Abstract
Natural killer (NK) cells are innate immune cells that express members of the leukocyte β2 integrin family in humans and mice. These CD11/CD18 heterodimers play critical roles in leukocyte trafficking, immune synapse formation, and costimulation. The cell-surface expression of one of these integrins, CD11b/CD18, is also recognized as a major marker of mouse NK-cell maturation, but its function on NK cells has been largely ignored. Using N-ethyl-N-nitrosourea (ENU) mutagenesis, we generated a mouse carrying an A → T transverse mutation in the Itgb2 gene, resulting in a mutation that prevented the cell-surface expression of CD18 and its associated CD11a, CD11b, and CD11c proteins. We show that β2 integrin–deficient NK cells have a hyporesponsive phenotype in vitro, and present an alteration of their in vivo developmental program characterized by a selective accumulation of c-kit+ cells. NK-cell missing-self recognition was partially altered in vivo, whereas the early immune response to mouse cytomegalovirus (MCMV) infection occurred normally in CD18-deficient mice. Therefore, β2 integrins are required for optimal NK-cell maturation, but this deficiency is partial and can be bypassed during MCMV infection, highlighting the robustness of antiviral protective responses.
Introduction
Natural killer (NK) cells are lymphocytes that contribute to the innate immune system. They can be cytotoxic and secrete an array of cytokines and chemokines such as interferon-γ (IFN-γ) and β-chemokines. NK cells are involved in tumor recognition, hematopoietic allograft rejection, control of microbial infections, and pregnancy.1 NK-cell activation is regulated by a large number of cell-surface receptors, including activating receptors, inhibitory receptors, and adhesion molecules.2,3 Upon interaction with neighboring cells, the integration of these pathways governs NK-cell effector function.4
NK cells undergo maturation through the recognition of self-molecules that are constitutively expressed under steady-state conditions. A prototypical example of this process is major histocompatibility complex (MHC) class I–induced NK-cell education. Indeed, NK cells need to be educated to become competent to recognize “missing-self” (ie, the absence of self-MHC class I molecules).5-7 In the mouse model, missing-self recognition is exemplified by the rejection of MHC class I–deficient hematopoietic cells by NK cells after their transfer to wild-type (WT) mice. This education process requires the engagement of inhibitory receptors such as killer cell immunoglobulin-like receptors (KIRs) in humans, Ly49 molecules in mice, and CD94/NKG2A in both species by cognate self-MHC class I molecules.8-10 In addition, like T cells, NK cells require priming for full activation. The molecules involved in NK-cell priming include such cytokines as interleukin-15 (IL-15) and IL-18 in the mouse.11,12
During their development, mouse NK cells sequentially acquire an array of cell-surface molecules that define distinct NK-cell subsets.13 Among them, the CD11b integrin has been defined as a major marker of NK-cell maturation.14,15 CD11blow NK cells, which are more abundant in the bone marrow and lymph nodes, develop into CD11bhigh NK cells with functional attributes of mature NK cells (ie, they are more prone to produce IFN-γ and to exert cell cytotoxicity).15 Despite these data, the actual role of CD11b in the maturation of NK cells has not been investigated.
Integrin family members are cell-adhesion molecules mediating cell-cell, cell-extracellular matrix, and cell-pathogen interactions that are critical for mounting an effective immune response.16,17 They are involved in leukocyte trafficking, migration, immune synapse formation, and costimulation. Integrins at the surface of lymphocytes undergo changes in their adhesive activity after stimulation. This adhesiveness can be regulated through a process called inside-out signaling—from the intracellular domains through the cell membrane to the extracellular region. Moreover, integrin binding to their ligands transduces signals from the extracellular domain to the cytoplasm in the classic outside-in signaling direction.16,17
In the present study, we describe an N-ethyl-N-nitrosourea (ENU)–induced mutation named Joker, first identified because of a lack of CD11b expression. The mutation was mapped to the Itgb2 locus encoding the β2 integrin CD18 that forms noncovalently linked dimers with integrin α subunits (CD11a, CD11b, CD11c, and CD11d) to generate functional integrin receptors.16 CD11/CD18 integrins bind to a number of receptors, such as intercellular adhesion molecule-1 (ICAM-1), ICAM-2, and ICAM-3, members of the immunoglobulin gene superfamily, and other proteins such as inactivated C3b and fibrinogen.16,17 Heterodimers of CD11a/CD18 (also referred as to LFA-1 [lymphocyte function–associated antigen-1]), CD11b/CD18 (also referred as to Mac-1 and CR3 [complement receptor 3]), and CD11c/CD18 (also referred as to CR4) are expressed on human and mouse NK cells. LFA-1 has been shown to be involved in tumor recognition and elimination by NK cells.18-21 In mice, unlike in humans, CD11d/CD18 is not expressed on lymphocytes.22 Using the Joker mutant, we tested the role of the β2 integrins on mouse NK-cell development and effector functions in vitro and in vivo.
Methods
Mice and ENU mutagenesis
C57BL/6J, C3H/HeN, Unc13djinx/jinx (MGI: 3628822; MMRRC: 016137-UCD), and Itgb2Jkr/Jkr (Joker; MGI: 3808883) mice were maintained and bred under standard housing at the Scripps Research Institute vivarium under the supervision of the Department of Animal Resources and at the Center of Immunology of Marseille-Luminy. ENU mutagenesis was performed on a C57BL/6J background, as described previously.23 All experimental procedures were approved by and performed in accordance with the rules of the Institutional Animal Care and Use Committee of The Scripps Research Institute and were approved by the local ethical committee at the Université de la Méditerranée. The Itgb2Jkr/Jkr mutant stock was transferred to the Mutant Mouse Regional Resources Centers for distribution (stock no. 016138-UCD).
Genetic mapping and positional cloning
Unc13djinx/jinx/Joker mice were outcrossed to C3H/HeN mice and F1 hybrids were backcrossed to Unc13djinx/jinx/Joker heterozygous and homozygous mice. Sixteen mice with the Joker phenotype were genotyped by fragment-length analysis with fluorescent primers of 128 microsatellite polymorphism markers. Genotyping and sequencing of the Itgb2 gene and cDNA were done using the Applied Biosystems 3100 DNA sequencer.
In vitro assay for NK-cell function
NK cells were isolated from splenocyte suspensions using the NK-cell isolation kit from Miltenyi Biotec (no. 130-090-864). NK cells were counted and distributed in a 96-well 2HB Immulon plate precoated with 25 μg/mL of purified anti-NK1.1 monoclonal antibodies (mAbs, PK136; eBiosciences), 5 μg/mL of purified anti-Ly49D mAbs (4E5; eBiosciences), or 25 μg/mL of purified goat anti–NKp46 antibodies (R&D Systems), or their respective isotype control antibodies. YAC-1, a Moloney virus–induced lymphoma of the A/Sn strain that down-regulates its MHC class I expression on prolonged in vitro culture, is a prototypical NK-cell target. YAC-1 cells express ICAM-1, one of the ligands of LFA-1. In some experiments, NK cells were activated by adding 200 000 YAC-1 cells/well or with 10 ng/mL of recombinant IL-12 alone or combined with 20 ng/mL of IL-18 in the presence of monensin (Golgi-Stop; BD Biosciences) or with a mix of phorbol 12-myristate 13-acetate (PMA; 200 ng/mL) and ionomycin (5 μg/mL). Degranulation of NK cells was detected by adding fluorescein isothiocyanate (FITC)–conjugated anti-CD107a mAbs (1D4B, eBiosciences), and incubating for 4-5 hours at 37°C in the dark. For intracellular IFN-γ detection, cells were fixed and permeabilized using the Cytofix/Cytoperm kit (BD Pharmingen), followed by intracellular staining using Perm/Wash (BD Pharmingen).
Antibodies and flow cytometry
mAbs used for flow cytometry were: anti-NKp46 (29A1.4), anti-NK1.1 (PK136)–allophycocyanin (APC), anti–CD49b-FITC (DX5), anti-CD3 (145-2C11)–PerCP-Cy5.5, anti–IFN-γ (XMG1.2)–PE, and anti–CD107 (1D4B)–FITC (BD Biosciences); and anti–NKp46 (29A1.4)–Alexa Fluor 647. NK-cell maturation was assessed by anti–KLRG1 (2F1)–APC, anti–CD27 (LG.3A10)–PE, anti–CD18-FITC (C71/16), anti–CD43-FITC (S7), anti–NKG2A-Biot (16a11), anti–Ly49A-FITC (A1), anti–Ly49C/I-PE (5E6), anti–Ly49D-FITC (4E5), anti–Ly49G2-FITC (4D11), anti–Ly49H-APC (3D10), NKG2D-Biot (CX5), anti–Ly49F-PE (HBF-719), and anti–c-kit-PE (2B8). Fc receptors were blocked by incubation with anti-FcγRII/III (2.4G2) during staining. Samples were analyzed using a FACSCalibur or a FACSCanto II (BD Biosciences) and FlowJo Version 7.6 software (TreeStar).
Generation of mixed BM chimeras
Recipient mice (C57BL/6-Ly5.1, 9-week-old male; Charles River Laboratories) were conditioned by 1000 rad of lethal irradiation. Donor BM cells were obtained from femurs and tibias of 11-week-old male C57BL/6J (Ly5.2 or Ly5.1) or Itgb2Jkr/Jkr (Ly5.2) mice, and Ly5.1+ and Ly5.2+ cells were mixed at a 1:1 ratio. These donor cells (4 × 106/mouse) were injected intravenously (retro-orbitally) into recipient mice 4 hours after irradiation, and these mixed–BM chimeras were kept on antibiotic-containing water (0.28% pediatric suspension of Bactrim; Roche) for 3 weeks after injection. Sixteen to 22 weeks later, spleens were collected for functional analysis. Donor cells were identified by anti–CD45.1 (Ly5.1; A20)–Pacific Blue and anti–CD45.2 (Ly5.2; 104)–Alexa Fluor 700 staining.
In vivo cytotoxicity assay
Two mixed populations of syngenic, MHC class I–deficient splenocytes from Tap1−/− and WT donors were differentially labeled with carboxyfluorescein succinimidyl ester (CFSE; Sigma-Aldrich). A total of 4 × 106 cells were injected intravenously into recipient mice, which were bled 2 days after injection. NK-mediated killing was assessed by flow cytometry by comparing the disappearance of Tap1−/− relative to WT cells.
MCMV infection, cytokine measurement, and viral titration
The mouse cytomegalovirus (MCMV) Smith strain was prepared from homogenates of BALB/cByJ salivary glands. Mice were infected intraperitoneally with 105 plaque-forming units (PFU) per mouse, a dose that allows discrimination between the naturally resistant strain C57BL/6J and the susceptible strain BALB/cByJ.24 The serum was collected 36-38 hours after infection, when cytokines peak in response to MCMV.25 Amounts of serum IFN-γ, IL-12p40, and IL-12p70 were measured by enzyme-linked immunosorbent assay (eBiosciences). Measurement of the viral titer in spleens was performed after serial dilutions of organ homogenates in Dulbecco modified Eagle medium and 3% fetal bovine serum that were incubated on 3T3-NIH cell overlays for 2 hours to allow virus attachment. Cells were covered with prewarmed carboxymethylcellulose Dulbecco modified Eagle medium, cultured for 5 more days, then fixed with formalin and stained with crystal violet.
Results
Joker, an ENU-induced phenotype caused by a partial deletion of the CD18 ectodomain
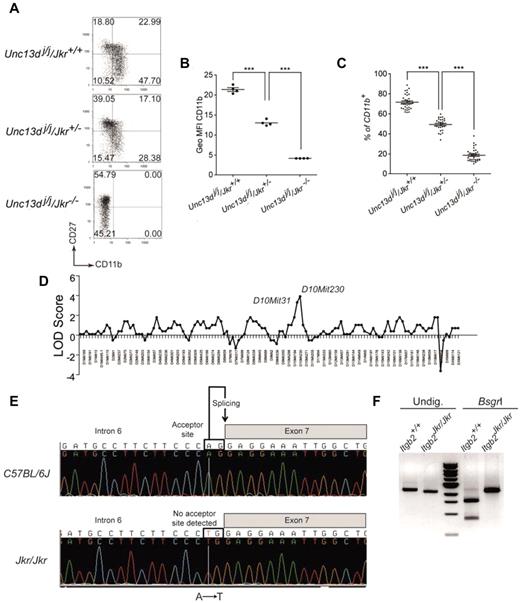
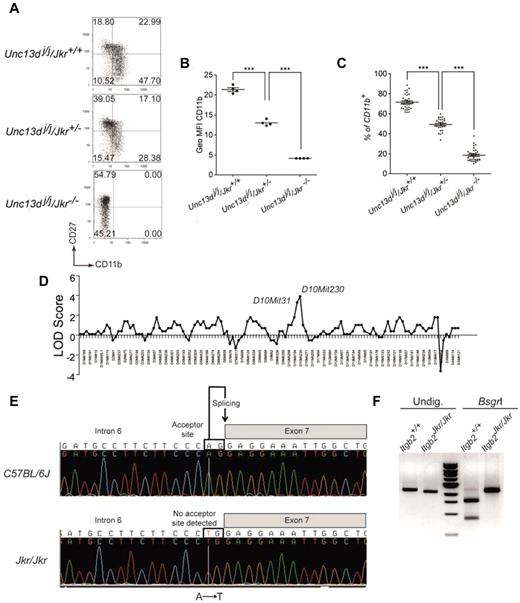
In the mouse, 4 subsets of circulating NK cells can be distinguished based on the expression of CD11b and CD27.15,26 NK cells differentiate from the CD11blowCD27low phenotype to the most mature CD11b+CD27low NK cells via CD11blowCD27+ NK cells and CD11b+CD27+ double-positive NK cells. The Joker (Jkr) phenotype was detected during an analysis of NK-cell maturation in the Jinx mutant background. The Jinx mutant mice, isolated during an ENU mutagenesis screen, exhibited defects in NK-cell degranulation because of a mutation altering Unc13d function.26,27 Unexpectedly, the CD11b/CD27 staining of splenic NK cells of several Jinx mice coming from the same litter showed disparate results. While some mice had NK cells showing a normal phenotype, others had NK cells displaying either a complete loss or an intermediate expression of CD11b (Figure 1A). Results from generation testing of Jinx breeding stock strongly suggested that the observed phenotype, called Joker, was caused by an autosomal recessive mutation affecting the normal expression of CD11a, CD11b, and CD11c on all types of leukocytes (data not shown). Using the cell-surface expression of CD11b on blood NK cells as a readout for the Joker phenotype (Figure 1B-C), the mutation was mapped to chromosome 10 between D10Mit31 (67.71 Mb) and D10Mit230 (89.65 Mb; Ensembl, Mouse Genome Assembly m37), a region encompassing 370 genes (Figure 1D). This region encompasses the Itgb2 gene, which contains 16 exons encoding the β2 integrin CD18. Sequencing of Itgb2 disclosed an A → T transversion within the acceptor splice site between exons 6 and 7 (Figure 1E). Although the resulting mRNA misses the entire exon 7 (Figure 1F), the coding region remains in-frame and the mutated protein is predicted to have an extracellular region devoid of its hybrid domain. The hybrid domain of the CD18 is thought to have bending properties that allow shape changes through the subunits of the integrins by moving the head of the heterodimer.28,29 This domain is critical in providing inside-out and outside-in signaling to the cells.30 Thus, the lack of surface expression of CD18 and its associated chains CD11a, CD11b, and CD11c in leukocytes from Joker mice (referred as to Itgb2Jkr/Jkr mice hereafter) suggests a role for the CD18 hybrid domain in the cell membrane expression of all β2 integrins. Our observations reported on the Jinx mutant mice were done on an Itgb2+/+ background27 ; all subsequent analyses of the Itgb2Jkr/Jkr mice were performed on a WT background.
CD11b expression on Joker leukocytes, genetic mapping, and identification of the Joker mutation. (A) CD27 and CD11b expression on Joker (Jkr) NK cells on the Jinx (Unc13dj/j) background. Splenic NK cells were defined as NK1.1+CD3ϵ− cells. The level of CD11b and CD27 expression on NK cells, as well as the distribution of each NK-cell subset in quadrants, are comparable in C57BL/6J mice (data not shown) and Unc13dj/j/Jkr+/+ mutants. Each dot plot represents data for 1 mouse of 5 tested for each genotype. (B) Geometric mean fluorescence intensity (Geo MFI) of CD11b expression on NK cells of Joker heterozygous (Jkr+/−) and homozygous mutants (Jkr−/−) on the Unc13dj/j background. (C) Percentages of CD11b+ NK cells in the blood of Joker homozygous and heterozygous mutants on the Unc13dj/j background. (D) The genetic linkage of Joker was performed on 16 mice using a panel of 128 informative markers. The Joker mutation was confined between D10mit31 and D10mit230 on chromosome 10. (E) Genomic sequence from a part of intron 6 of Itgb2 reveals an A → T transversion. Introns of eukaryotic genes have 2 distinct nucleotides at either end. At the 3′ end, the nucleotides are AG and constitute the acceptor splice site of the intron (depicted). The A → T transversion in intron 6 of Itgb2Jkr affects the A nucleotide of the AG acceptor site, impairing its recognition by the splicing machinery. In Joker mice, the donor site of intron 6 interacts with the acceptor site of intron 7, leading to the complete splicing of intron 6, exon 7, and intron 7 from the mRNA. (F) Effect of the Joker mutation at the mRNA level. The Joker cDNA lacks the 242 bp of exon 7. BsgrI cuts the WT cDNA once in exon 7 of 2456-bp cDNA amplification fragment, leading to 875-bp and 1581-bp bands. In the 242-bp deletion of exon 7 in Joker cDNA, the nucleotides are insensitive to BsgrI digestion and no WT transcript is detectable.
CD11b expression on Joker leukocytes, genetic mapping, and identification of the Joker mutation. (A) CD27 and CD11b expression on Joker (Jkr) NK cells on the Jinx (Unc13dj/j) background. Splenic NK cells were defined as NK1.1+CD3ϵ− cells. The level of CD11b and CD27 expression on NK cells, as well as the distribution of each NK-cell subset in quadrants, are comparable in C57BL/6J mice (data not shown) and Unc13dj/j/Jkr+/+ mutants. Each dot plot represents data for 1 mouse of 5 tested for each genotype. (B) Geometric mean fluorescence intensity (Geo MFI) of CD11b expression on NK cells of Joker heterozygous (Jkr+/−) and homozygous mutants (Jkr−/−) on the Unc13dj/j background. (C) Percentages of CD11b+ NK cells in the blood of Joker homozygous and heterozygous mutants on the Unc13dj/j background. (D) The genetic linkage of Joker was performed on 16 mice using a panel of 128 informative markers. The Joker mutation was confined between D10mit31 and D10mit230 on chromosome 10. (E) Genomic sequence from a part of intron 6 of Itgb2 reveals an A → T transversion. Introns of eukaryotic genes have 2 distinct nucleotides at either end. At the 3′ end, the nucleotides are AG and constitute the acceptor splice site of the intron (depicted). The A → T transversion in intron 6 of Itgb2Jkr affects the A nucleotide of the AG acceptor site, impairing its recognition by the splicing machinery. In Joker mice, the donor site of intron 6 interacts with the acceptor site of intron 7, leading to the complete splicing of intron 6, exon 7, and intron 7 from the mRNA. (F) Effect of the Joker mutation at the mRNA level. The Joker cDNA lacks the 242 bp of exon 7. BsgrI cuts the WT cDNA once in exon 7 of 2456-bp cDNA amplification fragment, leading to 875-bp and 1581-bp bands. In the 242-bp deletion of exon 7 in Joker cDNA, the nucleotides are insensitive to BsgrI digestion and no WT transcript is detectable.
NK-cell maturation in Itgb2Jkr/Jkr mice
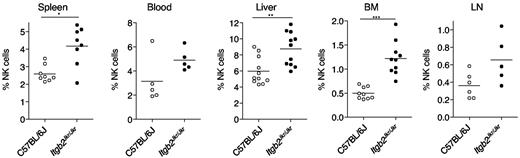
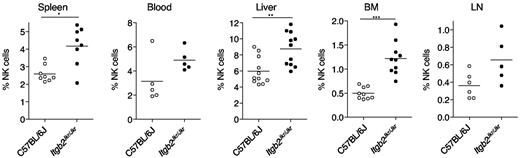
The cell-surface expression of CD11b is a major marker of NK-cell maturation, but its direct role in the maturation process is still obscure. We took advantage of Itgb2Jkr/Jkr mice to address this issue. Lymphocyte counts were normal in spleen, liver, BM, and blood of Itgb2Jkr/Jkr mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, we detected in the BM, liver, and spleen of Itgb2Jkr/Jkr mice a significant increase in NK-cell frequency compared with WT control mice (Figure 2). A similar trend was also observed in blood and LNs, but did not reach statistical significance.
Accumulation of NK cells in Itgb2Jkr/Jkr mice. The percentages of NK cells (CD3−NK1.1+ cells in the lymphocyte gate) present in indicated organs from WT (○) and Itgb2Jkr/Jkr (●) mice are indicated. Each dot represents the data obtained for 1 mouse.
Accumulation of NK cells in Itgb2Jkr/Jkr mice. The percentages of NK cells (CD3−NK1.1+ cells in the lymphocyte gate) present in indicated organs from WT (○) and Itgb2Jkr/Jkr (●) mice are indicated. Each dot represents the data obtained for 1 mouse.
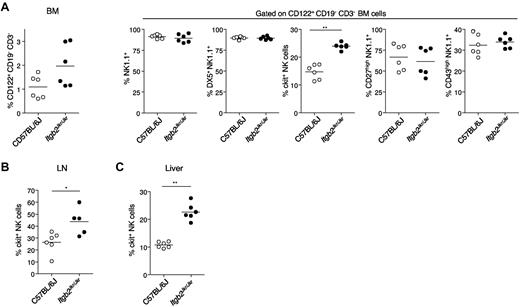
NK cells originate from the BM, where several stages of maturation have been described.14 Early BM NK progenitors (NKp, stage I), classically defined by the CD122+lin− cell-surface phenotype, differentiate into CD122+NK1.1+ immature NK cells (iNK, stages II and III). Stage II NK cells are characterized by the acquisition of NK.1.1, whereas stage III NK cells express c-kit (the stem cell factor receptor CD117). Stage IV NK cells further acquire at the cell surface the CD49b β1 integrin DX5 and the CD11b β2 integrin at late stage IV and the mature stage V.31 At the later stage of NK-cell maturation, c-kit and CD27 expression are lost, whereas CD43 is expressed. Using these maturation markers, we further analyzed the phenotype of CD122+CD3−CD19− cells that accumulate in the BM of Itgb2Jkr/Jkr mutant mice (Figure 3A). The increase in c-kit+ NK cells appeared to be selectively responsible for the increase in BM NK cells observed in Itgb2Jkr/Jkr mutant mice. The selective accumulation of c-kit+ NK cells was also detected in the LNs (Figure 3B), liver (Figure 3C), and spleen (data not shown) of Itgb2Jkr/Jkr mice. Therefore, β2 integrins are involved in NK-cell differentiation. The accumulation of c-kit+ NK cells in the Itgb2Jkr/Jkr mutants indicates that the cell-surface expression of β2 integrins at late stage IV help in the transition from c-kit+ stage IV NK cells into c-kit− stage V mature NK cells. In contrast to c-kit, the expression of CD49b β1 integrin, CD27, NK1.1, and CD43 was not affected in any of the organs tested (BM, spleen, LNs, liver; data not shown). In addition, the expression of several cell-surface receptors, including the activating receptors NKp46, Ly49H, Ly49D, and NKG2D and the inhibitory receptors Ly49C/I, Ly49F, Ly49G2, Ly49A, and NKG2A, was normal on splenic NK cells from Itgb2Jkr/Jkr mice (supplemental Figure 2).
Accumulation of c-kit+ NK cells in Itgb2Jkr/Jkr mice. (A) BM cells from WT (○) and Itgb2Jkr/Jkr (●) mice were analyzed for the cell-surface expression of CD122, CD3, and CD19. The cell-surface expression of NK1.1, CD49b (DX5), c-kit, CD27, and CD43 was analyzed on CD122+CD3−CD19− BM cells. (B) The percentages of c-kit+ NK cells present in LNs and liver is indicated. Each dot represents the data obtained for 1 mouse.
Accumulation of c-kit+ NK cells in Itgb2Jkr/Jkr mice. (A) BM cells from WT (○) and Itgb2Jkr/Jkr (●) mice were analyzed for the cell-surface expression of CD122, CD3, and CD19. The cell-surface expression of NK1.1, CD49b (DX5), c-kit, CD27, and CD43 was analyzed on CD122+CD3−CD19− BM cells. (B) The percentages of c-kit+ NK cells present in LNs and liver is indicated. Each dot represents the data obtained for 1 mouse.
Itgb2Jkr/Jkr mice present a NK-cell–intrinsic functional defect in vitro
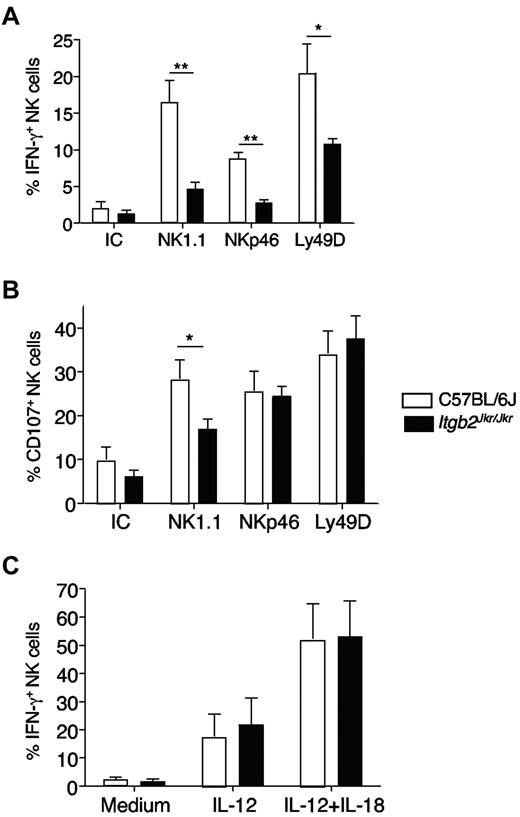
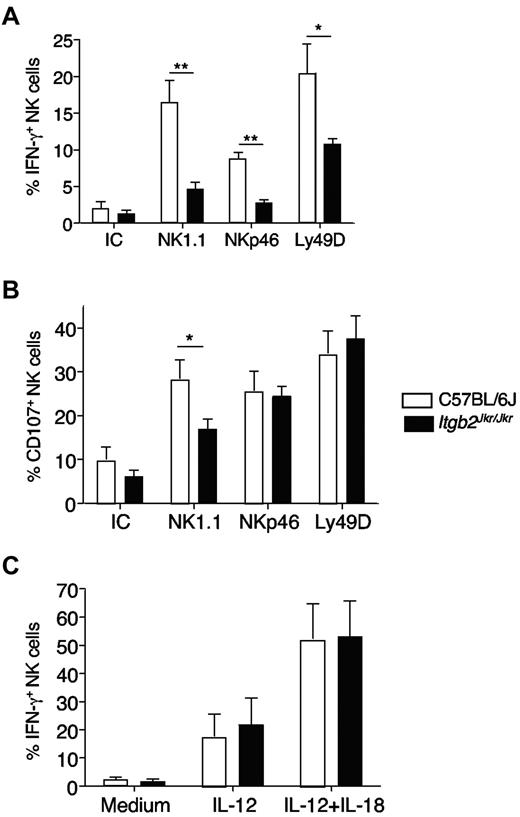
We next addressed the role of β2 integrins in NK-cell function. We first tested NK-cell responsiveness by measuring both NK-cell IFN-γ production and degranulation (monitored by CD107 surface exposure) by flow cytometry. We used a cell-free system based on the triggering of stimulatory receptors expressed on splenic NK cells (NK1.1, NKp46, Ly49D) by antibody-coated plates, because the Joker mutation did not affect the expression level of these receptors (supplemental Figure 2 and data not shown). Itgb2Jkr/Jkr NK cells were hyporesponsive to stimulation through the 3 activating receptors NK1.1, NKp46, and Ly49D (Figure 4). This defect was more pronounced when IFN-γ production was used as a readout for NK-cell activation (Figure 4A). NK-cell degranulation was affected only when NK1.1 was triggered (Figure 4B). The differences observed between IFN-γ secretion and CD107a exposure likely reflected a disparity in the activation threshold required to trigger these functions, as described previously.7 Finally, NK cells from Itgb2Jkr/Jkr mice were fully responsive to the cytokines IL-12 and IL-18, showing that not all of the pathways leading to IFN-γ production were altered (Figure 4C).
In vitro hyporesponsiveness of NK cells from Itgb2Jkr/Jkr mice. NK cells from WT (open histograms) or Itgb2Jkr/Jkr (black histograms) mice were purified from the spleen and tested for their responsiveness ex vivo. NK-cell degranulation (CD107a exposure) and IFN-γ production were tested by flow cytometry after surface and intracellular stainings, respectively. (A-B) Purified NK cells were activated by antibody-coated plates using anti-NK1.1, anti-NKp46, and anti-Ly49D antibodies or a mixture of the respective isotype controls (IC) for 4 hours. (C) NK cells were incubated with IL-12 or a mixture of IL-12 and IL-18 for 4 hours. Results are expressed as the percentage of NK cells (NK1.1+CD3− or NKp46+ cells) expressing CD107a or intracellular IFN-γ. Data represent means ± SEM. Statistical analysis was performed using a one-tailed Mann-Whitney test.
In vitro hyporesponsiveness of NK cells from Itgb2Jkr/Jkr mice. NK cells from WT (open histograms) or Itgb2Jkr/Jkr (black histograms) mice were purified from the spleen and tested for their responsiveness ex vivo. NK-cell degranulation (CD107a exposure) and IFN-γ production were tested by flow cytometry after surface and intracellular stainings, respectively. (A-B) Purified NK cells were activated by antibody-coated plates using anti-NK1.1, anti-NKp46, and anti-Ly49D antibodies or a mixture of the respective isotype controls (IC) for 4 hours. (C) NK cells were incubated with IL-12 or a mixture of IL-12 and IL-18 for 4 hours. Results are expressed as the percentage of NK cells (NK1.1+CD3− or NKp46+ cells) expressing CD107a or intracellular IFN-γ. Data represent means ± SEM. Statistical analysis was performed using a one-tailed Mann-Whitney test.
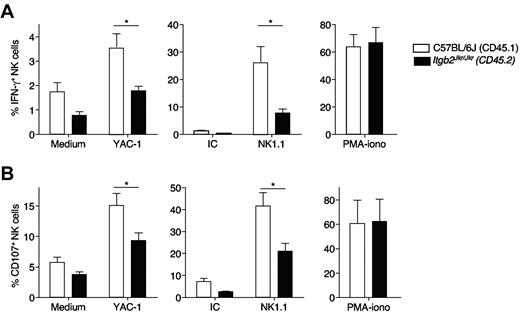
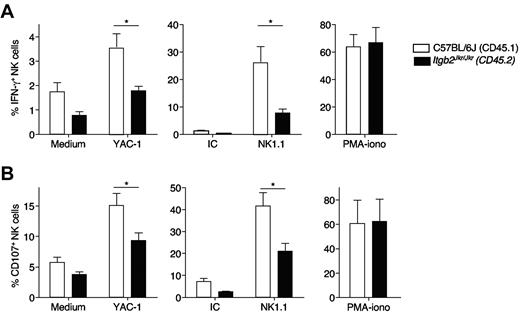
β2 integrins and their ligands are expressed on a large number of different cell types, not just on NK cells. Therefore, we investigated whether the NK-cell hyporesponsiveness observed in Itgb2Jkr/Jkr mice was because of the absence of β2 integrins either on NK cells or on cells present in their environment. To address this question, we performed experiments with mixed BM chimeras. Bone marrow cells from CD45.2+Itgb2Jkr/Jkr mice were mixed at an equal ratio with BM cells from WT mice expressing the allotypic marker CD45.1, and transferred into irradiated WT CD45.1+ recipients. Splenic NK cells were analyzed 16-22 weeks after reconstitution. Compared with CD45.1+ WT NK cells, CD45.2+Itgb2Jkr/Jkr NK cells developing in a β2 integrin–sufficient environment were hyporesponsive to NK1.1 stimulation both for degranulation and IFN-γ production (Figure 5A-B). These experiments showed that the defect is NK cell intrinsic and that β2 integrin expression on NK cells is necessary for full NK1.1 responsiveness in vitro. This defect affects the proximal signal transduction machinery, because the PMA/ionomycin stimulation that bypasses the surface receptors induced a normal activation in Itgb2Jkr/Jkr NK cells (Figure 5A-B). Moreover, Itgb2Jkr/Jkr NK cells were also hyporesponsive to stimulation with the tumor target cells YAC-1 (Figure 5A-B). Thus, the absence of β2 integrins is associated with an impaired reactivity of NK cells.
In vitro hyporesponsiveness of Itgb2Jkr/Jkr NK cells in mixed BM chimeras. Bone marrow cells from WT (CD45.1+; open histograms) or Itgb2Jkr/Jkr (CD45.2+; black histograms) mice were isolated and mixed at an equal ratio before transfer into irradiated WT (CD45.1+) recipients. Splenocytes from chimeric mice were analyzed 16-22 weeks after reconstitution. NK-cell IFN-γ production (A) and degranulation (CD107a exposure; B) were tested by flow cytometry after activation and surface and intracellular stainings. The responsiveness of WT (CD45.1 staining) and CD18-deficient (Itgb2Jkr/Jkr, CD45.2 staining) on stimulation with the tumor YAC-1, NK1.1 monoclonal antibody-coated plates, or PMA/ionomycin was compared after 4 hours of stimulation. Data are expressed as the percentage of IFN-γ+ (A) and the percentage of CD107a+ (B) in the NK cell gate. n = 7 for each genotype, means ± SEM are shown. Statistical analysis was performed using a one-tailed Mann-Whitney test.
In vitro hyporesponsiveness of Itgb2Jkr/Jkr NK cells in mixed BM chimeras. Bone marrow cells from WT (CD45.1+; open histograms) or Itgb2Jkr/Jkr (CD45.2+; black histograms) mice were isolated and mixed at an equal ratio before transfer into irradiated WT (CD45.1+) recipients. Splenocytes from chimeric mice were analyzed 16-22 weeks after reconstitution. NK-cell IFN-γ production (A) and degranulation (CD107a exposure; B) were tested by flow cytometry after activation and surface and intracellular stainings. The responsiveness of WT (CD45.1 staining) and CD18-deficient (Itgb2Jkr/Jkr, CD45.2 staining) on stimulation with the tumor YAC-1, NK1.1 monoclonal antibody-coated plates, or PMA/ionomycin was compared after 4 hours of stimulation. Data are expressed as the percentage of IFN-γ+ (A) and the percentage of CD107a+ (B) in the NK cell gate. n = 7 for each genotype, means ± SEM are shown. Statistical analysis was performed using a one-tailed Mann-Whitney test.
In vivo killing of TAP1−/− targets in Itgb2Jkr/Jkr mice
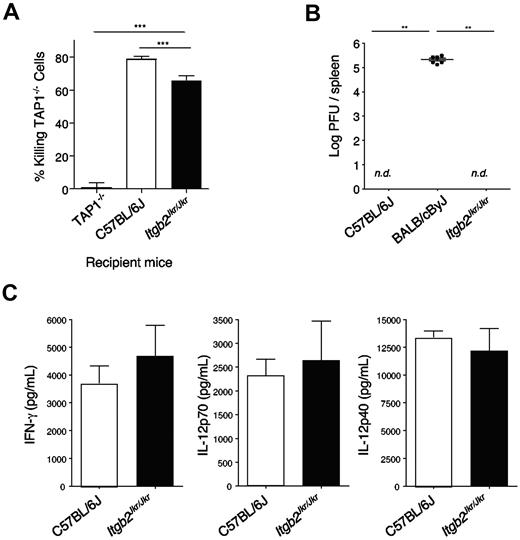
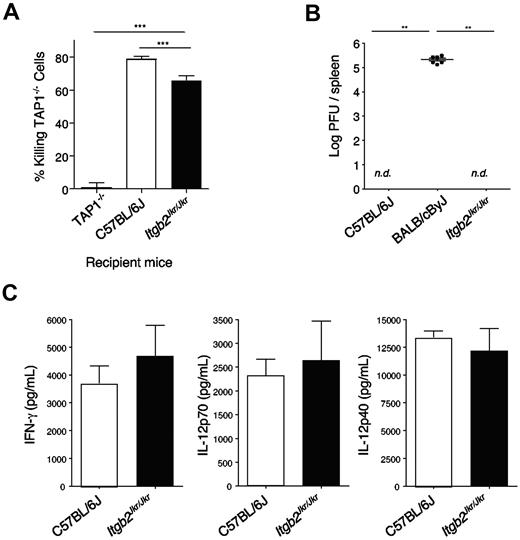
Like in Itgb2Jkr/Jkr mice, uneducated NK cells in MHC class I–deficient mice present a hyporesponsive phenotype after NK1.1 and YAC-1 stimulation,9,10,32 prompting us to test whether the defect observed in these 2 models was similar. A feature of the lack of NK-cell education observed in MHC class I–deficient mice resides in their inability to reject MHC class I–deficient grafts. Therefore, we compared the capacity of MHC class I–deficient (Tap1−/−), Itgb2Jkr/Jkr, and WT mice to reject MHC class I–deficient splenocytes in vivo. Itgb2Jkr/Jkr mice were able to reject these target cells slightly but significantly less efficiently than WT mice (Figure 6A). However, the phenotype was weak compared with the total absence of rejection observed in Tap1−/− mice (Figure 6A). Therefore, NK cell missing-self recognition was partially impaired in the absence of β2 integrins.
In vivo NK-cell function. (A) In vivo killing of MHC class I–deficient splenocytes. Splenocytes from WT or Tap1-deficient mice (Tap1−/−) were isolated, stained with 2 different concentrations of the fluorescent dye CFSE, and transferred intravenously into the recipient mice indicated (Tap1−/−, WT, or Itgb2Jkr/Jkr). Two days after transfer, the frequency on each CFSE+ population was assessed by flow cytometry. Data are represented as the percentage of killing of TAP-1−/− cells. (B-C) Itgb2Jkr/Jkr mice are not susceptible to MCMV infection. (B) Viral loads in spleens of C57BL/6J resistant mice, BALB/cByJ susceptible mice, and Itgb2Jkr/Jkr mutant mice were tested 5 days after infection with 105 PFU of MCMV inoculated intraperitoneally; nd, not detectable; n = 6 for each genotype. (C) Sera of C57BL/6J and Itgb2Jkr/Jkr mice infected with 105 PFU of MCMV were collected at 36 hours after infection, when systemic cytokines peak. IFN-γ, IL-12p70, and IL-12p40 were titrated by enzyme-linked immunosorbent assay performed on the serum; n = 6 for each genotype. These graphs are representative of 1 experiment of 2.
In vivo NK-cell function. (A) In vivo killing of MHC class I–deficient splenocytes. Splenocytes from WT or Tap1-deficient mice (Tap1−/−) were isolated, stained with 2 different concentrations of the fluorescent dye CFSE, and transferred intravenously into the recipient mice indicated (Tap1−/−, WT, or Itgb2Jkr/Jkr). Two days after transfer, the frequency on each CFSE+ population was assessed by flow cytometry. Data are represented as the percentage of killing of TAP-1−/− cells. (B-C) Itgb2Jkr/Jkr mice are not susceptible to MCMV infection. (B) Viral loads in spleens of C57BL/6J resistant mice, BALB/cByJ susceptible mice, and Itgb2Jkr/Jkr mutant mice were tested 5 days after infection with 105 PFU of MCMV inoculated intraperitoneally; nd, not detectable; n = 6 for each genotype. (C) Sera of C57BL/6J and Itgb2Jkr/Jkr mice infected with 105 PFU of MCMV were collected at 36 hours after infection, when systemic cytokines peak. IFN-γ, IL-12p70, and IL-12p40 were titrated by enzyme-linked immunosorbent assay performed on the serum; n = 6 for each genotype. These graphs are representative of 1 experiment of 2.
Itgb2Jkr/Jkr mice are resistant to MCMV infection
Mouse infection by many viruses can be controlled in part by NK cells.33 However, the most compelling evidence for a role of NK cells in early defense against viruses was obtained in studies showing increased susceptibility or resistance to the herpesvirus MCMV after NK-cell depletion or NK-cell adoptive transfer, respectively.34,35 Defects in NK-cell activity, such as decreased production of IFN-γ or cytotoxicity, also render mice more susceptible to MCMV infection.33,36,37 The recognition of the MCMV-infected cells by NK cells plays a central role in host resistance to infection.38 In C57BL/6J mice, resistance to MCMV infection relies on the expression of the activating NK receptor Ly49H, which binds the virally encoded molecule m157 found at the cell surface of infected cells.37,39
To test the role of β2 integrins in the early control of MCMV, we infected WT and Itgb2Jkr/Jkr (both on a C57BL/6J background) and BALB/cByJ mice with a dose of MCMV that allowed discrimination between C57BL/6J resistant mice and the naturally susceptible BALB/cByJ strain. When inoculated with 105 PFU per mouse, C57BL/6J mice are resistant to MCMV infection, whereas BALB/cByJ mice, which do not express the Ly49H receptor, are susceptible24 (Figure 6B). NK cell–depleted C57BL/6J mice died from MCMV at day 5 after infection, and no IFN-γ was detectable in their serum 36 hours after infection (data not shown), confirming that under these experimental conditions, resistance to MCMV infection is NK cell–dependent. No virus was detectable 5 days after infection in the spleen of WT or Itgb2Jkr/Jkr mice, showing that MCMV infection is controlled in the absence of β2 integrins. Moreover, cytokines were measured in the serum of infected mice 36-38 hours after infection, when cytokines peak in response to MCMV.25 IFN-γ, IL-12p70, and IL-12p40 production were not affected in Itgb2Jkr/Jkr mice (Figure 6C). β2 integrins are therefore dispensable for the early NK cell–dependent immune response against MCMV infection.
Discussion
The requirement for β2 integrins for optimal target-cell recognition and subsequent lysis has been highlighted by previous studies in cytotoxic T lymphocytes and NK cells, but the role of these molecules during the development of NK cells is still unclear.40-44 Considering the importance of β2 integrins for the establishment of cell-cell contacts, and the need for NK cells to be educated to acquire their full effector functions, we sought to investigate the consequences of an overall decrease in the cellular interacting capacity on NK-cell functions. We took advantage of the ENU-generated Itgb2Jkr/Jkr mouse mutant in which the β2 integrin CD18 is mutated, leading to the absence of expression of CD11a, CD11b, and CD11c at the cell surface of NK cells and all other hematopoietic cells. Although no complete block of NK-cell development was observed, we found an accumulation of c-kit+ NK cells in all organs, revealing the importance of β2 integrins at the transition between stage IV c-kit+ NK cells and stage V mature c-kit− NK cells. The appearance of surface CD11b at late stage IV of NK-cell development strongly supports the role of CD11b in the developmental defect of NK cells observed in Itgb2Jkr/Jkr mice.15
Our study also showed that β2 integrins are involved in tumor cell (YAC-1) recognition in vitro. This is corroborated by previous studies demonstrating that IL-2– or IL-12–activated, LFA-1–deficient NK cells are impaired in their ability to kill several tumors, including YAC-1.19,20 This defect was likely the consequence of a decreased capacity to form conjugates with target cells expressing some ligands of LFA-1.19 However, in a cell-free system in which only specific activating receptors are triggered, we found that Itgb2Jkr/Jkr NK cells were also hyporesponsive, suggesting that the ability of the various triggering receptors to transduce activating signals was not fully conserved in NK cells lacking β2 integrin expression. Interestingly, this hyporesponsive phenotype was NK cell intrinsic, because it was conserved even when Itgb2Jkr/Jkr NK cells developed in a WT environment. The lack of β2 integrins on NK cells therefore not only leads to an impaired capacity to recognize target cells, but also to a general decrease in NK-cell responsiveness. The higher percentage of c-kit+ NK cells could also account for the hyporesponsive phenotype of Itgb2Jkr/Jkr NK cells, because the effector functions of c-kit+ NK cells are lower than those of c-kit− NK cells. We favor this hypothesis; however, we cannot rule out a potential direct involvement of CD18 expression on ITAM-bearing receptor signaling. Nevertheless, NK cells from Itgb2Jkr/Jkr mice retain the capacity to reject MHC class I–deficient splenocytes in vivo. NK-cell education to missing-self recognition can thus occur in the absence of β2 integrins. However, we found that rejection of Tap1−/− splenocytes was slightly but significantly lower in Itgb2Jkr/Jkr recipients than in WT mice. This weak defect may be related to the hyporesponsiveness observed in vitro or to a partial role of β2 integrins in the recognition of these targets.
Using MCMV infection as a readout of NK-cell function in vivo, we showed that Itgb2Jkr/Jkr mice could clear the virus as efficiently as WT mice. LFA-1, CD11b, and CD11c are thus not required for in vivo elimination of MCMV. Similarly, hyporesponsive NK cells raised in a MHC class I–deficient environment are fully functional to control MCMV infection, although they are unable to reject MHC class I–negative target cells.45,46 These data suggest that the inflammatory environment generated during the course of a viral infection triggers alternative pathways of NK-cell activation that overcome the hyporesponsiveness of NK cells when they originate from a β2 integrin- or MHC class I–deficient environment. However, in the case of the Itgb2Jkr/Jkr mice, the increase in NK-cell numbers might also contribute to maintaining an efficient NK-cell response in vivo, as has been shown previously with memory CD8+ T cells, when the number of hyporesponsive cells could overcome the diminished per-cell reactivity.47
In humans, mutations in the CD18 gene are responsible for a rare autosomal recessive disorder called leukocyte adhesion deficiency type I (LAD1; Online Mendelian Inheritance in Man: OMIM116920). In the severe form of the disease, as in Itgb2Jkr/Jkr mice, leukocytes lack the cell-surface expression of all CD18/CD11 heterodimers. Interestingly, LAD1 NK cells express a normal density of triggering NK receptors and coreceptors,48 as we observed in Itgb2Jkr/Jkr mice. However, mAb-mediated engagement of the major activating molecules results in both IFN-γ release and in the induction of cytolytic activity.48 Thus, in vitro NK cell functions in CD18-deficient patients (LAD1) were not found to be drastically affected.48 In that study, IL-2–activated polyclonal NK cells from LAD1 patients were used, whereas in the present study, we used freshly isolated NK cells from Itgb2Jkr/Jkr mice. Therefore, it will be important in future studies to investigate the reactivity of freshly isolated NK cells from LAD1 patients to determine whether the IL-2–dependent culture of NK cells is responsible for the apparent differences between CD18-deficient humans and mice.
The generation and analysis of Itgb2Jkr/Jkr mice revealed that β2 integrins play nonredundant functions in NK-cell maturation and effector function. These data support a model in which CD11b participates in NK-cell maturation, whereas LFA-1 contributes to NK-cell reactivity toward YAC tumor cells. However, Itgb2Jkr/Jkr mice are still able to control MCMV infections, highlighting the efficiency of the compensatory mechanisms that are at play for mounting protective antiviral responses. Itgb2Jkr/Jkr mice might also be useful in illuminating other aspects of the biology of β2 integrins. Indeed, CD18-null mice on a mixed 129/Sv and C57BL/6 background exhibit spontaneous skin ulceration and chronic dermatitis with extensive submandibular erosions.49 The phenotype includes elevated neutrophil counts, increased immunoglobulin levels, lymphadenopathy, splenomegaly, and abundant plasma cells in the skin, LNs, gut, and kidney.49 We did not detect any obvious signs of spontaneous inflammation in Itgb2Jkr/Jkr mice, which were generated on a pure C57BL/6J background. The phenotypic differences observed between these strains of mice therefore suggest that differences at other loci must contribute to the reported pathology, and prompts further examination of these disease-modifier genes whose orthologs might be involved in the variability of the symptoms observed in LAD1 patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Corinne Beziers-Guigue (Center of Immunology of Marseille-Luminy [CIML], Marseille, France) for graphic assistance.
E.V. and S.U. are supported by grants from the Agence Nationale de la Recherche (ANR), Ligue Nationale contre le Cancer (Equipe labellisée “La Ligue”), and Fondation Del Duca, and by institutional grants from Inserm, Centre National de la Recherche Scientifique, and Université de la Méditerranée to the CIML. E.V. is a scholar from the Institut Universitaire de France. P.K. is supported by a fellowship from the Swiss National Science Foundation.
Authorship
Contribution: K.C., C.E., B.N.J., P.K., S.G., and S.U. performed the experiments and analyzed the data; B.B., E.V., and S.U. conceived of and supervised the study; and S.U., E.V., B.N.J., K.C., and B.B wrote the paper.
Conflict of interest disclosure: E.V. is a cofounder and a shareholder of Innate-Pharma. The remaining authors declare no competing financial interests.
The current affiliation for C.E. is Department of Immunology, Genentech Inc, South San Francisco, CA.
Correspondence: Dr Sophie Ugolini, Centre d'Immunologie de Marseille-Luminy (CIML), Campus de Luminy, Case 906, 13288 Marseille Cedex 09, France; e-mail: ugolini@ciml.univ-mrs.fr; or Pr Bruce Beutler, Department of Genetics, The Scripps Research Institute, 10550 North Torrey Pines Rd, SP-293, La Jolla, CA 92037; bruce@scripps.edu.
References
Author notes
K.C., C.E., and B.N.J. contributed equally to this study.