Abstract
In this study, we analyzed IL-2–activated polyclonal natural killer (NK) cells derived from 2 patients affected by leukocyte adhesion deficiency type I (LAD1), an immunodeficiency characterized by mutations of the gene coding for CD18, the β subunit shared by major leukocyte integrins. We show that LAD1 NK cells express normal levels of various triggering NK receptors (and coreceptors) and that mAb-mediated engagement of these receptors results in the enhancement of both NK cytolytic activity and cytokine production. Moreover, these activating NK receptors were capable of recognizing their specific ligands on target cells. Thus, LAD1 NK cells, similarly to normal NK cells, were capable of killing most human tumor cells analyzed and produced high amounts of IFN-γ when cocultured in presence of target cells. Murine target cells represented a common exception, as they were poorly susceptible to LAD1 NK cells. Finally, LAD1 NK cells could efficiently kill or induce maturation of monocyte-derived immature dendritic cells (DCs). Altogether our present study indicates that in LAD1 patients, 3 important functions of NK cells (eg, cytotoxicity, IFN-γ production, and DC editing) are only marginally affected and provides new insight on the cooperation between activating receptors and LFA-1 in the induction of NK cell activation and function.
Introduction
Leukocyte adhesion deficiency type I (LAD1; Mendelian Inheritance in Man [MIM] 116920) is a rare autosomal recessive disorder characterized by delayed separation of the umbilical cord at birth, persistent leukocytosis, and recurring bacterial and fungal infections involving skin and mucosa. The disease is caused by heterogeneous germ-line mutations of a gene located on human chromosome 21q22.3, which encodes for CD18, the β subunit shared by major leukocyte integrins. For example, in the lymphocyte function–associated antigen-1 (LFA-1), CD18 associates with CD11A. In the severe form of the disease, leukocytes lack the expression of CD18/CD11A, CD11B, and CD11C molecules at the cell surface and the course of the disease is dramatic resulting—in the absence of early stem cell transplantation—in death during the first years of life. The clinical features have been proposed to depend on abnormalities of a variety of adhesion-dependent functions of leukocytes including chemotaxis, phagocytosis, and the ability to adhere to the blood endothelium and consequently to migrate to the inflammatory sites.1–7
Of interest, LAD1 patients do not appear to display increased susceptibility to viral infections,8 although the function of cells involved in antiviral responses such as cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells is expected to be impaired by the lack of integrin expression.
The NK-mediated immune responses against virus-infected (or tumor) cells are mediated by the concerted action of multiple triggering receptors. These receptors are represented by NKp46, NKp30, and NKp44 molecules (collectively termed natural cytotoxicity receptors [NCRs]),9 NKG2D,10–13 and the leukocyte adhesion molecule DNAM-1 (CD226).14,15 While the expression of NCR is restricted to NK cells, NKG2D and DNAM-1 molecules are expressed also by T-cell subsets. Moreover, DNAM-1 is present also on monocytes. The simultaneous mAb-mediated masking of NCR, NKG2D, and DNAM-1 virtually abrogates the NK cytotoxicity against most tumor or virus-infected cells, suggesting that these receptors play a key role in NK cell–mediated recognition and killing of various susceptible target cells.16–19 However, additional surface molecules are involved in the process of natural cytotoxicity. These include 2B4 (CD244), NKp80,20 and NTB-A,21,22 which are considered as coreceptors since their triggering function is dependent on the simultaneous engagement of major activating receptors (eg, NCR).
Although the crucial role of adhesion molecules such as LFA-1 has been largely investigated in normal T-,23–33 B-,34–36, and NK37–39 cell–mediated immunologic responses, limited information is available on the NK cell–mediated responses in LAD1 patients. In particular, it has been reported that LAD1 NK cells would be characterized by an impaired activity of the DNAM-1 triggering receptor.40 This finding was explained by the lack of physical and functional association between DNAM-1 and LFA-1 molecules, which was shown to occur in normal NK cells. However, the functional analysis of LAD1 NK cells was confined to a single NK cell clone. Moreover, the only analysis performed on this clone was represented by a redirected killing assay against murine target cells.
In the present study, we analyzed IL-2–activated polyclonal NK cell populations derived from 2 LAD1 patients. These cells were evaluated for the expression of different triggering NK receptors and coreceptors. Moreover, LAD1 NK cells were analyzed for the ability of their activating NK receptors to induce natural cytotoxicity, cytokine production, as well as dendritic cell (DC) maturation.
Patients, materials, and methods
The study was conducted in accordance with a protocol approved by the University of Genova and by the Spedali Civili of Brescia institutional ethics board, and patients provided informed consent, in accordance with the Declaration of Helsinki.
LAD1 patients
Patient 1, a Tunisian 2-year-old male infant born from consanguineous parents, carried a homozygous deletion of 10 nucleotides (191-200) in the CD18 gene, leading to frameshift and premature termination at codon 49. The clinical phenotype was characterized by delayed umbilical cord separation; recurrent skin infections and ulcers without pus formation, associated with marked leukocytosis (38-60 × 109 white blood cells [WBCs]/L); and complete absence of CD18 expression on blood leukocytes. At the time of blood sampling, the child had no infections but was still on antibiotic treatment. The child was successfully treated with haploidentical stem cell transplantation, with full reconstitution of CD18 expression and immune function.
Patient 2, a 5-month-old Italian infant, presented soon after birth with severe omphalitis that required aggressive treatment with multiple antibiotics. Significant and persistent leukocytosis (40-65 × 109 WBCs/L) prompted to investigate LAD1 as a possible diagnosis. Defective expression of CD18 was disclosed. Molecular analysis at the CD18 locus showed a homozygous A151T mutation, resulting in premature termination at codon L27. At the time of blood sampling, the infant had not completely cleared the umbilical infection and was receiving antibiotics. At 7 months of age, the infant received a stem cell transplant from a matched unrelated donor, resulting in mixed chimerism and mild signs of cutaneous graft-versus-host disease (grade 2).
Neither of the 2 infants was under treatment with steroids or other immune-suppressive drugs at the time of blood sampling.
Monoclonal antibodies
C127 (IgG1, anti-CD16); BAB281 and KL247 (IgG1 and IgM anti-NKp46, respectively); AZ20 and F252 (IgG1 and IgM anti-NKp30, respectively); Z231 (IgG1 anti-NKp44); ON72 (IgG1 anti-NKG2D); KRA236 and F5 (IgG1 and IgM anti–DNAM-1, respectively); ECM17 (IgM anti-CD11a); QA196 (IgM anti-CD2); M5A10 (IgG1, anti-PVR); L14 (IgG2A, anti–Nectin-2); A6136 (IgM, anti–HLA class-I); and 289 (IgG2a anti-CD3) were produced in our lab.
Anti-CD1a (IgG1-PE), anti-CD14 (IgG2a), anti-CD83 (IgG2b), and anti-CD86 (IgG2b-PE) were purchased from Immunotech (Marseille, France).
IL-2–activated polyclonal NK cell populations and flow cytofluorimetric analysis
After informed consent, NK cells were purified from peripheral blood mononuclear cells (PBMCs) of the LAD1 patients or of 3 age-matched healthy donors using the Human NK Cell Enrichment Cocktail-RosetteSep (StemCell Technologies, Vancouver, BC). Healthy donors 1, 2, and 3 were 22, 19, and 7 months old, respectively. NK cells were cultured on irradiated PBMCs in the presence of RPMI-1640 medium supplemented with 2 mM glutamine, 50 mg/mL penicillin, 50 mg/mL streptomycin, and 10% heat-inactivated FCS (Invitrogen, Life technologies, Frederick, MD), and in the presence of 100 U/mL rIL-2 (Proleukin; Chiron, Emeryville, CA) and 1.5 ng/mL phytohemagglutinin (PHA; Gibco, Paisley, Scotland) in order to obtain IL-2–activated polyclonal NK cell populations. These cells were cultured for more than 3 weeks and then used for the described experiments.
For one-color cytofluorimetric analysis (FACSCalibur; Becton Dickinson, Mountain View, CA), NK cells were stained with the appropriate mAbs followed by phycoerythrin (PE)–conjugated isotype-specific goat antimouse secondary reagent (Southern Biotechnology Associated, Birmingham, AL).
Cytokine production
NK cells were cultured for 3 weeks in RPMI-1640 medium supplemented with 2 mM glutamine, 50 mg/mL penicillin, 50 mg/mL streptomycin, and 10% heat-inactivated FCS (Invitrogen, Life technologies) in the presence of 100 U/mL rIL-2 (Proleukin; Chiron). Cells were incubated overnight in 96-well flat-bottom tissue culture plates (7 × 105 cells/mL) either in the absence or in the presence of plate-bound purified mAbs of IgG1 isotype at concentrations of 10 μg/mL: C127 (anti-CD16); BAB281 (anti-NKp46); AZ20 (anti-NKp30); Z231 (anti-NKp44); ON72 (anti-NKG2D); KRA236 (anti–DNAM-1); c218 (anti-CD56). IL-2 was present in the medium during the stimulation.
For the evaluation of IFN-γ production by NK cells mixed with sensitive target cells, IL-2–activated NK cell populations were cocultured with human cell lines for 24 hours in 96-well round-bottom tissue culture plates, either in the absence or in the presence of the following mAbs of IgM isotype: F252 (anti-NKp30); F5 (anti–DNAM-1); ECM17 (anti-CD11A); A6220 (anti-CD56).
IFN-γ production by NK cells was measured in supernatants using enzyme-linked immunosorbent assay (ELISA) (IFN-γ; BIOSOURCE, Camarillo, CA).
Cell lines and cytolytic assays
IL-2–activated polyclonal NK cells were tested for cytolytic activity against the indicated target cells in a 4-hour 51Cr-release assay as previously described.16 mAbs were used at concentrations of 2.5 μg/mL (redirected killing assays) or 10 μg/mL (masking experiments). The effector-target (E/T) ratios are indicated in the figure captions.
Target cell lines used in the study were the FcγR+ P815 (murine mastocytoma) cell line (American type culture collection [ATCC], Manassas, VA) or the following FcγR-negative cell lines: HeLa (human cervical carcinoma; ATCC), FO-1, M14 (human melanomas; kindly provided by Dr P. Coulie, Ludwig Institute for Cancer Research, Brussels, Belgium), DAUDI (human Burkitt lymphoma; ATCC), BW5147, YAC (mouse lymphomas; European Collection of Cell Cultures, ECACC, Wiltshire, United Kingdom),16,41 and HTLA230 (human neuroblastoma; kindly provided by Dr V. Pistoia, Istituto G. Gaslini, Genoa, Italy).
The statistical analyses were done using nonparametric tests, Kruskal-Wallis for multiple independent samples, Mann-Whitney for 2 independent samples comparison, and chi-square test for cross-tab analysis. All the tests were performed at the 5% level of significance and were conducted using SAS software (SAS Institute, Cary, NC).
PVR cell transfectants
The cDNA encoding human PVR open reading frame was amplified starting from pSV2-PVRα plasmid (kindly provided by Dr M. Lopez, Marseille, France) using the following primers: 5′ GGAGGCCCAGCTGCTCG (PVR ORF up) and 5′ GGGGTCTTCATCCATTGGG (PVR ORF dw). Amplification was performed with TAQ DNA Polymerase (Invitrogen, Carlsbad, CA) for 30 cycles (30 seconds at 95°C, 30 seconds at 58°C, and 30 seconds at 72°C) followed by a 7-minute elongation step at 72°C. The 1351-bp PCR product was subcloned in pcDNA3.1/V5-His TOPO TA vector (Invitrogen) and sequenced using a d-Rhodamine Terminator Cycle Sequencing Kit and a 377 Applied Biosystems Automatic Sequencer (Perkin Elmer Applied Biosystems, Jersey City, NJ). This construct was stably transfected into the murine thymoma cell line BW5147 (ECACC) using Polyfect (Qiagen, Valencia, CA) following the manufacturer's instructions. After 48 hours, cells were cultured in selective medium (RPMI/G418 1.5 mg/mL), and after 3 weeks positive cells expressing PVR were subcloned by limiting dilution.
Generation of immature dendritic cells
Immature dendritic cells (iDCs) were generated as follows: PBMCs were derived from healthy donors and were seeded in 25-mm2 plastic flasks at a density of 5 × 106 cells/mL. After 30 minutes at 37°C nonadherent cells were removed. To obtain a nearly pure adherent cell population, extensive repeated washes were performed. Plastic adherent cells were then cultured in the presence of IL-4 and GM-CSF (Peprotech, London, United Kingdom) at the final concentration of 20 ng/mL and 50 ng/mL, respectively. After 6 days of culture, iDC purity was assessed by cytofluorimetric analysis. Cells displaying high FSC-SSC values ranged from 80% to 95% and were all CD14−CD1a+CD83− iDCs.
Induction of maturation of dendritic cells
iDCs were plated in 96-well round-bottomed microtiter plates at 5 × 104 cells/well either in the absence or in the presence of IL-2–activated polyclonal NK cells derived from LAD1 patient 1 or from a healthy donor 1 (DC/NK ratio, 5:1). After 2 days, DCs were harvested and assessed for the expression of the maturation markers CD83, CD86, and HLA class I. As control, optimal DC maturation was induced by the addition of LPS (Sigma-Aldrich, St Louis, MO) at the final concentration of 1 μg/mL.
Results
Expression of triggering receptors and coreceptors on LAD1 NK cells and evaluation of their ability to induce cytokine release
NK cells were isolated from 2 LAD1 patients characterized by homozygous mutations of the CD18 gene leading to a premature end of the transcription. Purified NK cells were cultured in the presence of rIL-2 in order to obtain IL-2–activated polyclonal NK cell populations. As expected, LAD1 NK cells were characterized by the absence of LFA-1 molecules at the cell surface as shown by the lack of reactivity with CD18 (not shown) and CD11A-specific mAbs (Figure 1A). On the other hand, the natural cytotoxicity receptors (ie, NKp46, NKp30, and NKp44 receptors), NKG2D, DNAM-1 (Figure 1A), the coreceptors 2B4, NTBA, NKp80, as well as CD2 (not shown) were expressed at levels comparable to those of NK cells isolated from healthy donors.
Surface expression of the major triggering receptors and IFN-γ release by LAD1 NK cells. (A) IL-2–activated NK cell populations from LAD1 patients and from representative age-matched healthy donors were stained with monoclonal antibodies specific for the indicated molecules followed by PE-conjugated goat anti–mouse isotype-specific secondary reagent and analyzed by flow cytometry. NK cells represented virtually all purified cells as demonstrated by the homogeneous expression on viable cells of different NK markers including the NK-restricted NCR. White profiles indicate cells incubated with the secondary reagent only. (B) IL-2–activated NK cell populations from LAD1 patient 1 or the representative age-matched healthy donor 1 were stimulated with plate-bound mAbs specific for the indicated molecules. After 24 hours of culture, IFN-γ production was assessed by ELISA. Data represent the mean of 5 independent experiments (standard deviation < 5%).
Surface expression of the major triggering receptors and IFN-γ release by LAD1 NK cells. (A) IL-2–activated NK cell populations from LAD1 patients and from representative age-matched healthy donors were stained with monoclonal antibodies specific for the indicated molecules followed by PE-conjugated goat anti–mouse isotype-specific secondary reagent and analyzed by flow cytometry. NK cells represented virtually all purified cells as demonstrated by the homogeneous expression on viable cells of different NK markers including the NK-restricted NCR. White profiles indicate cells incubated with the secondary reagent only. (B) IL-2–activated NK cell populations from LAD1 patient 1 or the representative age-matched healthy donor 1 were stimulated with plate-bound mAbs specific for the indicated molecules. After 24 hours of culture, IFN-γ production was assessed by ELISA. Data represent the mean of 5 independent experiments (standard deviation < 5%).
We next analyzed whether in LAD1 IL-2–activated NK cells, mAb-mediated cross-linking of one or another triggering receptor could generate activating signals leading to cytokine release. As shown in Figure 1B, anti-NCR and anti-CD16 mAbs induced production of IFN-γ both in normal and LAD1 NK cells. On the other hand, in agreement with previous data obtained using NK cells from healthy donors, anti-NKG2D42 or anti–DNAM-143 mAbs were unable to induce cytokine release.
Analysis of LAD1 NK cells in redirected killing assays
Next, we evaluated whether mAb-mediated cross-linking of the various triggering receptors could induce cytolytic activity in LAD1 NK cell population. Thus, IL-2–activated LAD1 NK cells were used as effectors in a redirected killing assay against the murine (FcγR+) P815 target cells. This analysis was performed either in the absence or in the presence of mAbs specific for one or another triggering NK receptor. As shown in Figure 2A, mAb-mediated engagement of all the triggering receptors analyzed induced redirected lysis by LAD1 NK cells. Note, however, that the triggering effect shown in Figure 2 was obtained at a 40:1 E/T ratio, while that detected in control NK cells derived from age-matched healthy donors, was at a 2:1 E/T ratio. Accordingly, both the spontaneous (ie, with no mAb added) and the redirected lysis induced by the addition of triggering mAbs (see, for example, the representative anti-CD16 mAb) were significantly lower in LAD1 NK cells than in control NK cells when analyzed at the same E/T ratio (Figure 2B) (P ≤ .05). These data were confirmed by the analysis of the lytic activity of LAD1 NK cells against other murine target cells including BW5147 (Figures 2C and 3A) (P < .05) and YAC (not shown).
Cytolytic response of LAD1 NK cells in a redirected killing assay. (A) IL-2–activated NK cell populations derived from the LAD1 patients and from representative age-matched healthy donors were analyzed in a redirected killing assay against the FcγR+ P815 murine target cell line either in the absence (white bars) or in the presence of mAbs (IgG isotype) specific for the indicated molecules (black bars). The E/T ratios used were 40:1 and 2:1 for LAD1 patients and healthy donors, respectively. (B) NK cells from LAD1 patient 1 and healthy donor 1 were analyzed in redirected killing assays against the P815 cell line at various E/T ratios. Experiments were performed either in the absence (white circle) or in the presence of a mAb (IgG isotype) specific for CD16 (black circle) (2.5 μg/mL). Mann-Whitney test was used for statistical analysis. (C) IL-2–activated NK cell populations derived from the LAD1 patients and from representative age-matched healthy donors were analyzed for cytolytic activity against the BW5147 murine target cell line either in the absence of mAb (white bar) or in the presence of mAbs (IgM isotype) specific for the indicated molecules (gray bars). The E/T ratios used were 40:1 and 20:1 for LAD1 patients and healthy donors, respectively. (D) NK cells from healthy donor 1 were analyzed for cytolytic activity against the P815 cell line either in the absence of mAb (white bar) or in the presence of mAbs specific for the indicated molecules (black bars) used alone or in combination. mAbs of IgG or IgM isotype were used in order to either trigger or mask the different NK surface molecules, respectively. The E/T ratio used was 2:1. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%.
Cytolytic response of LAD1 NK cells in a redirected killing assay. (A) IL-2–activated NK cell populations derived from the LAD1 patients and from representative age-matched healthy donors were analyzed in a redirected killing assay against the FcγR+ P815 murine target cell line either in the absence (white bars) or in the presence of mAbs (IgG isotype) specific for the indicated molecules (black bars). The E/T ratios used were 40:1 and 2:1 for LAD1 patients and healthy donors, respectively. (B) NK cells from LAD1 patient 1 and healthy donor 1 were analyzed in redirected killing assays against the P815 cell line at various E/T ratios. Experiments were performed either in the absence (white circle) or in the presence of a mAb (IgG isotype) specific for CD16 (black circle) (2.5 μg/mL). Mann-Whitney test was used for statistical analysis. (C) IL-2–activated NK cell populations derived from the LAD1 patients and from representative age-matched healthy donors were analyzed for cytolytic activity against the BW5147 murine target cell line either in the absence of mAb (white bar) or in the presence of mAbs (IgM isotype) specific for the indicated molecules (gray bars). The E/T ratios used were 40:1 and 20:1 for LAD1 patients and healthy donors, respectively. (D) NK cells from healthy donor 1 were analyzed for cytolytic activity against the P815 cell line either in the absence of mAb (white bar) or in the presence of mAbs specific for the indicated molecules (black bars) used alone or in combination. mAbs of IgG or IgM isotype were used in order to either trigger or mask the different NK surface molecules, respectively. The E/T ratio used was 2:1. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%.
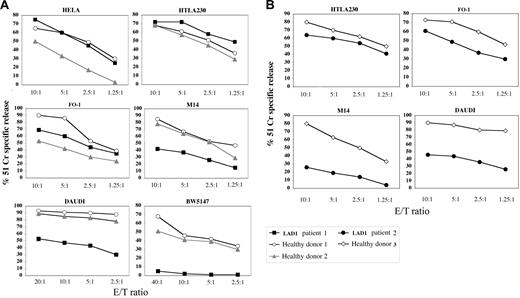
Comparison between natural cytotoxicity mediated by IL-2–activated LAD1 NK cells and normal NK cells against human tumor cell targets. IL-2–activated NK cell populations derived from LAD1 patient 1 (A), LAD1 patient 2 (B), and the representative age-matched healthy donors were analyzed for cytolytic activity against the indicated (FcγR-negative) target cell lines at different E/T ratios. Target cells are of human origin with the exception of murine BW5147 cells. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%. Kruskal-Wallis test and Mann-Whitney test were used for statistical analyses of the data showed in panel A and B, respectively.
Comparison between natural cytotoxicity mediated by IL-2–activated LAD1 NK cells and normal NK cells against human tumor cell targets. IL-2–activated NK cell populations derived from LAD1 patient 1 (A), LAD1 patient 2 (B), and the representative age-matched healthy donors were analyzed for cytolytic activity against the indicated (FcγR-negative) target cell lines at different E/T ratios. Target cells are of human origin with the exception of murine BW5147 cells. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%. Kruskal-Wallis test and Mann-Whitney test were used for statistical analyses of the data showed in panel A and B, respectively.
In this context, it should be pointed out that the spontaneous NK-mediated lysis of murine cells (such as P815, BW5147, and YAC) by normal human NK cells depends uniquely on the engagement of NKp4641 and LFA-1 by conserved ligand(s) expressed on murine targets cells. Indeed, in NK cells from healthy donors, mAb-mediated masking of LFA-1 or NKp46 (but not of other triggering receptors) virtually abolished the NK-mediated lysis of murine target cells (Figure 2C-D). Moreover, the disruption of LFA-1/ICAM-1 interactions strongly reduces the redirected lysis induced by the engagement of activating molecules such as CD16 and NKp46 (Figure 2D).
Altogether, the above results indicate that LAD1 NK cells express normal levels of triggering NK receptors and coreceptors. Moreover, mAb-mediated engagement of the major activating molecules results in both IFN-γ release (Figure 1B) and in the induction of cytolytic activity. This suggests that the ability of the various triggering receptors to transduce activating signals is conserved in NK cells lacking LFA-1 expression. It is of note that in contrast to previous data,40 mAb-mediated cross-linking of DNAM-1 could also enhance the cytolytic activity of LAD1 NK cells. However, it is to underline that in the xenogenic setting used for redirected killing assay, a high E/T ratio was necessary in order to obtain optimal LAD1 NK-mediated cytotoxicity against murine targets. This is likely due to the lack of LFA-1/ICAM interactions that are crucial for human NK/mouse target cell-to-cell contact.
Heterogeneity in the susceptibility of human tumor cell lines to LAD1 NK cell–mediated killing
Different from the xenogenic setting, killing of most human target cell lines by normal human NK cells occurs thanks to the establishment of multiple molecular interactions between effector and target cells. Thus, we further analyzed the cytolytic activity of LAD1 NK cells using as targets human tumor cell lines of different histotype including carcinomas, neuroblastomas, melanomas, and lymphomas. Lysis of HeLa, HTLA230, and FO-1 target cells mediated by LAD1 NK cells was comparable to that elicited by control NK cells (Figure 3). LAD1 NK cells were less efficient only in killing of M14 and DAUDI cell lines (Figures 3 and 4) (P < .05).
These experiments indicate that, in LAD1 patients, the lack of LFA-1 expression differentially affects the ability of NK cells to lyse human tumor target cells in vitro. It is of note, however, that none of the different human target cell lines used was completely resistant to LAD1 NK cells. These data suggest that in the human setting, besides the occurrence of multiple receptor/ligand triggering interactions, other adhesion molecules could possibly substitute LFA-1 in the formation of cell-to-cell conjugates necessary for NK cell–mediated lysis.
Contribution of different triggering NK receptors in the lysis of tumor cells by LAD1 NK cells
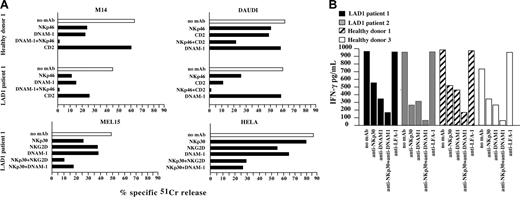
We next analyzed the ability of different triggering NK receptors expressed by LAD1 NK cells to transduce an efficient activating signal after engagement with the specific cellular ligands. To this end, the experiments shown in Figure 3 were performed in the presence of mAbs (of IgM isotype) capable of disrupting the interaction of one or another triggering receptor with its specific ligand(s). Similar to healthy donors,16,41 the spontaneous cytolytic activity of LAD1 NK cells against M14 cell line was strongly inhibited by mAb-mediated masking of NKp46 or DNAM-1 molecules. Moreover, the combined masking of both receptors led to the virtual abrogation of lysis (Figure 4A).
Involvement of the major triggering receptors in the recognition of different human tumor cell lines by LAD1 NK cells. (A) IL-2–activated NK cell populations derived from LAD1 patient 1 and healthy donor 1 were analyzed for cytolytic activity against the indicated (FcγR-negative) target cell lines either in the absence (white bar) or in the presence of the mAbs specific for the indicated molecules (black bars). The E/T ratios used were 20:1 (LAD1 patient) and 5:1 and 1:1 (healthy donor) for M14 and DAUDI cell lines, respectively; 10:1 (LAD1 patient) for MEL15 and HeLa tumor cells. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%. The chi-square test was used for statistical analysis. (B) IL-2–activated NK cell populations from LAD1 patients and age-matched healthy donors were cocultured with the representative HTLA230 (human) target cell lines, either in the absence or in the presence of mAbs (IgM isotype) specific for the indicated molecules. After 24 hours of coculture, IFN-γ production was assessed by ELISA. The IFN-γ production by effector or target cells alone was below the limit of detection of the assay. The NK/HTLA230 ratio used was 1:1. Data represent the mean of 3 independent experiments (standard deviation < 5%).
Involvement of the major triggering receptors in the recognition of different human tumor cell lines by LAD1 NK cells. (A) IL-2–activated NK cell populations derived from LAD1 patient 1 and healthy donor 1 were analyzed for cytolytic activity against the indicated (FcγR-negative) target cell lines either in the absence (white bar) or in the presence of the mAbs specific for the indicated molecules (black bars). The E/T ratios used were 20:1 (LAD1 patient) and 5:1 and 1:1 (healthy donor) for M14 and DAUDI cell lines, respectively; 10:1 (LAD1 patient) for MEL15 and HeLa tumor cells. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%. The chi-square test was used for statistical analysis. (B) IL-2–activated NK cell populations from LAD1 patients and age-matched healthy donors were cocultured with the representative HTLA230 (human) target cell lines, either in the absence or in the presence of mAbs (IgM isotype) specific for the indicated molecules. After 24 hours of coculture, IFN-γ production was assessed by ELISA. The IFN-γ production by effector or target cells alone was below the limit of detection of the assay. The NK/HTLA230 ratio used was 1:1. Data represent the mean of 3 independent experiments (standard deviation < 5%).
The ability of activating molecules expressed by LAD1 NK cells to recognize the specific ligands was further documented by the use, as target, of the DAUDI lymphoma cell line. Similar to NK cells from healthy donors, the LAD1 NK-mediated lysis of this target primarily involves NKp46 and CD2, a surface molecule known to be involved in lysis of most hematopoietic cell lines. Indeed, mAb-mediated disruption of their interactions with the specific ligand(s) resulted in inhibition of lysis. Note that the masking of CD2 in LAD1 NK cells resulted in a reduction of lysis even higher than that observed in the controls (P < .001 and P < .05, respectively). Indeed, in LAD1 NK cells the lysis was strongly reduced by the masking of CD2 alone (Figure 4A). According to the lack of the specific ligands, DNAM-1 was not involved in NK-mediated lysis of DAUDI.
The function of 2 other major triggering receptors, NKp30 and NKG2D in LAD1 NK cells, was analyzed by using as targets MEL15 and HeLa tumor cell lines.44,45 As shown in Figure 4A, NKp30 played a major role in the lysis of MEL15 mediated by LAD1 NK cells. A contribution to lysis of this target was also provided by both NKG2D and DNAM-1. The ability of NKG2D molecules to recognize their specific ligands was confirmed also by using HeLa as target cells. Indeed, mAb-mediated masking of NKG2D resulted in reduction of lysis. In addition, NKp30 and DNAM-1 also contributed to lysis of HeLa cells. This was indicated by the further inhibitory effect detectable when these receptors were masked simultaneously with NKG2D (Figure 4A).
Altogether these data demonstrate that in LAD1 NK cells, the absence of LFA-1/ICAM interactions does not substantially affect the ability of NCR, NKG2D, and DNAM-1 receptors to interact with their ligands expressed at the cell surface of various human tumor cells resulting in NK cell activation and target cell killing.
The interaction of LAD1 NK cells with human target cells results in strong IFN-γ release
IL-2–activated polyclonal NK cells from LAD1 patients or from age-matched healthy donors were cocultured with human target cell lines. After 24 hours, IFN-γ production by NK cells was measured in the supernatants. As shown in Figure 4B, the representative HTLA230 human cell lines induced strong IFN-γ production in NK cells derived from both healthy donors and LAD1 patients. This effect was down-regulated by mAb-mediated masking of NKp30 and DNAM-1 (ie, the receptors that were involved mainly in the NK-mediated lysis of this target cell). Moreover, masking of both molecules virtually abrogated target-induced IFN-γ production. Of interest, masking of LFA-1 on normal NK cells did not affect target-induced IFN-γ production. IFN-γ release by LAD1 NK cells was also observed when coculturing NK cells with other LAD1 NK-susceptible targets such as FO-1 (not shown),
These results further document the ability of LAD1 NK cells to efficiently interact with different human tumors. Moreover, they suggest that LAD1 NK cells are able to form with sensitive target cells immune synapses leading to both cytotoxicity and cytokine release.
The expression of human PVR molecules renders the BW5147 murine cell line susceptible to LAD1 NK cells
Under our experimental conditions, DNAM-1/ligand interactions delivered activating signals capable of inducing cytolytic activity and were crucial for IFN-γ production after effector-to-target cell contacts.
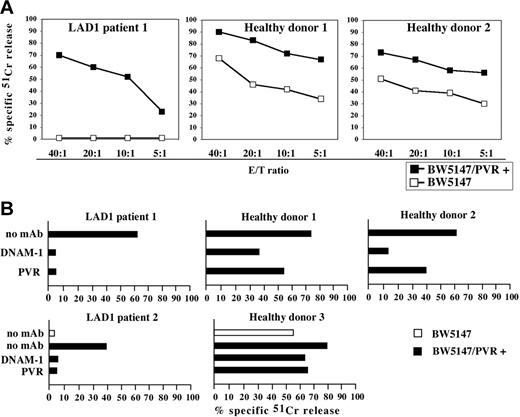
In order to further document that DNAM-1 could efficiently recognize its specific ligands on tumor target cells, we analyzed the cytolytic activity of LAD1 NK cells against BW5147 mouse cells that had been stably transfected with human PVR (CD155), a specific DNAM-1 ligand.16 Untransfected BW5147 cells, similar to other murine target cells, were poorly susceptible to LAD1 NK–mediated lysis (Figures 3 and 5A). Remarkably, however, they became susceptible to lysis after transfection with PVR (Figure 5A). Furthermore, the cytolytic activity of LAD1 NK cells against PVR-transfected BW5147 cells was virtually abrogated by mAb-mediated masking of either DNAM-1 on NK cells, or of PVR on target cells (Figure 5B).
Analysis of the DNAM-1–induced cytolytic activity in IL-2–activated LAD1 NK cells. (A) IL-2–activated NK cell populations derived from LAD1 patient 1 and from 2 representative age-matched healthy donors were analyzed for cytolytic activity against BW5147 murine cell line either untransfected or transfected with the PVR molecule (BW5147/PVR+) at different E/T ratios. (B) IL-2–activated NK cell populations from LAD1 patients and from representative age-matched healthy donors were analyzed for cytolytic activity against BW5147 cell line untransfected (white bar) or PVR transfected (black bars) either in the absence of mAb or in the presence of mAbs specific for the indicated molecules. The E/T ratios used were 20:1 for LAD1 NK cell populations, 5:1 for healthy donor 1, 10:1 for healthy donor 2, and 5:1 for healthy donor 3. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%.
Analysis of the DNAM-1–induced cytolytic activity in IL-2–activated LAD1 NK cells. (A) IL-2–activated NK cell populations derived from LAD1 patient 1 and from 2 representative age-matched healthy donors were analyzed for cytolytic activity against BW5147 murine cell line either untransfected or transfected with the PVR molecule (BW5147/PVR+) at different E/T ratios. (B) IL-2–activated NK cell populations from LAD1 patients and from representative age-matched healthy donors were analyzed for cytolytic activity against BW5147 cell line untransfected (white bar) or PVR transfected (black bars) either in the absence of mAb or in the presence of mAbs specific for the indicated molecules. The E/T ratios used were 20:1 for LAD1 NK cell populations, 5:1 for healthy donor 1, 10:1 for healthy donor 2, and 5:1 for healthy donor 3. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%.
These data confirm that in LAD1 NK cells (ie, in the absence of LFA-1), the function of DNAM-1 receptor is conserved.
LAD1 NK cells are capable of killing immature DCs and of inducing DC maturation
It has been shown that NK cells are capable of interacting with DCs, leading to a bidirectional cross talk resulting in NK cell priming and proliferation as well as in DC editing and maturation.46 We analyzed whether NK/DC interaction was conserved in LAD1 patients. In agreement with data obtained using healthy donors,43,47,48 IL-2–activated LAD1 NK cells could efficiently lyse allogeneic monocyte-derived immature DCs (iDCs). Moreover, as in normal NK cells, both NKp30 and DNAM-1 contributed to this process since the cytolytic activity was strongly inhibited by the addition of blocking anti-NKp30 and/or anti–DNAM-1 mAbs (Figure 6A).
Cytolytic activity against iDCs and induction of DC maturation by IL-2–activated LAD1 NK cells. (A) IL-2–activated NK cell populations derived from the LAD1 patient 1 and from the representative healthy donor 1 were analyzed for cytolytic activity against CD14−CD1a+ immature monocyte-derived iDCs either in the absence of mAb (white bar) or in the presence of the mAbs specific for the indicated molecules (black bars). The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%. (B) Immature DCs were cocultured for 2 days with NK cell populations derived from either the LAD1 patient or the representative healthy donor. DCs were analyzed by indirect immunofluorescence and cytofluorimetric analysis for expression of the indicated maturation markers. The percent of positive cells is indicated. Negative and positive controls (eg, iDCs cultured in medium alone [CTR] or in the presence of LPS, respectively) are shown.
Cytolytic activity against iDCs and induction of DC maturation by IL-2–activated LAD1 NK cells. (A) IL-2–activated NK cell populations derived from the LAD1 patient 1 and from the representative healthy donor 1 were analyzed for cytolytic activity against CD14−CD1a+ immature monocyte-derived iDCs either in the absence of mAb (white bar) or in the presence of the mAbs specific for the indicated molecules (black bars). The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%. (B) Immature DCs were cocultured for 2 days with NK cell populations derived from either the LAD1 patient or the representative healthy donor. DCs were analyzed by indirect immunofluorescence and cytofluorimetric analysis for expression of the indicated maturation markers. The percent of positive cells is indicated. Negative and positive controls (eg, iDCs cultured in medium alone [CTR] or in the presence of LPS, respectively) are shown.
In order to explore whether LAD1 NK cells were capable of also inducing DC maturation, iDCs were cocultured with IL-2–activated polyclonal NK cells derived from LAD1 patient 1. After 2 days of coculture, the DC maturation markers CD83, CD86 (Figure 6B), as well as HLA class I (not shown) were significantly up-regulated at the cell surface, although their levels of expression were lower than in DCs cocultured with normal NK cells.
Discussion
In the present study, we analyzed the functional capability of IL-2–activated NK cells derived from 2 patients affected by LAD1. Our results indicate that different important functions of NK cells are only marginally impaired both in NK/tumor and in NK/DC interactions. Indeed, all of the triggering receptors maintained their ability to transduce activating signals and could mediate both cytotoxicity and lymphokine release. As a consequence, most human tumors analyzed as well as iDCs were susceptible to LAD1 NK cell–mediated lysis similarly to normal NK cells. A remarkable exception was represented by murine target cells that were poorly susceptible to LAD1 NK cells. The LAD1 NK cell–mediated killing of monocyte-derived iDCs was comparable to that of control NK cells and was inhibited both by anti-NKp30 and by anti–DNAM-1 mAbs. Finally, maturation of iDCs upon coculture with LAD1 NK cells was only partially reduced compared to normal NK cells.
CD18, the gene affected in LAD1 patients, encodes a β chain that may combine with 3 α chains, CD11A, CD11B, and CD11C, resulting in different integrins, namely LFA-1, MAC-1, (or CR3), and CR4, respectively. These are integral cell-surface proteins expressed on leukocytes known to participate in different processes including cell adhesion, cell-surface–mediated signaling, chemotaxis, and phagocytosis. Thus, the susceptibility of LAD patients to recurrent infections is likely the result of abnormalities of a wide variety of CD18/CD11-dependent functions of cells such as granulocytes, monocytes, and lymphocytes, which are crucial in inflammatory responses. In NK cells, LFA-1 molecule is involved in the formation of conjugates with target cells as well as in the induction of the early signals mediating cytotoxicity.38 This role is confirmed by our experiments in which normal NK cells were analyzed for their ability to kill murine target cells. In this case, the number of different receptor-ligand interactions occurring during the process of NK cell activation is drastically reduced, being confined to the engagement of human NKp46 and LFA-1 with ligands expressed on murine cells. Indeed, mAb-mediated blocking experiments revealed that killing of murine target cells by normal human NK cells can be abrogated by the addition of either anti-NKp46 or anti–LFA-1 “masking” mAb. In line with these data, NKp46 receptor alone was not sufficient to induce optimal killing of murine targets (including P815, BW5147, and YAC) by LAD1 NK cells. On the other hand, in LAD1 NK cells, NKp46 could transduce activating signals resulting in both cytotoxicity and cytokine release, when triggering was induced by NKp46-specific mAbs or by NKp46-specific ligands expressed on human target cells. It is of note, however, that previous data demonstrated that optimal killing of human target cells results from a functional cooperation between different triggering receptors.19 Thus the overall ability of NK cells to kill targets clearly depends on the number of receptor/ligand interactions involved in this process. In the present report, a partial decrease of NK cell–mediated cytotoxicity was observed in the absence of LFA-1/ICAM interactions. This was particularly evident in the spontaneous lysis and redirected killing assays against murine targets as well as in the spontaneous lysis of certain human target cells such as M14 and DAUDI where NKp46 plays a dominant role in NK-mediated recognition and killing. Indeed, lysis of these target cells by normal NK cells was strongly inhibited by anti-NKp46 mAb. On the contrary, LAD1 and normal NK cells killed, to a similar extent, target cells such as HeLa, FO-1, and HTLA230. Lysis of these tumor cells by both types of NK cells was only partially affected by anti-NKp46 mAb since additional receptors including NKp30 and NKG2D cooperated in the induction of lysis. Thus, it is conceivable that the concerted action of different receptors is sufficient to compensate for the slightly reduced function of NKp46 in LAD1 patients.
Previous studies suggested that, in LAD1 patients, the DNAM-1 receptor might be nonfunctional. It was proposed that the impaired DNAM-1 function could be due to the requirement of a direct physical interaction between DNAM-1 and LFA-1.40 In the patients analyzed, we did not detect any impairment of DNAM-1 function. Remarkably, DNAM-1 function has been assessed by using different experimental approaches and further confirmed by using the murine BW5147 cells transfected with PVR (ie, a DNAM-1 ligand) as target cells. This murine lymphoma, resistant to lysis by LAD1 NK cells, became highly susceptible after transfection with PVR. Moreover, cytolytic activity could be abolished in the presence of either anti–DNAM-1 or anti-PVR mAbs. Of interest, in most instances, LAD1 NK cells displayed functional capabilities indistinguishable from normal NK cells. For example, the finding that LAD1 NK cells preserved the ability to kill different tumor cells suggests that in these patients LFA-1 may be dispensable for recognition and killing of most human targets. Thus, at variance with murine target cells, human tumors often express a complete set of surface ligands recognized by various activating molecules that may compensate for the lack of LFA-1/ICAM interactions.19 Indeed, besides the presence of ligands for different activating receptors including NCR and NKG2D, human tumors also express ligands for receptors involved not only in cell activation but also in cell adhesion. An example is provided by the ligands for DNAM-1 (eg, PVR and Nectin-2). With the exception of target cells of hematopoietic origin (for example, DAUDI lymphoma), both molecules are expressed on the majority of tumor cells.16,17,43 The use of specific blocking mAbs indicated that in LAD1 NK cells, DNAM-1 is involved in killing of most tumors. This further supports the concept that, at least in the LAD1 patients analyzed, the function of DNAM-1 is substantially unaffected. LFA-3 (CD58) is another example of a target cell ligand recognized by a triggering/adhesion molecule (ie, CD2), whose function is not simply conserved in LAD1 NK cells but might be even increased compared to normal NK cells. Indeed, triggering of LAD1 NK cells via CD2 resulted in levels of activation of cytotoxicity even higher than in normal NK cells (Figure 2). Moreover, the disruption of CD2/LFA-3 interaction strongly inhibited the LAD1 NK cell–mediated killing of cell lines such as DAUDI (Figure 4A). These data are in agreement with a previous observation that in LAD patients, CTL-mediated lysis of allogeneic target cells could require CD2/ LFA-3 interactions.47 Thus, our present data also suggest that the expression of ligands for receptor/adhesion molecules such as DNAM-1 and CD2 could compensate for the lack of LFA-1/ICAM interactions in NK-mediated recognition of target cells. It is of note, however, that CD18/CD11 integrins are involved also in other leukocyte functions such as the firm adhesion to endothelium during the process of extravasation.1,2 In this context, while a defect in neutrophil migration has been described in LAD1 patients,1 so far no information is available on the molecular interactions that participate in the various steps of transendothelial migration of normal as well as of LAD1 NK cells. Further studies will clarify whether in LAD1 patients NK cells might be able to reach the sites of inflammation or tumor growth. Our study was performed using IL-2–cultured polyclonal NK cell populations (ie, cells that likely correspond to fully activated cells present at the inflammatory sites). It could be interesting to analyze also the function of resting or short-term activated NK cells derived from LAD1 patients. In this context, it has been recently shown that the cytotoxicity of resting NK cells is independent of LFA-1/ICAM interactions. Indeed, costimulation of NK cells by CD16 and 2B4 was sufficient to induce release of cytolytic granules.48 Unfortunately, we were unable to perform experiments on resting LAD1 NK cells, as there were too few available patients and those who were available underwent stem cell transplantation soon after diagnosis. Moreover, because all patients analyzed were infants, only a few milliliters of blood could be drawn.
Data on the ability of LAD1 NK cells to interact with monocyte-derived DCs suggest that they are potentially capable of inducing both editing and maturation of iDCs during the early phases of innate immune responses.46 These events have been shown to depend primarily on the interaction of NKp30 and DNAM-1 with ligands expressed on DCs.43,49,50 In line with previous data on NK cells from healthy individuals, the use of blocking antibodies against these receptors could inhibit the cytotoxicity of LAD1 NK cells against iDCs and/or their ability to promote iDC maturation.
In conclusion, our present study not only provides novel information on the functional capabilities of NK cells in LAD1 patients, but also offers new insight into the relationships existing between LFA-1 and activating NK receptors in the induction of NK cell activation and functional interaction with tumor cells or DCs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro (AIRC), Istituto Superiore di Sanità (ISS), Ministero della Sanità, Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), European Union FP6, LSHB-CT-2004-503319-AlloStem, and EURO-POLICY-PID (contract no. 006411). (The European Commission is not liable for any use that may be made of the information contained.) Also the financial support of Fondazione Compagnia di San Paolo, Turin, Italy, is gratefully acknowledged. A.D. is the recipient of a Fondo Investimenti Ricerca di Base (FIRB) fellowship awarded by MIUR, and M.D.C. is the recipient of a fellowship awarded by Federazione italiana per la Ricerca sul Cancro (FIRC).
We thank Dr Giorgio Reggiardo and Dr Roberto Fasani (Data Management Unit, Medi Service, Genoa, Italy) for statistical analyses.
Authorship
Contribution: R.C. and A.D. performed most of the experimental work and contributed to paper writing; C.C. performed molecular analysis; M.D.C. performed experiments on DCs; C.P. and M.N. performed molecular analysis; M.F. and Lucia N. recruited study subjects; S.P. provided selected samples; L.M. supervised the project, provided economic support, and contributed to paper writing; Luigi N. recruited study subjects, and contributed to discussion and to paper writing; A.M. contributed to interpretation of the results, provided economic support, and contributed to paper writing; and C.B. designed and coordinated experimental work, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
R.C. and A.D. contributed equally to this study.
Correspondence: Cristina Bottino, Istituto Giannina Gaslini, L.go G. Gaslini 5, 16147 Genova, Italy; e-mail: cristina.bottino@unige.it.



![Figure 6. Cytolytic activity against iDCs and induction of DC maturation by IL-2–activated LAD1 NK cells. (A) IL-2–activated NK cell populations derived from the LAD1 patient 1 and from the representative healthy donor 1 were analyzed for cytolytic activity against CD14−CD1a+ immature monocyte-derived iDCs either in the absence of mAb (white bar) or in the presence of the mAbs specific for the indicated molecules (black bars). The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the standard deviation of the mean of the triplicates was less than 5%. (B) Immature DCs were cocultured for 2 days with NK cell populations derived from either the LAD1 patient or the representative healthy donor. DCs were analyzed by indirect immunofluorescence and cytofluorimetric analysis for expression of the indicated maturation markers. The percent of positive cells is indicated. Negative and positive controls (eg, iDCs cultured in medium alone [CTR] or in the presence of LPS, respectively) are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/11/10.1182_blood-2006-08-038760/4/m_zh80110703530006.jpeg?Expires=1765908000&Signature=X1wQh~6IC~zTbkamUfbVZaxZg3Q0Bwuo2crak2QdFV5zzsjXL2s67p1DDOPtuUiZKzXl8eiK0WXbPcDnajUx3N5fi6ugvSCVwqH7krym5LBjEIpyulfhJOBnmr~w6B3-GWLivkM6brlFGlD0nEpsx8G3hO9hcKLS05tFpbg9byjIG3y0RY0nCAE3delbUNz7sAsk3iTdRzTzYFNzo0ef7O9cAJE0274QoYSFVZdg96KnfdR5zrFbupDzZ9tY873kXPHyaRMvXEuS8rBgXlLw1aFL7bOsU5czBPJN5CKwuBWkSMmgAnuhCICko~5rIQ5fKnykPfExi5NQd6L~r4uItg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal