Abstract
Cutaneous involvement is seen in ∼ 50% of adult T-cell leukemia/lymphoma (ATLL) patients. We investigated the association between skin eruption type and prognosis in 119 ATLL patients. ATLL eruptions were categorized into patch (6.7%), plaque (26.9%), multipapular (19.3%), nodulotumoral (38.7%), erythrodermic (4.2%), and purpuric (4.2%) types. When the T stage of the tumor-node-metastasis-blood (TNMB) classification of mycosis fungoides/Sézary syndrome was applied to ATLL staging, 16.0% were T1, 17.7% T2, 38.7% T3, and 4.2% T4, and the remaining 23.5% were of the multipapular and purpuric types. For the patch type, the mean survival time (median survival time could not be estimated) was 188.4 months. The median survival times (in months) for the remaining types were as follows: plaque, 114.9; multipapular, 17.3; nodulotumoral, 17.3; erythrodermic, 3.0; and purpuric, 4.4. Kaplan-Meier curves of overall survival showed that the erythrodermic type had the poorest prognosis, followed by the nodulotumoral and multipapular types. The patch and plaque types were associated with better survival rates. Multivariate analysis demonstrated that the hazard ratios of the erythrodermic and nodulotumoral types were significantly higher than that of the patch type, and that the eruption type is an independent prognostic factor for ATLL. The overall survival was worse as the T stage became more advanced: the multipapular type and T2 were comparable, and the purpuric type had a significantly poorer prognosis than T1.
Introduction
Adult T-cell leukemia/lymphoma (ATLL) is a malignancy of mature CD4+ T cells caused by the human T-cell lymphotropic virus type I (HTLV-1).1-3 HTLV-1 infection is prevalent in southern Japan, especially in Kyushu,4,5 and in the Caribbean region and Africa.6,7 Based on the number of abnormal lymphocytes, organ involvement, and severity, ATLL is divided into 4 clinical categories: acute, lymphoma, chronic, and smoldering (Shimoyama classification).8 This classification is the most common tool used for estimating the prognosis of ATLL patients. The smoldering type has the best prognosis, followed by the chronic type, lymphoma type, and acute type. The median survival times (MSTs) of the acute, lymphoma, and chronic types are 6.2, 10.2, and 24.3 months, respectively.8 Thus, the acute and lymphoma types of ATLL are associated with remarkably poor prognoses despite advances in chemotherapy and allogeneic hematopoietic stem cell transplantation.9-11 In contrast, the chronic and smoldering types are relatively indolent and can usually be managed with “watchful waiting” until the disease progresses to acute crisis, just as smoldering (asymptomatic) myeloma is managed.12
Studies have attempted to identify other prognostic factors for survival of ATLL patients. Advanced performance status, high blood lactate dehydrogenase (LDH) level, age of 40 years or more, more than 3 involved lesions, and hypercalcemia have all been associated with shortened survival.13 The existence of hepatosplenomegaly and lymphadenopathy also indicates poor prognosis.8,14 However, there has been no large study on the correlation between the type and spread of skin eruptions and the prognosis of ATLL.
Because cutaneous involvement can be recognized in approximately 50% of ATLL patients,15,16 the evaluation of skin lesions in relation to prognosis is important. Tumor cells infiltrating the skin exhibit several differences in phenotype and function.17,18 ATLL patients can develop various types of eruptions, including nodules, tumors, plaques, erythrodermas, and even purpuric lesions,19,20 and the categorization of these eruption types remains unclear. In this study, we retrospectively analyzed the prognosis of ATLL on the basis of the skin manifestations. We classified the skin eruptions and applied the T stage of the tumor-node-metastasis-blood (TNMB) classification for mycosis fungoides (MF) and Sézary syndrome (SS) to the type of skin lesions of ATLL. Our results indicate that eruption type is a predictor for prognosis.
Methods
Patients
We analyzed 119 patients with newly diagnosed, untreated ATLL who had skin eruptions and were seen at the University of Occupational and Environmental Health and Kyushu Kosei Nenkin Hospital from April 1979 to December 2009. The cutoff date for analysis was June 2010. The diagnosis of ATLL was based on clinical features, histopathologically and cytologically proven mature T-cell malignancy, presence of anti–HTLV-1 antibody, and monoclonal integration of HTLV-1 proviral DNA into the blood and/or skin tumor cells, as described previously.2,8,21,22 The subtypes of ATLL were classified according to the criteria established by the Lymphoma Study Group of Japan Clinical Oncology Group (Shimoyama classification).8 Our retrospective, nonrandomized, observational study using existing data was granted an exemption from the institutional review board and was exempt from the requirement for written informed consent in accordance with the Declaration of Helsinki.
Clinical evaluation and definitions
The patients were categorized into 2 age groups: younger than 60 years and 60 years or older. Complications at diagnosis were classified into present and absent. Leukocytosis and lymphocytosis were defined as white blood cell count more than 12 × 109/L, and total lymphocyte count more than 6.5 × 109/L, respectively. LDH and calcium levels were classified into 2 groups according to a standard index.13 We categorized skin eruptions of ATLL into 6 different types: patch, plaque, multipapular, nodulotumoral, erythrodermic, and purpuric (Figure 1). We defined the criteria for categorizing ATLL-related skin involvement into the patch type as no infiltrated erythema, the plaque type as infiltrated erythema, the multipapular type as multiple papules with diameter less than 1 cm, the nodulotumoral type as nodules or tumors with diameters more than 1 cm, the erythrodermic type as generalized erythema involving 80% or more of the patient's skin, and the purpuric type as red or purple discolorations that did not change with diascopy.
Statistical analyses
Overall survival (OS) was defined as the time from the date of first diagnosis to the date of death or the latest contact with the patient. Survival curves were drawn using the Kaplan-Meier method and were compared with the log-rank test. P values were calculated using the generalized Wilcoxon test. MST was defined as the time point at which the Kaplan-Meier survival curves crossed 50%. Mean survival time was provided when MST could not be calculated. To examine the multiple comparisons of the factors and of the pairs of groups, univariate and multivariate Cox regression analyses were applied to evaluate prognosis factors for survival. The effects of clinical parameters were evaluated as hazard ratios (HRs) and their 95% confidence intervals. All statistical analyses were performed using Dr SPSS II software (SPSS). A P value < .05 was considered statistically significant.
Results
Patient clinical characteristics
The clinical data of 119 patients with skin eruptions (ratio of male:female = 1.2:1) are summarized in Table 1. The mean age of the patients was 64.0 years (range, 23-91 years; SD, 12.00 years). According to Shimoyama classification, 40 (33.6%) patients were diagnosed with the acute type of ATLL, 6 (5.0%) with the chronic type, 17 (14.3%) with the lymphoma type, and 56 (47.1%) with the smoldering type. Twenty-three patients had complications at the time of diagnosis, including 7 patients with diabetes mellitus, 10 with hypertension, 3 with stroke, and 9 with opportunistic infections. Blood examination revealed that 36 patients (30.3%) had leukocytosis, 26 (21.9%) had lymphocytosis, and 49 (41.2%) had high LDH levels. Hypercalcemia was found in 70 patients (58.8%).
Patient skin lesions
We categorized the skin eruptions into the patch, plaque, multipapular, nodulotumoral, erythrodermic, and purpuric types (Figure 1). The most highly incident was the nodulotumoral type in 46 patients (38.7%), followed by the plaque type in 32 patients (26.9%), the multipapular type in 23 patients (19.3%), the patch type in 8 patients (6.7%), the erythrodermic type in 5 patients (4.2%), and the purpuric type in 5 patients (4.2%). Because the categorized skin eruptions of ATLL have similarities to those of MF/SS (with the exception of the multipapular and purpuric types), and because the TNMB classification for MF/SS16 has been widely used, we attempted to apply the T stage of the TNMB classification to ATLL skin lesions. According to the MF/SS classification,16 eruptions are classified into: T1 (patch/plaque, less than 10% of body surface), T2 (patch/plaque, more than 10% of body surface area), T3 (nodulotumoral type), and T4 (erythrodermic type). Ninety-one (76.5%) of our 119 patients could be classified using this system: 19 patients (16.0%) belonged to T1, 21 (17.7%) to T2, 46 (38.7%) to T3, and 5 (4.2%) to T4. The remaining 28 patients (23.5%) had multipapular (19.3%) and purpuric (4.2%) types, which are peculiar for ATLL and are not described in the T classification of MF/SS. We also evaluated these 2 types to investigate whether they are comparable with either the T1 or T4 category of the MF/SS classification system.
Clinical features of ATLL with skin eruptions. (A) Patch type, (B) plaque type, (C) multipapular type, (D) nodulotumoral type, (E) erythrodermic type, and (F) purpuric type.
Clinical features of ATLL with skin eruptions. (A) Patch type, (B) plaque type, (C) multipapular type, (D) nodulotumoral type, (E) erythrodermic type, and (F) purpuric type.
We examined the frequencies of the clinical subtypes of Shimoyama classification in each of the eruption types and T stages (Table 2). All patients with the erythrodermic type belonged to the acute type, whereas most of the patients with the patch type were grouped into the smoldering subtype. As the T stage advanced, the frequencies of the aggressive types (the acute and lymphoma types) increased, whereas those of the smoldering type decreased.
Survival by baseline clinical factors
Sixty-nine of our 119 patients died during the observation period, with a median follow-up duration of 3.0 years (range, 30 days-20.3 years). The MSTs of the acute, lymphoma, chronic, and smoldering types were 7.7, 15.0, 16.6, and 154.0 months, respectively (Table 1). Of the 69 fatal cases during the observation, 45 patients died of acute ATLL, 17 of acute crisis from the other subtypes, 5 of other diseases (3 of chronic pulmonary diseases and 2 of acute respiratory disease syndrome [ARDS]), and 2 patients of unknown causes.
The effects of various clinical factors on prognosis in the 119 patients were analyzed using the Kaplan-Meier method (Table 1). There was no statistically significant difference in survival rates between the absence and presence of any complication (P = .114), between the ≥ 60 years and < 60 years age groups (P = .702), or between males and females (P = .956). The survival rate was poor in patients with leukocytosis (P < .001), lymphocytosis (P < .001), and higher LDH levels (P < .001). Blood calcium level did not significantly affect survival in this study.
Survival and multivariate analyses in each eruption type
The MSTs were different between the types of skin eruptions. In the erythrodermic type, all 5 patients died of the disease with 3.0 months of MST. In the nodulotumoral type, the MST was 17.3 months, and 38 of 46 patients died, 17 of acute ATLL, 16 of acute crisis, 1 of ARDS, 2 of chronic pulmonary disease, and 2 of unknown causes. In the plaque type, the MST was 114.9 months, and 9 of 32 died of the disease. The multipapular type showed the same MST (17.3 months) as the nodulotumoral type, and 9 died of acute ATLL, 1 of acute crisis, 1 of ARDS, and 1 of chronic pulmonary disease. The patch type exhibited a good prognosis, with 188.4 months of mean survival time (the MST was not estimable). The purpuric type was found to have a poor prognosis, with an MST of 4.4 months and 3 of 5 patients dying of the disease.
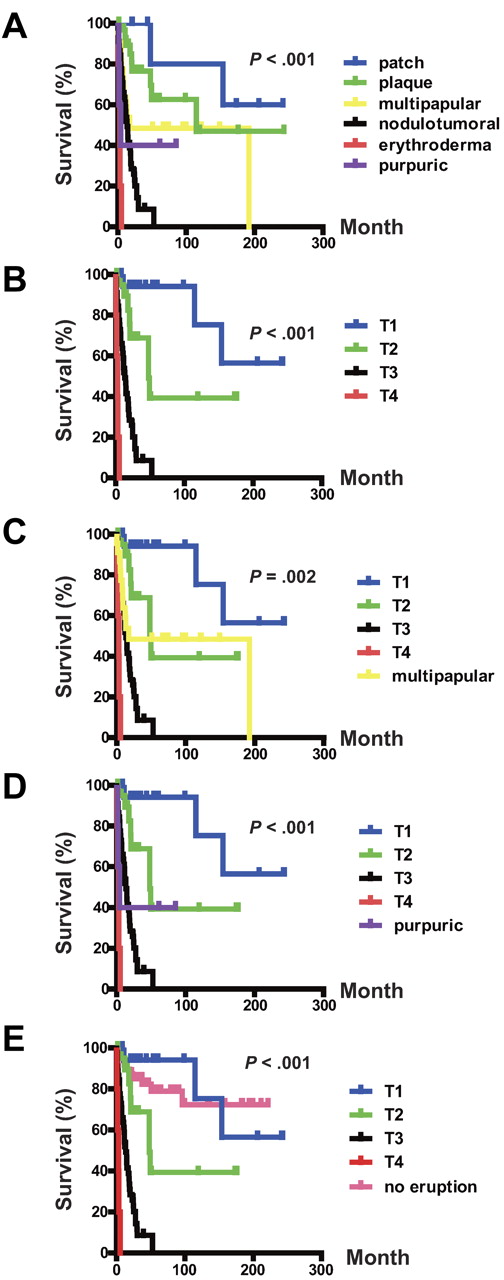
Kaplan-Meier curves of the OS for each eruption type are shown in Figure 2A. The OS rate of the erythrodermic type was significantly lower than those of the other eruption types (P < .001, erythrodermic type vs the nodulotumoral, multipapular, plaque, or patch types). The OS rate of the nodulotumoral type was significantly lower than those of the multipapular, plaque, or patch types (P = .010, nodulotumoral type vs multipapular type; P < .001, nodulotumoral type vs plaque or patch type). The OS rate of the multipapular type was significantly lower than that of the patch type (P = .045). Therefore, the erythrodermic type of ATLL is associated with the poorest prognosis, followed by the nodulotumoral and multipapular types. The patch and plaque types showed better survival rates.
OS of ATLL patients with skin eruptions. (A) OS rates of skin eruption types. (B) OS rate of T stage. (C) OS rate of the T stage and the multipapular type. (D) OS rate of the T stage and the purpuric type. (E) OS rate of the T stage and the no-eruption type.
OS of ATLL patients with skin eruptions. (A) OS rates of skin eruption types. (B) OS rate of T stage. (C) OS rate of the T stage and the multipapular type. (D) OS rate of the T stage and the purpuric type. (E) OS rate of the T stage and the no-eruption type.
We performed univariate and multivariate analyses of the eruption types in a comparison with Shimoyama classification, sex, age, complications, leukocyte counts, lymphocyte counts, LDH level, and calcium level (Table 3). In the multivariate analysis, the smoldering type proved to be a good prognostic factor. We fixed the HR of the patch type to be 1, and then compared it with those of the other eruption types. In the univariate analysis, the HRs of the other eruption types were significantly higher than that of the patch type. In the multivariate analysis, the HRs of the nodulotumoral and erythrodermic types were significantly higher than that of the patch type. The purpuric type also showed such a tendency; however, this result provided limited power for tests against the other groups. The analysis demonstrated that the eruption type is an independent prognostic factor for ATLL.
Survival and univariate and multivariate analyses in each T stage
We also performed the univariate and multivariate analyses of T stage and other clinical and laboratory parameters for OS. Of 19 patients in the T1 stage, 3 died of the disease, and the mean survival time (the MST was not estimable) was 192.6 months (Table 1). In the T2 stage, 8 of 21 died of the disease and the MST was 47.9 months. In the T3 stage, the MST was 17.3 months and 38 of 46 patients died: 17 of acute ATLL, 16 of acute crisis, 1 of ARDS, 2 of chronic pulmonary disease, and 2 of unknown etiology. In the T4 stage, 5 patients died of the disease with 3.0 months of MST. The OS of the patients was worse as the T stage became more advanced (Figure 2B). Patients in the T1 stage had the longest OS, followed by patients in the T2-T4 stages (P = .034, T1 vs T2; P < .001, T1 vs T3 or T4; P < .001 T2 vs T3 or T4; and P < .001, T3 vs T4).
The multipapular and purpuric types are missing in the T stage of the MF/SS system due to their peculiarity. We therefore compared the OS of these 2 eruption types with those of the T stages. Patients with the multipapular type and T2 had a similar outcome (Figure 2C), and there was no statistical significance (P = .415). Patients with the purpuric type had a significantly poorer prognosis than those with T1 (P = .001); Figure 2D). The differences in OS between the purpuric type and the other T stages were not statistically significant (P = .412, purpuric type vs T2; P = .257; purpuric type vs T3; P = .099, purpuric type vs T4).
We performed univariate and multivariate analyses of T stage and clinical and laboratory parameters with the HR of T1 set as 1 (Table 3). The univariate analysis revealed that the prognoses of T2, T3, T4, and the multipapular and purpuric types were significantly higher than that of T1. In the multivariate analysis, the HR of T3 and T4 and the multipapular and purpuric types were significantly higher than that of T1.
Survival and univariate and multivariate analyses in each T stage and in the no-eruption group
We performed univariate and multivariate analyses of T stage by comparing them with the no-eruption group and other clinical and laboratory parameters for OS. Of 51 patients without skin eruptions, 10 died of the disease and the mean survival time (the MST was not estimable) was 66.5 months. When classifying the no-eruption patients into each clinical Shimoyama subtype, 7 patients (13.7%) belonged to the acute type, 5 (9.8%) to the lymphoma type, 12 (23.5%) to the chronic type, and 27 (52.9%) to the smoldering type. The OS of the patients without eruption was better than those at T2-T4 (Figure 2E; no-eruption group vs T2, P = .033; no eruption group vs T3 or T4, P < .001). There was no statistically significant difference in OS between the no-eruption group and T1.
We performed univariate and multivariate analyses of T stage, including the no-eruption group and clinical and laboratory parameters, by assigning a value of 1 to the HR of T1 (Tables 4 and 5). The univariate and multivariate analyses revealed that the prognoses of T3 and T4 were significantly worse than that of T1.
Discussion
In the present study, we investigated the association of each type of skin eruption with prognosis in ATLL patients and attempted to apply the T stage of MF/SS classification to the assessment of ATLL skin lesions. We classified ATLL skin eruptions into 6 categories: patch, plaque, multipapular, nodulotumoral, erythrodermic, and purpuric. Table 2 shows that the frequencies of the clinical subtypes of Shimoyama classification were different for each eruption type and T stage. All erythrodermic patients belonged to the acute type, whereas most of patients with the patch type were of the smoldering type. This raised the possibility that prognosis is different among the individual eruption types. Our results revealed the poorest prognosis in the erythrodermic type, followed by the nodulotumoral and multipapular types. The patch and plaque types exhibited better survival rates. Moreover, our multivariate analysis demonstrated that the HRs of the erythrodermic and nodulotumoral types were significantly higher than that of the patch type, and that skin eruption is an independent prognostic factor for ATLL.
It has been reported that the smoldering type of ATLL with skin eruptions, especially those of the nodulotumoral type, has a poorer prognosis than ATTL without skin involvement.19 Another group of investigators reported that the MSTs of the nodulotumoral and maculopapular types were 26 and 80 months, respectively, which are significantly shorter than those in ATLL without cutaneous eruptions.23 Our findings are in agreement with these observations, and further clarify the relationship between type of skin lesion and prognosis. Skin-targeted therapy using topical steroids, psoralen photochemotherapy, or narrow-band UVB therapy19 may improve the prognosis of ATLL for patients with skin eruptions.
The purpuric type of ATLL is one of the rarest skin eruptions of ATLL,24 and has been reported to occur in 1.6% of ATLL patients with skin lesions.19 However, its incidence is higher than was previously thought, because we documented a 4.2% frequency in this study. The production of granzyme B by ATLL cells may lead to the destruction of vessels and the development of purpuric eruptions in these patients.24 The prognosis for the purpuric type of skin lesion has not been investigated because of the rarity of this type. There have been 9 cases of the purpuric type reported in the literature.24-31 When these are divided into the punctate and macular subtypes, the prognosis of the punctate purpuric subtype might be better than the macular purpuric subtype.24-31 In our 5 purpuric cases, 2 cases of the punctuate purpuric subtype survived, with a 73.4-month mean survival time (the MST was not estimable), whereas 3 cases of the macular purpuric subtype died with 2.1 months of the MST. This suggests that the punctuate subtype has a good clinical prognosis, and the poor prognosis of the total purpuric type is derived from the macular subtype.
In addition to the purpuric type, the erythrodermic type is a rare skin manifestation in ATLL patients, with a prevalence of 3.5% reported in a previous study19 and 4.2% in the present study. The majority of ATLL cases associated with the erythrodermic type of skin lesion are aggressive. In our study, all patients with erythrodermic lesions also belonged to the acute type and had the poorest prognosis among all skin eruption types. In patients with cutaneous T-cell lymphoma (CTCL), the erythrodermic type is typically termed SS and also has a poor prognosis.16 In some erythrodermic CTCL patients, the decreased expression of intercellular adhesion molecule-1 by keratinocytes may lead to an inability of malignant T cells to enter the epidermis and infiltrate the blood and other organs.32 This pathomechanism in erythrodermic CTCL can also be applied to ATLL, resulting in poor prognosis. Skin biopsy specimens of the erythrodermic type of ATLL revealed scant tumor cell infiltration into the epidermis.33,34
We applied MF/SS classification T stages to ATLL assessment, and demonstrated that the OS was worse as the T stage became more advanced. The results shown in Table 3 indicated that the prognosis of T1 stage was better than that for T2, suggesting that the difference in the body surface area of skin lesions is associated with the prognosis of ATLL. Moreover, the prognosis of T3 patients was poorer than those of T1 and T2, indicating that the depth of tumor-cell infiltration is associated with survival rate. T-stage classification accurately reflects the prognosis of ATLL and MF/SS. However, the multipapular and purpuric types are not applicable to T stage. We found that the multipapular type and T2 had similar outcomes, and that the purpuric type had a significantly poorer prognosis than T1. This may provide clinically useful information for patient management and choice of therapy. Moreover, our present study demonstrated that skin eruption is an independent prognostic factor for ATLL patients: the presence of skin eruptions may indicate poorer outcome compared with no eruptions. Therefore, evaluation of skin lesions and treatments targeting the skin may be important for improving clinical outcome.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank R. Ide (Department of Work Systems and Health, Institute of Industrial Ecological Sciences, University of Occupational and Environmental Health) and Y. Miyamura (Department of Environmental Epidemiology, University of Occupational and Environmental Health) for advising on the statistical analyses.
This work was supported by Grants-in-Aid for Science Research from the Ministry of Education, Science, Sports, and Culture of Japan.
Authorship
Contribution: Y.S. collected and analyzed the data and wrote the manuscript; R.H. analyzed the data; K.H. collected the data; S.O., H.F., S.Y., S.F., M.T., R.K., M.Y., D.N., K.S., R.Y., T.S., T.M., K.I., M.K., and M.N. diagnosed and treated ATLL patients; and Y.T. organized the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yu Sawada, MD, Department of Dermatology, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8555, Japan; e-mail: long-ago@med.uoeh-u.ac.jp; or Ryosuke Hino, MD, PhD, Department of Dermatology, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8555, Japan; e-mail: hinoti@med.uoeh-u.ac.jp.