In this issue of Blood, Brentjens and colleagues report on the feasibility, tolerability, and persistence of autologous CD19-directed chimeric antigen receptor (CAR) T cells in patients with relapsed chronic lymphocytic leukemia (CLL) and B-cell acute lymphocytic leukemia (B-ALL).1 These diseases are generally considered incurable, unless treated with allogeneic stem cell transplantation, which is evidence of the efficacy of immune-mediated mechanisms in their treatment. Alternative immunotherapy approaches have been investigated, including antitumor vaccines and adoptive transfer of CD19 CAR-T cells reported in this paper.
The engineering of CAR-T cells is unique in that T cells are collected from a patient and genetically modified to express a receptor that will bind to a surface antigen expressed on the patient's own tumor cells. After infusion, autologous CAR-T cells home to sites of disease and also persist over time (see figure). The earliest CARs consisted of an extracellular antigen recognition domain (typically a single chain Fv variable fragment from a monoclonal antibody) linked via a transmembrane domain to an intracellular signaling domain (usually the CD3ζ endodomain), resulting in the redirection of T-cell specificity toward target antigen-positive cells.2 While effective in lysing tumor cells in vitro, the clinical utility of these first-generation CAR-T cells was limited by their inability to sufficiently activate and sustain themselves in vivo. Second generation CAR-T cells, with the addition of costimulatory domains including CD28, 4-1BB, or OX40 to the intracellular portion, are engineered to enhance cytokine secretion and effector cell expansion, and prevent activation-induced apoptosis and immune suppression by tumor-related metabolites.3
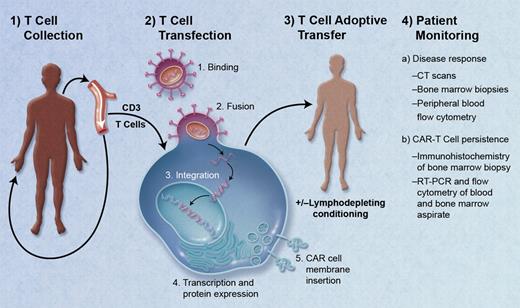
Schematic of the treatment of a patient with chimeric antigen receptor (CAR) T cells. (1) Isolation of peripheral T cells from patient via apheresis. (2) Transfection of T cells with a lentivirus containing genes for CAR directed against the tumor target antigen: binding of virus to T-cell membrane, fusion of virus with cell membrane, reverse transcription, DNA integration, and transcription/protein expression of CAR genes, and insertion of CAR into cell membrane. (3) Adoptive transfer of autologous CAR-T cells via infusion with or without prior lymphodepleting conditioning. (4) Patient monitoring for treatment response, and for persistence of CAR-T cells. Professional illustration by A. Y. Chen.
Schematic of the treatment of a patient with chimeric antigen receptor (CAR) T cells. (1) Isolation of peripheral T cells from patient via apheresis. (2) Transfection of T cells with a lentivirus containing genes for CAR directed against the tumor target antigen: binding of virus to T-cell membrane, fusion of virus with cell membrane, reverse transcription, DNA integration, and transcription/protein expression of CAR genes, and insertion of CAR into cell membrane. (3) Adoptive transfer of autologous CAR-T cells via infusion with or without prior lymphodepleting conditioning. (4) Patient monitoring for treatment response, and for persistence of CAR-T cells. Professional illustration by A. Y. Chen.
Here, Brentjens and colleagues report the use of their CD19-CD28z CAR construct in 10 patients with CLL (n = 8) or B-ALL (n = 2). CD19 is present on the malignant CLL and B-ALL cells, as well as healthy B cells, but not on hematopoietic stem cells, plasma cells, and other healthy tissue. CLL patients, with bulky disease that recurred after at least 1 prior chemotherapy regimen, were enrolled in a phase 1 trial. The first 3 patients were treated with 1.2 to 3.0 × 107 19-28z+ T cells/kg without lymphocyte-depleting conditioning; the second cohort of 5 patients was to receive the same dose of T cells after dose-escalating cyclophosphamide conditioning. However, after an unexpected death because of a sepsis-like syndrome immediately after T-cell infusion in the first patient in this cohort, all subsequent patients received 0.4 to 1.0 × 107 19-28z+ T cells/kg over 2 days. Of 2 ALL patients with relapsed disease, only 1, in a second complete remission, was treated with cyclophosphamide conditioning followed by infusion of 3 × 106 19-28z+ T cells/kg. All patients experienced transient fevers within 24 hours after T-cell infusion, which was otherwise well tolerated. There were no clinical responses in the first CLL cohort, but there was 1 patient with marked reduction in lymphadenopathy, and 2 patients with stable disease in the second cohort. The ALL patient, who was in a complete remission at the time of infusion, had persistent B-cell aplasia after T-cell infusion, and subsequently had a planned allogeneic stem cell transplantation. These results correlated with 19-28z+ T-cell persistence in bone marrow and blood: there were no CAR-T cells at 1 month after infusion in the first CLL cohort, compared with retention of CAR-T cells up to 6 weeks after infusion in the blood and marrow of patients treated in the second CLL and ALL cohorts. When these CAR-T cells were collected 8 days after infusion and cultured with antigen-expressing fibroblasts, they exhibited marked expansion and cytotoxic effects. Furthermore, these T cells were found to have infiltrated tumor beds in the 1 patient who died shortly after T-cell infusion.
This study is one of a number of studies investigating the use of second-generation anti-CD19 CAR-T cells in B-cell neoplasms.4 The results of a series of 3 patients with refractory CLL treated with CD19-CD137 (41BB) CAR-T cells have been reported.5,6 While all 3 patients treated in this study had a large tumor burden, 1 achieved a partial response and 2 achieved complete responses that were ongoing 7 to 11 months after treatment. All patients experienced tumor lysis syndrome 7 to 21 days after T-cell infusion, and B-cell aplasia and hypogammaglobulinemia were observed. CAR-T cells were evident in the blood and bone marrow for at least 4 months after treatment, and these cells maintained their effector functions.
These protocols differ in their use of alternate costimulatory molecules, the conditioning regimen, and the number of CAR-T cells infused, but together they demonstrate the safety and potential efficacy of a novel anticancer treatment strategy. The different approaches used in these studies may impact significantly on the efficacy of this new technology and more patients need to be treated to identify the optimal treatment protocol. Given the small numbers treated to date, we do not yet know the possible unanticipated toxicities, nor can we predict how reproducible these findings will be in more patients. CAR targets must be present on the surface of tumor cells, but also should contribute to their malignant phenotype and have limited expression on healthy host tissues to avoid tumor immune evasion by loss of target expression and off-target effects, respectively. While CD19 is an attractive target for B-cell malignancies, given its specificity for cells of B-cell lineage and the general tolerability of B-cell aplasia and hypogammaglobulinemia, finding similarly specific antigens in other malignancies may prove difficult. Furthermore, whether there are unforeseen effects of genetically modified self-sustaining T cells is not yet known, and the ability to ameliorate such effects by immunosuppression or inducible suicide signals is also unknown. The ability of these cells to persist is attractive from an antitumor perspective, but we do not yet know how long they will persist and how long their affects on healthy tissue may last; CAR-T cells with a central memory phenotype have been described.6 These cautions aside, if these results can be replicated in further studies in larger numbers of patients, this technology could be modified to be used in a variety of hematologic and solid malignancies.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal