The transmembrane protein CD47 is a potential therapeutic target for treatment of a variety of hematologic malignancies. In this issue of Blood, Chao and colleagues use a human non-Hodgkin lymphoma (NHL) xenotransplant mouse model to demonstrate that CD47 is involved in dissemination of NHL and that blocking its interaction with the signal regulatory protein α (SIRPα) by anti-CD47 antibody therapy can prevent spread of this lymphoma.1
CD47, formerly known as integrin associated protein (IAP), is a ubiquitously expressed penta-transmembrane domain Ig-like protein. Apart from its ability to associate with integrins, it serves as a receptor for the extracellular matrix protein Thrombospondin. Furthermore, CD47 is a ligand for SIRPα, an inhibitory ITIM-motif receptor prominently expressed by phagocytic cells. This interaction inhibits phagocytosis by macrophages. Not surprisingly, given this diversity of interacting partners, CD47 plays an important role in a wide variety of biologic processes, including leukocyte motility, adhesion and migration, phagocytosis and recognition of “self,” and as such (xeno) transplant rejection and hematopoietic stem cell engraftment.2
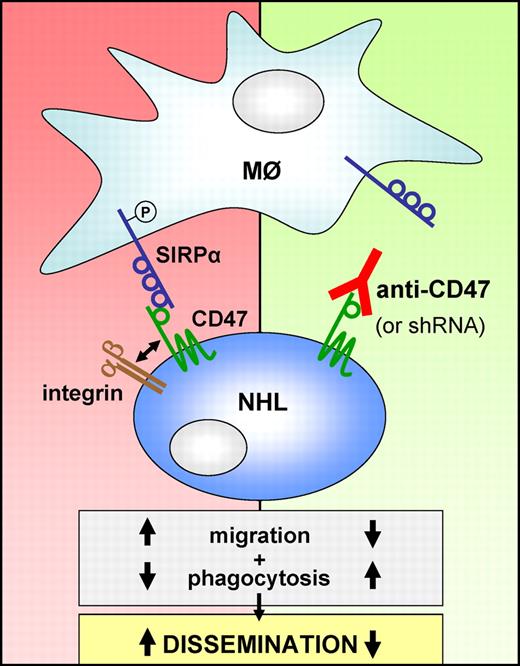
CD47 is an integrin-associated protein and a ligand for the phagocytosis-inhibitory receptor SIRPα (left). Various types of NHL overexpress CD47, which correlates with poor prognosis. In this issue of Blood, Chao et al show that CD47 is more prominently expressed by disseminated lymphoma cells and that targeting CD47 with a blocking antibody or by RNAi impairs chemokine-controlled migration, promotes macrophage-mediated phagocytosis and prevents dissemination of the malignant B cells (right).
CD47 is an integrin-associated protein and a ligand for the phagocytosis-inhibitory receptor SIRPα (left). Various types of NHL overexpress CD47, which correlates with poor prognosis. In this issue of Blood, Chao et al show that CD47 is more prominently expressed by disseminated lymphoma cells and that targeting CD47 with a blocking antibody or by RNAi impairs chemokine-controlled migration, promotes macrophage-mediated phagocytosis and prevents dissemination of the malignant B cells (right).
In previous studies, the groups of Weissman and Majeti reported that CD47 is up-regulated on circulating hematopoietic stem cells during inflammation-mediated mobilization and on myeloid leukemic cells, enabling them to evade phagocytosis by macrophages.3 Furthermore, they established that increased expression of CD47 on the self-renewing leukemia stem cells in acute myeloid leukemia (AML) and on acute lymphoblastic leukemia (ALL) cells is an independent poor prognostic factor, and targeting of CD47 with a blocking antibody in a human xenograft mouse model depleted AML and ALL by enabling phagocytosis of the malignant cells.4,5 Recently, Chao and colleagues demonstrated that CD47 is also overexpressed on multiple B-cell NHL subtypes, including diffuse large B-cell lymphoma (DLBCL), B-cell chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), and follicular lymphoma (FL), with adverse prognosis in DLBCL, CLL, and MCL.6 Using human NHL xenograft mouse models with the human Burkitt lymphoma cell line Raji and primary DLBCL and FL cells, either injected intraveneously (“disseminated model”) or subcutaneously (“localized model”; Raji only), they showed that anti-CD47 treatment reduced lymphoma growth and prolonged mouse survival. In combination with the therapeutic anti-CD20 antibody rituximab, anti-CD47 displayed strong synergy in promotion of phagocytosis in vitro, and complete lymphoma eradication and long-term disease-free survival of the mice.6
In their current study, Chao et al investigated a possible role for CD47 in NHL dissemination, and whether dissemination could be inhibited by therapeutic targeting of CD47. Using the “localized” mouse model in which Raji and now also primary DLBCL cells are transplanted subcutaneously, dissemination to secondary sites was studied: Raji cells spread to most major organs, including liver and bone marrow (BM), whereas spread of the primary DLBCL cellswas limited to BM and peripheral blood. Interestingly, the disseminated lymphoma cells express higher levels of CD47. Moreover, RNAi-mediated silencing of CD47 and treatment of the mice with a CD47-blocking antibody only mildly decreased the growth rate of the primary disease but completely prevented extra-nodal and hematogenous dissemination of the Raji and DLBCL cells, respectively (see Figure).1
Next, the authors explored the possible mechanism(s) underlying this impaired dissemination. Given the critical role of chemokines and integrins in lymphoma dissemination,7 combined with the interaction of CD47 with integrins, they investigated if the observed effect may involve chemokine-controlled migration and/or integrin-mediated adhesion. Interestingly, their results convincingly show that silencing or blocking of CD47 severely impaired migration of the Raji cells toward the chemokines CXCL12 and CXCL13.1 Basal integrin-mediated adhesion was not affected but, unfortunately, chemokine-induced integrin activation was not studied. Future experiments will have to elucidate the underlying molecular mechanism of this novel feature of CD47 (eg, CXCR4/5 signaling, cytoskeletal reorganization, or integrin activation). Furthermore, by phagocyte depletion with the bisphosphonate clodronate and by means of anti-CD47 antibodies capable or not of blocking the interaction of CD47 with the phagocytosis inhibitory receptor SIRPα, compelling evidence was provided that the impaired dissemination involves enhanced macrophage-mediated phagocytosis.1
These findings are particularly interesting given the potential application of anti-CD47 therapy as treatment for lymphoma or leukemia. Obviously, there may be many pitfalls on the road to a possible clinical application. Regarding specificity and clinical safety, however, it is important to note that despite the ubiquitous expression of CD47, CD47-deficient mice have a rather mild phenotype, a blocking anti–mouse CD47 antibody does not exert toxicity in mice, and a blocking anti–human CD47 does not affect phagocytosis of normal human PB cells and CD34+ BM cells in vitro.4,6 This apparent selectivity of anti-CD47 for malignant cells most likely involves the differential co-expression of a pro-phagocytic signal, for example, of the membrane protein Calreticulin (also overexpressed in NHL).8 However, given the critical role of the CD47-SIRPα interaction in discriminating “non-self” from “self” or “eat me” from “do not eat me,” let's not forget that in the xenografted mice, the mouse phagocytes will be more eager to attack and eat the “non-self” malignant human B cells. Therefore, in the end, the specificity, safety, efficacy and the underlying mechanism of action of the blocking anti-CD47 antibody therapy can only be reliably determined in a completely human setting, that is, in clinical trials.
If clinical trials turn out to be safe and promising, there will also be many interesting options for rational combination therapy. Apart from the rather obvious combination of anti-CD47 with rituximab, there may be more enticing candidates. An example? Some novel efficacious small molecule drugs that target the BCR signaling pathway, that is, pharmacologic inhibitors of Syk (R788/R406), Btk (PCI-32765), and PI3K (CAL-101), show an unexpected mode of action in clinical trials with NHL. In, for example, CLL, rather than directly killing the cells the rapidly reduced lymphadenopathy is accompanied by transient lymphocytosis. Given the role of Btk, Syk, and PI3K in both BCR- and chemokine-controlled integrin-mediated adhesion and migration of B cells,9,10 the observed transient lymphocytosis and tumor regression may be the direct consequence of overcoming BCR- and/or chemokine-controlled retention of the malignant B cells in their tumor microenvironment (lymph nodes and bone marrow), thereby depriving the cells of critical growth- and survival signals. Indeed, recent studies with CLL and MCL have provided support for this explanation (Buchner et al11 ; M. F. M. de Rooij, J. J. Buggy, and M.S., manuscript in preparation). In this perspective, it is tempting to speculate that combining these agents with anti-CD47 may turn out to be a highly efficacious treatment for NHL patients: once the malignant B cells have been forced out of their protective growth- and survival-supporting microenvironment into the circulation, they are more accessible and vulnerable for the action of anti-CD47, preventing their dissemination and priming the fully exposed malignant B cells for being attacked and eaten by the macrophages. This would make a promising rational combination therapy for complete eradication of lymphoma, spread or not!
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal