In this issue of Blood, 2 groups (McDermott et al1 and Dale et al2 ) independently report the results of phase 1 clinical trials using the CXCR4-specific chemokine receptor antagonist plerixafor (Mozobil) to target the hyperfunctional CXCR4 signaling axis in patients with the rare immunodeficiency disease WHIM syndrome.
Warts, hypogammaglobulinemia, immundeficiency, and myelokathexis (WHIM) syndrome is an unusual disorder whose cardinal features are severe neutropenia despite an abundance of mature neutrophils in the bone marrow (myelokathexis), lymphopenia, and susceptibility to human papillomavirus infection.3 Initial speculation regarding the mechanism of neutropenia centered on inappropriate retention of mature neutrophils4 versus premature apoptosis.5 Genetic studies have revealed that the disease is caused by terminal truncations of the chemokine receptor CXCR4 cytoplasmic domain, a domain important for receptor down-regulation.6 This discovery suggested that hyperactivation of the mutant receptor was the underlying pathogenic mechanism and that inhibition of signaling might be therapeutically effective (see figure). The highly specific CXCR4 antagonist plerixafor is currently approved as a stem cell mobilizing agent. The use of this agent as treatment for WHIM syndrome required establishing the safety of CXCR4 antagonism for chronic use and testing whether partial blockade would be effective in mobilizing hematopoietic populations that carried mutant CXCR4 receptors.
In the present studies, patients with truncating mutation of CXCR4 who presented with the characteristic features of WHIM syndrome were treated with daily intramuscular injections of plerixafor over an escalating dose range. The baseline data in both study populations confirmed the profound neutropenia and lymphopenia that is characteristic of the disorder. A clinical response was observed in both cohorts, even at the lowest plerixafor dose, as assessed by serial complete blood counts, and dose responsiveness was observed up to the maximal dose used. Interestingly, lymphocyte populations showed a more robust response to treatment than neutrophils, with normalized or supranormal counts obtained even at submaximal doses. Neutrophil counts were responsive to treatment but never normalized, even at maximal plerixafor dosing. This pattern was unexpected as neutrophil mobilization in healthy subjects was greater than that of lymphocytes.7 Nonetheless, the neutrophil counts attained were in excess of 500 neutrophils/μL of blood in both studies, suggesting that therapeutic dosing was attainable within the range reported in these studies. In the study by McDermott and colleagues,1 drug pharmacokinetic and pharmacodynamic properties were confirmed to be similar in the WHIM patients as in previously reported healthy controls. The safety profile after 1 week of use in both patient cohorts was acceptable, supporting further investigation of plerixafor as a therapeutic agent in WHIM syndrome.
In addition to the safety data presented, the results of the current clinical studies confirmed an interesting discrepancy between the relative responsiveness of lymphocyte, monocyte, and neutrophil populations in control versus WHIM syndrome subjects. In particular, B lymphocytes were highly mobilized from nearly undetectable levels to supranormal levels. It is not clear yet from which compartment lymphocytes were released by plerixafor treatment, but McDermott and colleagues speculate that the source is also the bone marrow.1 The effect of chronic plerixafor lymphocyte mobilization on immune function in WHIM patients was not addressed in these studies, but the stage is now set for efficacy studies in which immune function can be characterized during a therapeutic trial. Clinical efficacy is likely with regard to prevention of neutropenia-related bacterial infections in light of the results obtained in both studies.
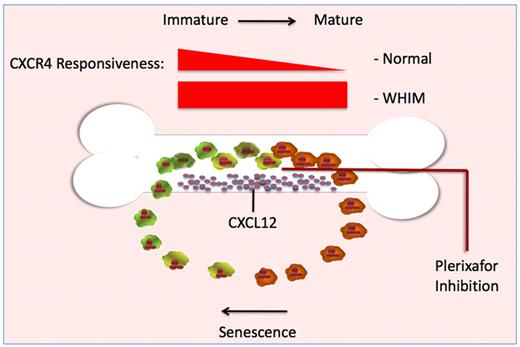
Illustration outlining the relationship between neutrophil maturation, bone marrow release, and cellular responsiveness of CXCR4 receptor to the ligand CXCL12. As neutrophils mature in healthy individuals, the cell-surface expression of CXCR4 and responsiveness to CXCL12 are reduced (darker cells). Once in the periphery, cell-surface expression and responsiveness to CXCL12 increase as these neutrophils age (lighter cells), allowing relocalization to CXCL12 secreting niches. In cells expressing CXCR4 truncation mutants, down-regulation after receptor activation is impaired. As a result, the mutant receptor remains continuously responsive to CXCL12 produced by bone marrow stromal cells. Blockade of the CXCR4 receptor with plerixafor permits mutant neutrophils to escape the constant signaling loop that otherwise keeps mature neutrophils trapped in the bone marrow. This schema is presented as a probable mechanism for correction of the neutrophil trafficking defect. McDermott et al speculate that release from the bone marrow may also be the mechanism by which plerixafor corrects the lymphopenia of WHIM syndrome.1
Illustration outlining the relationship between neutrophil maturation, bone marrow release, and cellular responsiveness of CXCR4 receptor to the ligand CXCL12. As neutrophils mature in healthy individuals, the cell-surface expression of CXCR4 and responsiveness to CXCL12 are reduced (darker cells). Once in the periphery, cell-surface expression and responsiveness to CXCL12 increase as these neutrophils age (lighter cells), allowing relocalization to CXCL12 secreting niches. In cells expressing CXCR4 truncation mutants, down-regulation after receptor activation is impaired. As a result, the mutant receptor remains continuously responsive to CXCL12 produced by bone marrow stromal cells. Blockade of the CXCR4 receptor with plerixafor permits mutant neutrophils to escape the constant signaling loop that otherwise keeps mature neutrophils trapped in the bone marrow. This schema is presented as a probable mechanism for correction of the neutrophil trafficking defect. McDermott et al speculate that release from the bone marrow may also be the mechanism by which plerixafor corrects the lymphopenia of WHIM syndrome.1
The data presented by these groups culminate a series of investigations into the molecular pathogenesis of WHIM syndrome. In vitro studies confirmed that not only did mutant receptors signal more robustly than wild-type receptors, they also failed to internalize normally after stimulation with the CXCR4 ligand, CXCL12 (SDF-1).8 Two animal models of WHIM syndrome have been described in which a recurrent 19-amino acid truncation mutant (R334X) was used to generate a murine xenotransplant model9 and a transgenic zebrafish model.10 These preclinical models both suggested that neutrophil retention was the basis of myelokathexis. Furthermore, transgenic expression of the mutant receptor did not accelerate apoptosis directly in the mouse model, suggesting that the apoptotic cells observed in WHIM syndrome were a secondary phenomenon of neutrophil sequestration in the bone marrow. In the zebrafish model, inhibition of the endogenous CXCL12 expression corrected the neutrophil retention, providing in vivo evidence that suppression of CXCR4-CXCL12 signaling could correct the myelokathexis phenotype. Preclinical studies with plerixafor in cell culture models expressing the R334X mutant further supported the validity of this approach.11 The development of plerixafor stemmed from earlier work to develop chemokine receptor antagonists that could be useful as agents that inhibited HIV cellular entry. The approval of the agent for hematopoietic precursor cell mobilization has now fortuitously permitted the rapid development of a molecularly targeted therapy for use in an extremely rare immunodeficiency disease.
Not all cases of myelokathexis or WHIM syndrome result from mutation of CXCR4, but increased signaling by the receptor has been implicated even in cases with distinct genetic etiologies.12 Genetic evaluation of patients with WHIM syndrome or myelokathexis may eventually be of direct clinical use to identify those patients whose inappropriately sequestered leukocytes can be released by plerixafor therapy, potentially releasing these patients from the grasp of their disease.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■