In this issue of Blood, Yu and colleagues demonstrate that combined targeted disruption of transcription factors T-bet and RORγt have defective differentiation of donor CD4+ T cells toward T helper 1 (Th1) and Th17, and ameliorated graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (SCT).1
GVHD, a major complication after allogeneic hematopoietic SCT, is mediated by donor-derived T cells. On activation with alloantigens expressed on host tissues, donor-derived naive CD4+ T cells differentiate into Th-cell subsets of effector T cells expressing distinct sets of transcriptional factors and cytokines. Although acute GVHD has been classically assumed to be Th1-mediated on the basis of findings from animal models, we now face a more complex scenario involving possible roles of newly identified Th17 cells as well as regulatory T cells (Tregs) in GVHD (see figure). The role of Th17 responses in GVHD has been evaluated using IL-17–deficient mice or ex vivo polarized Th17 cells, which produced somewhat inconsistent results. In sum, a series of studies using IL-17–deficient T cells suggest that IL-17 is not essential for all manifestations of acute GVHD but it does play a role in some organ-specific GVHD, particularly in skin GVHD.2-7 Studies using ex vivo polarized Th17 cells also suggest that Th17 cells are sufficient but not necessary for GVHD induction.8,9 However, these results should be carefully interpreted because of study design limitations. For example, IL-17 is not the only cytokine secreted by Th17 cells and Th17 differentiation can occur in the absence of IL-17. Th17 cells polarized ex vivo are not antigen-specific and do not always maintain their cytokine profiles in vivo after transfer.
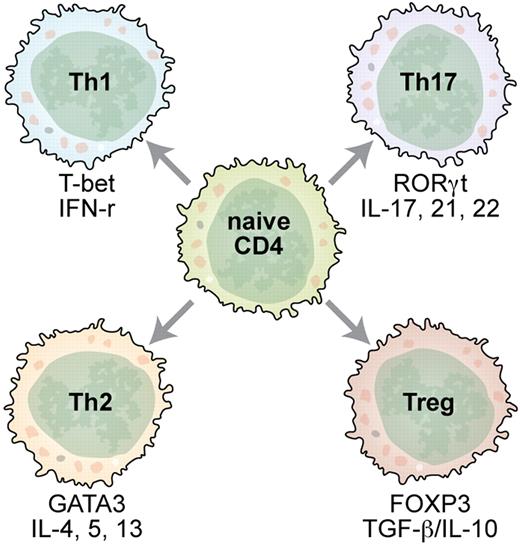
Antigen-activated naive CD4+ T cells adopt various phenotypes as directed by cytokine cues and transcriptional factors. Professional illustration by Kenneth X. Probst.
Antigen-activated naive CD4+ T cells adopt various phenotypes as directed by cytokine cues and transcriptional factors. Professional illustration by Kenneth X. Probst.
The article by Yu and colleagues uses T cells with targeted disruption of the transcription factors, which are critical for Th differentiation.1 T-bet and RORγt are critical transcription factors for Th1 and Th17 differentiation, respectively. T cells with target disruption of T-bet skewed differentiation toward Th2, Th17, and Treg phenotypes in vivo after transplantation and attenuate GVHD, particularly gut GVHD, compared with wild-type T cells. In contrast, RORγt deficiency of T cells has little impact on the manifestations of systemic or organ-specific GVHD. Importantly, T-bet and RORγt doubly deficient T cells have defective differentiation toward Th1 and Th17, skewed differentiation toward Th2 and Treg phenotypes, and produced less severe GVHD than T-bet–deficient T cells. These results demonstrate that Th17 plays less of a role than Th1 but synergizes with Th1 in GVHD induction. Reduction of GVHD was evident in the liver and lungs as well as the intestines in recipients of the doubly deficient T cells. On stimulation, T cells can rapidly switch chemokine receptor expression, acquiring new migratory capacity. Chemokine receptors are differentially expressed on subsets of activated/effector T cells. The differential expression of chemokine receptors and selectin ligands on each Th subset may ensure the unique ability of each Th subset to induce tissue-specific GVHD. Thus, it seems that a delicate balance among Th1, Th2, Th17, and Tregs after transplantation is an important determinant of the severity, manifestation, and tissue distribution of GVHD.
The article by Yu et al1 additionally demonstrates that T cells deficient for T-bet and RORγt have preserved graft-versus-leukemia (GVL) activity that is primarily dependent on CD8+ cytolytic T lymphocytes (CTLs) and their perforin and granzyme pathways.10 This study thus suggests that combined blockade of Th1 and Th17 differentiation is a promising strategy to improve outcome of allogeneic SCT by inhibiting GVHD, while preserving GVL activity of donor T cells. This strategy to block transcription factors regulating Th1 and Th17 differentiation appears to be much more effective for GVHD prevention than a strategy for inhibiting Th1 and Th17 cytokines.4 However, there are still several issues that need to be addressed. It is not clear if the changes seen in this model are due to the inhibition of Th1/Th17 differentiation or accelerated Th2/Treg differentiation. The role of recently identified Th9 cells also remains unclear. On the other hand, combined deficiency of T-bet and RORγt did not completely inhibit GVHD development. What are the effector mechanisms involved in this residual GVHD? The underlying mechanisms of full differentiation of naive CD8+ T cells into functional CTLs that usually require T-bet expression and Th1 help are also unclear. Lastly, this strategy should be tested in more clinically relevant MHC-matched models of allogeneic SCT. It should be noted that almost all previous studies addressing the role of each Th subset in GVHD have been also using MHC-mismatched models of GVHD. Impacts of this strategy on infection immunity are also clinically important. Certainly, further studies are required for better understanding of the dynamic process of reciprocal differentiation of Th and regulatory cell subsets as well as their complex interactions for establishing novel strategies that modulate the fine balance between Th and Treg subsets to improve outcome of allogeneic SCT.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal