lowest velocity at which a cell traveling close to the vessel wall can move without receptor/ligand-mediated adhesive interactions, was calculated as Vmeanϵ(2 − ϵ).21,22 All labeled cells moving above Vcrit were considered noninteracting, whereas cells moving below Vcrit were engaged in adhesive interactions with the vessel wall and were considered rolling. Venular wall shear rate (γ) was calculated, assuming Newtonian flow: 4.9 (8 Vmean/d), where Vmean is the mean blood flow velocity and d the diameter of the vessel. The constant 4.9 is a mean empirical correction factor obtained from recently described velocity profiles measured in microvessels in vivo.23,24 The venular wall shear stress(τ) was γ × blood viscosity (η), where η was assumed to be 0.025 poise.25
Statistical analysis
For multiple pairwise comparisons, a 2-tailed Student t test was used with the Bonferroni correction. Two-way ANOVA was used to assess statistical significance of cell preparations performed on different days and isotype versus antibody blocking for in vivo antibody blocking experiments. Error bars in graphs represent SDs.
Results and discussion
Surface modification of MSCs with a rolling ligand, sLex
Given that culture-expanded MSCs do not express PSGL-1,7 we chemically immobilized sLex on the MSC surface to investigate the targeting of systemically administered MSCs to sites of inflammation. We used a 3-step modification to immobilize sLex that involves (1) covalent biotinylation of the cell surface through the reaction of cell surface amine groups with N-hydroxy-succinimide functionalities, (2) functionalization of the biotin with streptavidin, and (3) attachment of a nanometer-scale polymer construct containing biotinylated sLex (Figure 1A). The covalent functionalization of MSC resulted in successful immobilization of biotin and streptavidin on the MSC surface that was subsequently used to immobilize sLex on the MSC surface. The covalent conjugation of biotin on the MSC surface was characterized by the fluorescence signal of rhodamine-streptavidin (SR) attached to the biotin after BNHS and SR treatment (day 0) and after 7 days after BNHS and SR treatment (supplemental Figure 2). The temporal stability and accessibility of the covalently conjugated biotin on the MSC surface were examined by quantifying the fluorescence signal of SR added to the cells at different time points, which indicated that covalent immobilization of biotin with BNHS treatment resulted in a stable and accessible biotin on the cell surface up to 7 days (supplemental Figure 3). The relatively sustained fluorescence intensity from rhodamine-conjugated streptavidin up to day 7 may indicate an excess of biotin on the cell surface; as cells proliferated, the surface concentration of biotin remained higher than what was required to saturate the streptavidin, a much larger molecule compared with biotin. To maximize the sLex density on the MSC surface, the efficiency of conjugation for all 3 steps was optimized with flow cytometric analysis (supplemental Figure 1). The optimized condition resulted in 10, 1, and 5 minutes of reaction time for steps 1, 2, and 3, respectively, for the cell surface modification (Figure 1A).Under the optimized condition, the sLex density on MSC surface, determined with purified HECA-452 and the secondary antibody (FITC mouse anti–rat IgM), was approximately 38.5 ± 19.5 sLex moieties/μm2 (ie, ∼ 27 500 ± 13 600 sLex ligands per cell), whereas the site density of sLex on neutrophils is approximately 400 sLex moieties/μm2, resulting in approximately 90 700 sLex ligands on the cell surface.26
Cell surface modification has minimal impact on MSC phenotype
Importantly, the methods used to engineer cell homing did not impact the phenotype of the MSCs. The results demonstrate that covalent immobilization of sLex on the MSC surface has minimal impact on the cells. Specifically, the covalent immobilization of sLex through biotin-streptavidin did not affect the viability, kinetics of adhesion to tissue culture plastic, proliferation, and multilineage differentiation potential (supplemental Figure 4). The role of MSC-secreted paracrine factors is important for providing immunomodulatory function and for maintaining the cellular microenvironment of tissues,27 which does not appear to be compromised in cells that have been modified with sLex. Specifically, the secretion of paracrine factors by the MSCs was examined by quantifying SDF-1, IGF-1, and PGE2 secretion in the culture media using ELISA assays, and no significant difference was observed between the levels of expression of the paracrine factors for the MSCs modified with sLex compared with the PBS-treated cells (supplemental Figure 5). A temporary reduction in the expression of MSC markers CD90, CD29, and a homing receptor CD49d on the MSC surface was observed immediately after sLex modification by flow cytometry analysis (supplemental Figure 5). The reduced antibody binding indicates that these surface proteins are altered through the chemical modification or that the accessibility of the surface antigens to antibodies is restricted. The recovery of the surface antigens after 24 hours indicates that the reduced binding is only temporary.
sLex-modified MSCs roll on P-selectin substrate in vitro
The dynamic adhesive interactions between immobilized sLex on the MSC surface and P-selectin were characterized with an in vitro flow chamber assay. The enhanced interaction of sLex-modified MSCs was evident from the lower velocity and increased number of interacting cells compared with unmodified MSCs. The chemically immobilized sLex reduced the velocity of MSCs on P-selectin substrates from approximately 74 ± 15 μm/s to approximately 0.5 ± 0.3 μm/s at a wall shear stress of 0.36 dyne/cm2 (P < .001, Figure 1B). The number of interacting cells (ie, rolling or firmly adherent cells on the surface within a 0.45-mm2 surface area in a 10-second interval) increased from 20 cells/mm2 for PBS-treated MSCs to 150 cells/mm2 for sLex-modified MSCs (P < .05, Figure 1C). This indicates that MSCs engineered with sLex interact with P-selectin through increased adhesive interactions. To examine the specificity of sLex as a rolling ligand, a nonrolling ligand, biotinylated monosaccharide (glucose), was immobilized on the MSC surface (instead of sLex). The velocity of glucose-modified MSCs (Figure 1B) was substantially higher than sLex-MSCs, and significantly fewer glucose-modified MSCs (Figure 1C) interacted with P-selectin compared with sLex-MSCs. As the shear stress was increased from 0.36 dyne/cm2 to 1.89 dyne/cm2, the velocity of the sLex-modified cells increased modestly from 0.5 μm/s to 2 μm/s (Figure 1D). Remarkably, the cell rolling response of sLex-modified MSCs was similar to the rolling response of HL60 cells28 (a model cell line used to examine cell rolling on selectin-coated substrates in vitro) up to a shear stress of approximately 2 dyne/cm2. In addition, the number of sLex-MSCs interacting with P-selectin surface remains relatively constant up to approximately 2 dyne/cm2 (supplemental Figure 6). Thus, the sLex-modified MSCs induced a rolling response through promoting specific interactions with P-selectin by mimicking P-selectin–mediated leukocyte rolling.
sLex-modified MSCs roll on inflamed endothelium in vivo
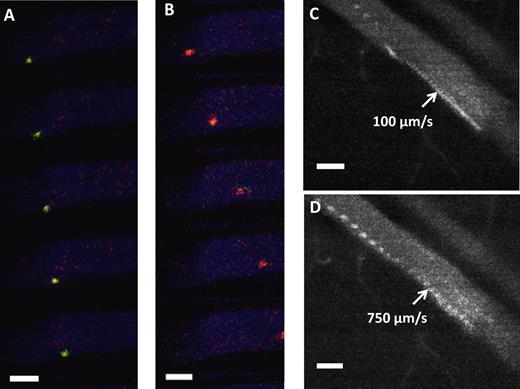
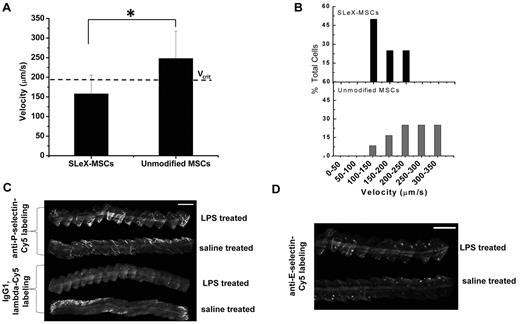
After demonstrating enhanced rolling interactions in vitro, we examined the rolling of the engineered MSCs on activated endothelium in vivo with dynamic real-time intravital confocal microscopy using injection of LPS into the ear of a mouse as the model for inflammation. sLex-modified and unmodified MSCs were pretreated with tracker dyes infused via the tail vein simultaneously. To avoid potential bias for imaging, the type of dye used to stain the sLex-modified and unmodified MSCs was alternated between experiments. The rolling interactions of the MSCs were analyzed within the first hour after infusion of cells. The velocity of the sLex-modified MSCs and unmodified MSCs that interacted with the venular endothelium was determined within the same field of view to minimize the differences because of flow conditions, the level of inflammation, and differences in imaging. The interaction of sLex-modified MSCs with the inflamed endothelium induced the cells to roll with a reduced velocity compared with the unmodified MSCs along the vessel surface (Figure 2A-B). Figure 2C-D shows representative time-lapsed images showing sLex-modified MSCs rolling along the inflamed endothelium while unmodified MSCs did not exhibit any interactions. The reduced velocity of sLex-modified MSCs on the activated endothelium indicates enhanced interaction with the vessel wall and is characteristic of cell rolling observed in vivo. This demonstrates that MSCs engineered with sLex were able to interact with the endothelium in vivo and thus signifies the importance of cell membrane modification to induce a rolling response. Rolling interactions in vivo were analyzed using a standard critical velocity calculation, which represents the lowest velocity at which a cell traveling close to the vessel wall can move without specific receptor/ligand-mediated adhesive interactions.22,28 For example, the critical velocity for a vessel with a 47-μm diameter was 191 μm/s. The average velocity of the sLex-modified MSCs along the activated endothelium of the vessel was 158 ± 48 μm/s compared with unmodified MSCs which had an average velocity of 247 ± 70 μm/s (Figure 3A, P < 0.05). Thus MSCs engineered with sLex were classified as rolling, whereas unmodified cells traveled at a significantly higher velocity. In addition, the distribution of velocities of the cells (Figure 3B) shows that 75% of sLex-MSCs in the field of view that were interacting with the vessel wall within inflamed tissue were rolling, whereas only 25% of the unmodified MSCs were rolling. The wall shear stress for the 47-μm-diameter vessel was approximately 8 dyne/cm2. To examine the specificity of the interactions between the sLex and selectins, the rolling interactions were examined with MSCs modified with a nonrolling ligand, glucose. Compared with glucose-modified MSCs, sLex-modified MSCs exhibited enhanced interaction with the inflamed endothelium. This indicates that the enhanced interaction of sLex-MSCs is the result of the specific interactions between the sLex immobilized on the MSC surface and the selectins expressed on the activated endothelium. Specifically, the average velocity of the glucose-modified MSCs was significantly higher than sLex-MSCs (average glucose-MSC velocity, 748 ± 217 μm/s; and average sLex-MSCs velocity, 529 ± 226 μm/s, P < 0.0001; within a 60-μm-diameter vessel with a critical velocity of 571 μm/s and wall shear stress ∼ 17 dyne/cm2), and the distribution of the velocities of the cells shows only 23% glucose-modified MSCs were rolling compared with 63% of sLex-MSCs on the activated endothelium (supplemental Figure 7A-B). Under inflamed conditions, 50% of leukocytes have been shown to roll with a velocity 60 ± 36 μm/s29 under shear stress of 8 dyne/cm2, and specifically 40% of neutrophils have been shown in a separate study to roll on a 31-μm-diameter vessel with a velocity 151 ± 10 μm/s.28 Thus, the immobilized sLex on the surface of the MSCs specifically induced rolling interactions on the inflamed endothelium compared with unmodified MSCs. Because MSCs exhibit reduced expression of key homing ligands during culture expansion that contributes to their inefficient homing potential,10 our results signify the importance of engineering the MSC surface with rolling ligands to reduce the velocity of cells in the bloodstream within sites of inflammation. These results also indicate that sLex-modified MSCs are able to induce adhesive interactions with inflamed endothelium at high shear stress (up to 17 dyne/cm2) and in large blood vessels. This is probably in part the result of the extension of the sLex moieties from the MSC surface, which is estimated to be approximately 43 nm (the length of the multivalent sLex polymeric construct is estimated to be 30 nm,30 the globular dimension of streptavidin diameter is ∼ 11 nm, and the BNHS sulfonated biotin with spacer is ∼ 2.2 nm). Thus, the extension of sLex moieties from the MSC surface is close to the 60-nm reported length of PSGL-1 on the surface of neutrophils that is required to bypass the formidable source of steric and electrostatic repulsion provided by the endothelial cell glycocalyx.31
In vivo rolling of surface engineered MSCs. (A) In vivo confocal video images of sLex-MSCs with velocity 250 μm/s (green) were taken at 30 frames/s within the inflamed ear vessel (blue) after injection of MSCs. (B) In vivo confocal video images of unmodified MSCs with velocity 1100 μm/s (red) were taken at 30 frames/s within the inflamed ear vessel (blue) after injection of MSCs. (A-B) The vessel diameter is approximately 60 μm, and the critical velocity is 571 μm/s. (C) Representative image of sLex-MSCs interacting with inflamed endothelium in approximately 60 μm vessel resulting in velocity of 100 μm/s (103 frames stacked with 30 fps; ie, sLex-MSC remains in the field of view for > 3.3 seconds). (D) Representative image of unmodified MSCs interacting with inflamed endothelium in approximately 60 μm vessel resulting in velocity of 750 μm/s (27 frames stacked with 30 fps; ie, unmodified MSC remains in the field of view for < 0.9 seconds). Bar represents 50 μm.
In vivo rolling of surface engineered MSCs. (A) In vivo confocal video images of sLex-MSCs with velocity 250 μm/s (green) were taken at 30 frames/s within the inflamed ear vessel (blue) after injection of MSCs. (B) In vivo confocal video images of unmodified MSCs with velocity 1100 μm/s (red) were taken at 30 frames/s within the inflamed ear vessel (blue) after injection of MSCs. (A-B) The vessel diameter is approximately 60 μm, and the critical velocity is 571 μm/s. (C) Representative image of sLex-MSCs interacting with inflamed endothelium in approximately 60 μm vessel resulting in velocity of 100 μm/s (103 frames stacked with 30 fps; ie, sLex-MSC remains in the field of view for > 3.3 seconds). (D) Representative image of unmodified MSCs interacting with inflamed endothelium in approximately 60 μm vessel resulting in velocity of 750 μm/s (27 frames stacked with 30 fps; ie, unmodified MSC remains in the field of view for < 0.9 seconds). Bar represents 50 μm.
In vivo rolling velocity of surface engineering MSCs and selectin expression. (A) Velocity of sLex-modified MSCs and unmodified MSCs on inflamed endothelium within a vessel of 47 μm diameter where the critical velocity (Vcrit) is 191 μm/s. (B) Representative distribution of velocity showing 75% of sLex-MSCs and 25% of unmodified MSCs are below the critical velocity. Cells traveling below the critical velocity decelerate on the vessel wall through receptor/ligand-mediated adhesive interactions. (C) In vivo confocal images of anti–P-selectin–labeled postcapillary venules 24 hours after LPS stimulation. Top: Two venules represent anti–P-selectin–Cy5 labeling in LPS-treated ear and saline-treated ear. Bottom: Two venules represent IgG1, λ-Cy5 labeling in LPS-treated ear and saline-treated ear. Bar represents 500 μm. (D) In vivo confocal images of anti–E-selectin–labeled postcapillary venules 24 hours after LPS stimulation. Top venule represents anti–E-selectin–Cy3 labeling in LPS-treated ear, and bottom venule represents anti–E-selectin–Cy3 labeling in saline-treated ear. Bar represents 500 μm *P < .05.
In vivo rolling velocity of surface engineering MSCs and selectin expression. (A) Velocity of sLex-modified MSCs and unmodified MSCs on inflamed endothelium within a vessel of 47 μm diameter where the critical velocity (Vcrit) is 191 μm/s. (B) Representative distribution of velocity showing 75% of sLex-MSCs and 25% of unmodified MSCs are below the critical velocity. Cells traveling below the critical velocity decelerate on the vessel wall through receptor/ligand-mediated adhesive interactions. (C) In vivo confocal images of anti–P-selectin–labeled postcapillary venules 24 hours after LPS stimulation. Top: Two venules represent anti–P-selectin–Cy5 labeling in LPS-treated ear and saline-treated ear. Bottom: Two venules represent IgG1, λ-Cy5 labeling in LPS-treated ear and saline-treated ear. Bar represents 500 μm. (D) In vivo confocal images of anti–E-selectin–labeled postcapillary venules 24 hours after LPS stimulation. Top venule represents anti–E-selectin–Cy3 labeling in LPS-treated ear, and bottom venule represents anti–E-selectin–Cy3 labeling in saline-treated ear. Bar represents 500 μm *P < .05.
It is important to consider that rolling interactions in vivo are typically highly variable and generally depend on the vessel type and dimension, velocity of blood flow and wall shear stress, level of inflammation, and particular animal model. Importantly, the rolling velocity of sLex-modified MSCs closely resembled that of leukocytes and was mediated through specific interactions between the chemically immobilized sLex on the MSC surface and selectins within vessels in inflamed tissue. To verify that P-selectin was up-regulated after LPS treatment, in vivo immunolabeling was performed on inflamed and saline-treated ears 24 hours after LPS stimulation. A total of 3- or 4-mm of postcapillary venules were imaged for both anti–P-selectin and the isotype control (Figure 3C). The LPS-treated anti–P-selectin–labeled venule showed increased fluorescence signal compared with anti–P-selectin–labeled saline-treated venules and isotype-labeled venules. Although E-selectin has been shown to be up-regulated on endothelium 4 days after intraperitoneal administration of 50 μg LPS and within 4 hours after injecting 20 μg LPS in the footpad of rodents,32,33 E-selectin up-regulation was not observed in the present study (Figure 3D).
sLex modification facilitates MSC homing to inflamed tissue
To determine whether the engineered rolling response could be used to increase the homing of systemically administered MSCs to inflamed tissue, we examined the total number of cells, both sLex-modified MSCs and unmodified MSCs, that homed to the inflamed (LPS) ear 24 hours after infusion. Figure 4A shows that sLex-modified MSCs localized to the inflamed ear with 56% increased efficiency compared with the unmodified MSCs, whereas no significant difference was observed between the numbers of sLex-MSCs and unmodified MSCs within the noninflamed (saline) ear. The enhanced homing of sLex-MSCs to inflamed tissue compared with unmodified MSCs shows the significance of the engineered adhesion ligands to improve homing of the cells. Furthermore, no difference in homing of the sLex-MSCs and unmodified MSCs within the noninflamed ear signifies the role of the immobilized sLex as homing ligand. It is also important to consider that the sLex modification did not impact homing to the noninflamed ear, suggesting that the modification process did not compromise the native homing ligands on the MSC surface. Noninflamed endothelium of mouse ear vessels constitutively express selectins,32,34 which probably contributed to the basal levels of homing observed for both the unmodified MSCs and sLex-MSCs within the noninflamed ear. On investigation of the specificity of sLex interactions with selectins within the inflamed ear, we observed that homing of sLex-MSCs was significantly more efficient than the glucose-modified MSCs and homing of the glucose-modified MSCs showed a similar response to the unmodified MSCs (supplemental Figure 7C). In addition, treatment with blocking antibody against P-selectin resulted in a 25% reduction of the homing of sLex-MSCs to the inflamed ear compared with the isotype control (P < .05). Specifically, the average number of sLex-MSCs (per the field of view) dropped from approximately 30 in the isotope control to approximately 23 after blocking P-selectin in the inflamed ear. This partial but significant decrease of the homing of sLex-MSCs to the inflamed ear further confirms the specific role of the P-selectin in mediating the homing response of sLex-modified MSCs. These results indicate that enhanced homing efficiency for sLex-MSCs was mediated in part by rolling interactions between selectin and sLex. However, the incomplete response to the anti–P-selectin treatment also suggests that, in addition to P-selectin, homing sLex-MSCs to the inflamed ear is also mediated by other receptors expressed by the inflamed endothelium. Figure 4B shows increased number of sLex-MSCs (red) homed to the inflamed ear compared with unmodified MSCs (green). To ensure efficient homing, it is critical for the MSCs to extravasate through the endothelium into the tissue. The majority (> 90%) of sLex-MSCs localized within the inflamed tissues had extravasated from the blood vessels after 24 hours, indicating that the ability of the MSCs to transmigrate through the endothelium was not affected by the covalent modification of the cell surface with sLex. In addition, no significant difference was observed in the extravasation efficiency between the sLex-MSCs (88% ± 11%) and unmodified MSCs (83% ± 19%) in the noninflamed ear. The increased homing response is probably critical for improving the engraftment of MSCs, within diseased tissues after systemic injection, leading to improved therapeutic outcome. The proof of principle for this hypothesis is provided by approaches that have involved enzymatic and genetic modifications of cells to overexpress adhesion or chemokine receptors, leading to improved homing and functional outcome in animal models.7,17,35
Targeted homing of surface engineering MSCs to inflamed tissue. (A) Percentage increase in sLex-modified MSCs compared with unmodified MSCs that homed to the inflamed and saline ear (noninflamed) 24 hours after systemic infusion. The average number of sLex-modified MSCs (per field of view) that homed to the inflamed ear was 48 compared with 31 unmodified MSCs, whereas the average number of sLex-modified MSCs (per field of view) that homed to the saline ear (noninflamed) was 31 compared with 29 unmodified MSCs. (B) Representative image of MSC localization in the inflamed ear at 24 hours after injection of DiD-labeled sLex-MSCs (red) and DiR-labeled unmodified MSCs (green). Most cells extravasated though the vessel walls (visualized by FITC-dextran, blue). No differences in extravasation efficiency were observed, thus indicating that the enhanced homing was because of an engineered rolling response through MSC surface functionalization with sLex *P < .05.
Targeted homing of surface engineering MSCs to inflamed tissue. (A) Percentage increase in sLex-modified MSCs compared with unmodified MSCs that homed to the inflamed and saline ear (noninflamed) 24 hours after systemic infusion. The average number of sLex-modified MSCs (per field of view) that homed to the inflamed ear was 48 compared with 31 unmodified MSCs, whereas the average number of sLex-modified MSCs (per field of view) that homed to the saline ear (noninflamed) was 31 compared with 29 unmodified MSCs. (B) Representative image of MSC localization in the inflamed ear at 24 hours after injection of DiD-labeled sLex-MSCs (red) and DiR-labeled unmodified MSCs (green). Most cells extravasated though the vessel walls (visualized by FITC-dextran, blue). No differences in extravasation efficiency were observed, thus indicating that the enhanced homing was because of an engineered rolling response through MSC surface functionalization with sLex *P < .05.
The success of MSC systemic therapy is limited by poor homing efficiency.10 This study demonstrates that chemical modification of primary human MSCs under controlled conditions can promote a robust rolling response in vivo leading to increased homing to inflamed tissue. Most significantly, it is demonstrated that inducing a rolling response is critical to increase the homing of systemically delivered MSCs. We have shown that the conditions that maximize cell rolling preserve the MSC phenotype, including their multilineage differentiation potential and their ability to secrete paracrine factors. Although the modification with sLex increased homing of MSCs to a site of inflammation, we do not anticipate that this will directly reduce entrapment of MSCs in the microvascular of organs, such as the lungs. These results provide a roadmap for introducing functional adhesion ligands on the surface of cells to promote a robust homing response. This method offers a simple approach to explore engineered cell homing and potentially target any cell type to specific tissues via the circulation.
This article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the American Heart Association (grant 0970178N) and the National Institutes of Health (grants DE019191, HL095722, and HL097172, J.M.K.) as well as the Massachusetts Institute of Technology Undergraduate Research Opportunities Program. P.K.V. was supported by Kauffman Foundation Entrepreneur (Postdoctoral Fellowship). W.Z. was supported by the Human Frontier Science Program (Postdoctoral Fellowship).
National Institutes of Health
Authorship
Contribution: D.S., J.A.S., C.P.L., and J.M.K. designed the research and the experiments; D.S., S.S., J.A.S., J.A.P., and D.P.S. performed the experiments; D.S., J.A.S., J.A.P., L.J.M., S.K., W.Z., P.K.V., R.S., C.P.L., R.K., and J.M.K. analyzed the data; and D.S., J.A.S., C.P.L., R.K., and J.M.K. wrote the manuscript and contributed to the interpretation of the results.
Conflict-of-interest disclosure: J.M.K. is a co-owner of Megacell Therapeutics, a company that has an option to license IP generated by J.M.K. J.M.K. may benefit financially if the IP is licensed and further validated. The interests of J.M.K. were reviewed and are subject to a management plan overseen by the Brigham & Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey M. Karp, Center for Regenerative Therapeutics & Department of Medicine, Brigham & Women's Hospital, Harvard Medical School, Harvard Stem Cell Institute, Harvard–Massachusetts Institute of Technology, Division of Health Sciences and Technology, 65 Landsdowne St, Cambridge, MA 02139; e-mail: jkarp@rics.bwh.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal