Abstract
In humans, the majority of peripheral blood γδ T cells expresses Vγ9Vδ2 T-cell receptors (TCR) and recognize nonpeptidic phosphorylated antigens. In contrast, most tissue-derived γδ T cells, which are located mainly in spleen and epithelia, preferentially use Vδ1 or Vδ3 chains paired with diverse Vγ chains to form their TCR. Our knowledge about the antigenic specificity and costimulation requirements of human Vδ2− γδ T cells remains limited. In an attempt to address this important issue, we characterized the specificity of a monoclonal antibody (mAb 256), screened for its ability to specifically inhibit cytolytic responses of several human Vδ2− γδ T-cell clones against transformed B cells. We show that mAb 256 does not target a TCR ligand but blocks key interactions between non-TCR molecules on effector γδ T cells and ILT2 molecule, expressed by tumor targets. In line with the previously reported specificity of this NK receptor for classic and nonclassic major histocompatibility complex (MHC) class I molecules, blockade of MHC class I/ILT2 interactions using MHC class I- or ILT2-specific mAbs and ILT2-Fc molecules inhibited tumor-induced activation of Vγ8Vδ3 T-cell clones. Therefore, this study describes a new cytotoxic T lymphocyte activation pathway involving MHC class I engagement on γδ T cells.
Introduction
One important feature of γδ T cells is the preferential expression of T-cell receptor (TCR) V regions in distinct tissue locations. In humans, γδ T cells are distinguished by usage of one of the 3 main TCR Vδ1, Vδ2, or Vδ3 regions. Vδ2+ T cells (Vγ9Vδ2) represent the major peripheral γδ T-cell population in blood (0.5%-5% of the peripheral blood leukocyte [PBL] pool).1,2 In a nonpathologic context, Vδ1+ subsets are preferentially located in thymus, spleen, liver, gut epithelia, and dermis, and to a lesser extent in blood, whereas Vδ3+ subsets are mainly found in liver and gut epithelia. Tissue-localized γδ T cells are considered as unique self-reactive sensors of cell stress and transformation that may recognize endogenous molecules whose expression is up-regulated in particular physiopathologic conditions. Accordingly, a growing number of studies support a critical role played by γδ T cells in protective immunity against extracellular and intracellular pathogens, tumor surveillance, modulation of innate and adaptive immune responses, tissue healing or epithelial cell maintenance, and regulation of physiologic organ function.3,4
Vδ2+ T cells, the best characterized human γδ T-cell subset, recognize in vitro a wide array of transformed or infected target cells and, accordingly, are activated in vivo in various tumoral and infectious contexts.5-7 Human Vδ2− γδ T cells express TCR with greater combinatorial and junctional diversity, consistent with recognition of a wide range of antigens. Human Vδ1+ T cells reactive against major histocompatibility complex (MHC), MHC-like, or non-MHC molecules, such as MICA/B, CD1c or HLA-DR,8-11 have been described in tumor and infection contexts, but formal evidence for cognate interactions between Vδ2− γδ TCRs and their suspected ligands has not been provided so far. As for their peripheral Vδ2+ counterpart, the precise modalities of Vδ2− γδ T-cell activation remain poorly understood. Importantly, efficient triggering of TCR-mediated human γδ T-cell activation is induced with the help of nonclonal surface receptors, such as adhesion molecules (eg, ICAM-1/2), TLRs (eg, TLR3), Fc receptors (eg, CD16), and natural killer receptors.12-16 Indeed, most human Vδ2+ and Vδ2− γδ T cells, which do not express CD4 or CD8 coreceptors, use activating (eg, NKG2D) and inhibitory natural killer receptors (eg, CD94/NKG2A or ILT2) to fine-tune their TCR-mediated activation threshold and ensure discrimination between normal and altered self.17-19
In an attempt to analyze the activation modalities of Vδ2− γδ T cells, we generated monoclonal antibodies (mAbs) against a transformed B-cell line not recognized by Vδ2+ γδ T cells, and screened them for their ability to selectively block tumor cell recognition by reactive Vδ2− γδ T-cell clones. Characterization of the tumor ligand recognized by one such blocking mAb revealed a key role played by the ILT2 (or LILRB1/CD85j) molecule, a type I transmembrane protein of the immunoglobulin superfamily of receptors that is broadly expressed by hemopoietic cells and that binds for most classic and nonclassic MHC class I molecules,20-24 in transformed B-cell recognition by several Vδ2− T-cell clones. We further showed that target cell-derived ILT2 costimulated Vδ2− γδ T-cell activation through interaction with non-TCR molecules (eg, MHC class I) expressed by γδ T-cell effectors. Altogether, these results suggest the existence of a new cytotoxic T lymphocyte (CTL) activation pathway involving MHC class I engagement on many subsets of human Vδ2− γδ T cells and provide new insights into mechanisms regulating γδ T-cell functional responses.
Methods
Antibodies and reagents
The following antibodies were used: CD107a-fluorescein isothiocyanate (FITC; clone H4A3), CD107b-FITC (H4B4), and mouse isotypes IgG1 and IgG2 purchased from BD Biosciences. CD3ϵ-phycoerythrin (PE), -PC5, -FITC, and purified (UCHT1), ILT2 (GHI-75), ILT2-PE and purified (HP-F1), CD14-PE (RMO52), CD56-PE (N901), CD69-PC5 (TP1.55.3), and pan TCRγδ-PC5 (IMMU510) were obtained from Immunotech/Beckman Coulter. MHC class I mAb (W6/32) was obtained from eBioscience. Goat anti–human Fc-FITC and goat anti–mouse-PE secondary mAbs were from Jackson ImmunoResearch Laboratories. Goat anti–mouse-Alexa 647 and -Alexa 488 mAbs, Live/Dead cell marker, and carboxyfluorescein succinimidyl ester were from Invitrogen. Recombinant human ILT2-Fc molecule was purchased from R&D Systems. Mouse serum, monensin A, phorbol myristate acetate, and ionomycin were from Sigma-Aldrich. Full-length human ILT2 cDNA was kindly provided by M. Lopez-Botet (IMIM, Barcelona, Spain).
Cells
Human γδ T cells were cultivated in complete RPMI 1640 medium (2mM l-glutamine, 10 μg/mL streptomycin, and 100 IU/mL penicillin) with 10% fetal calf serum and supplemented with 300 IU/mL of recombinant human interleukin-2 (Proleukin; Chiron Therapeutics). γδ T cells were expanded in vitro in complete RPMI 1640 medium containing 10% human serum and supplemented with 0.5 μg/mL of purified leucoagglutinin (Sigma-Aldrich) and irradiated (30 Gy) allogeneic feeder cells. These human γδ T cells, Vγ8Vδ3 (clones 73R9, R3R11, and 73R13), Vγ9Vδ1 (73R14), and Vγ3Vδ3 (84R33), were obtained from standard mixed lymphocyte (10 × 106 fresh human PBL from healthy donors)-tumor (5 × 106 irradiated Raji Burkitt lymphoma cells) cultures performed in the presence of recombinant human interleukin-2. After 2 weeks, γδ T cells were magnetically sorted using a pan γδ TCR mAb (IMMU510, Beckman Coulter), cloned by limiting dilution (0.3 cells/well) and amplified. Peripheral blood mononuclear cell (PBMC)-Vγ9Vδ2 (G12) and -Vγ2–3Vδ3 (94–5-14) and thymic-18S3.1 (Vγ2–3Vδ3), -18S3.5 (Vγ8Vδ3), -21S3.15 (Vγ4Vδ3) γδ T-cell clones were similarly sorted, cloned, and expanded. B cells were kept in complete RPMI 1640 medium. Daudi, Raji, and its MHC class II deficient mutant RJ225 (Burkitt lymphomas), RPMI8226 (myeloma), and 721.221 (B-lymphoblastoid cell line [B-LCL]) were from ATCC. Epstein-Barr virus+ (EBV+)–B-LCLs were generated by culturing 2 × 106 PBMCs from healthy human donors (identified as Do#) with EBV-containing supernatant from the virus-producing B95.8 marmoset cell line. Cultures were performed in the presence of 1 μg/mL cyclosporine A; 00136, BM16, GRC212, IBW9, GRC138, BM9, GO85, 26/27, SP0010, JESTHOM, and KT17 were homozygous typing cell human B-cell lines that were derived from the International Histocompatibility Working Group. The human C91 T2.2 T-cell line was kindly provided by D. Olive (Inserm Unité Mixte de Recherche 891, Marseille, France). U2OS cells (human osteosarcoma) were kindly provided by D. Heymann (Inserm Unité Mixte de Recherche 957, Nantes, France). ILT2+ and ILT2− 721.221 B cells were sorted using a FACSAria cell sorter (BD Biosciences) and next cloned by limiting dilution.
Proliferation
Proliferative activity of responder γδ T cells was measured after a 48-hour culture with irradiated target cells followed by an overnight pulse with 3H thymidine. Cells were harvested, and 3H thymidine incorporation was detected by liquid scintillation counting.
Cytolytic activity
Cytolytic activity of γδ T cells was measured by a standard 4-hour 51Cr release assay. Percentage of tumor target cell lysis was calculated according to the following formula: ((experimental release-spontaneous release)/(maximum release − spontaneous release)) × 100. Maximum and spontaneous release was determined by, respectively, adding 1% Triton X-100 or medium to 51Cr-labeled tumor target cells in the absence of γδ T cells. When indicated, cytolytic activity of human γδ T cells was also analyzed by measuring CD107a/b surface mobilization by flow cytometry. γδ T cells were cocultured for 4 hours together with target cells (1:1 cell ratio) at 37°C in complete RPMI 1640 medium in the presence of monensin (5μM) and a combination of FITC-conjugated CD107a/b mAbs. T cells were harvested, stained with CD3-PC5 mAb and Live/Dead cell marker, and analyzed by flow cytometry. Data were collected on a FACSCanto cytometer (BD Biosciences) and analyzed with FlowJo software Version 8.7.1 (TreeStar).
Generation and production of mAb 256
Six-week-old BALB/c mice were intraperitoneally immunized in 3 periods with B-LCL (5 × 106 cells, Do#AD) cells. Hybridoma cells were a fusion product between splenic lymphocytes of immunized Balb/c mice and SP2/0 myelomas, according to standard procedures. Supernatant of hybridoma cells were screened for detection of antibody by ELISA. The suitable clones were selected for limiting dilution, and batches of mAb 256 (IgG1) were produced in vitro and in ascitic fluids.
Vγ8Vδ3 TCR Jurkat transductants
The γ and δ chains of the 73R9 (Vγ8Vδ3) T-cell clone were cloned by reverse-transcribed polymerase chain reaction using specific primers that amplify the full-length molecule. The polymerase chain reaction products were subcloned in the pCR2.1 vector (Invitrogen) and sequenced. The γ8 and δ3 cDNAs were cloned into the lentiviral vector pTEEW (vectorology facility of Bordeaux 2 University, Bordeaux, France; www.ifr66.u-bordeaux2.fr/pages/vectorologie.html) under the control of the promoter EF1a. Transduction of human Jurkat T cells (J.RT3-T3.5, TCR β-chain-deficient variant) was performed using lentiviral particles, which were produced by transient transfection of 293T HEK cells as previously described,25 at a multiplicity of infection of 2, 4, and 8 in RPMI 1640 medium with 8% fetal calf serum and 8 mg/mL protamine sulfate (Sigma-Aldrich). After 24 hours of infection, cells were washed twice, amplified for 5 days, and sorted for TCR expression by flow cytometry (BD FACSAria). Jurkat T cells stably expressing, or not, the Vγ8Vδ3 TCR (supplemental Figure 3, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were activated for 4 hours at 37°C in complete RPMI 1640 medium, harvested, and stained with a CD69-PC5 mAb. Surface expression of CD69 on Jurkat T cells was analyzed by flow cytometry on LSR-I cytometer (BD Biosciences) and FlowJo software Version 8.7.1. Carboxyfluorescein succinimidyl ester staining was used to exclude target cells.
Western blots
Whole cell extracts were obtained by cell pellet lysis in RIPA buffer (50mM Tris HCl, 150mM NaCl, 0.25% deoxycholate (DOC), 1% Triton X-100, 0.1% sodium dodecyl sulfate, 100μM Na3VO4, 1mM phenylmethylsulfonyl fluoride/Pefabloc, 1 μg/mL leupeptin, and 1 μg/mL aprotinin). Proteins (50 μg for cell lysates or 0.5 μg for purified recombinant proteins) were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon polyvinylidene difluoride membranes (Millipore). Unbound sites were blocked with Western Blocking Reagent (Roche Applied Science), and blots were probed by overnight incubation at 4°C with purified primary mAbs (1 μg/mL). After washing with Tris-buffered saline–Tween 0.1%, membranes were further incubated 1 hour at room temperature, with a horseradish peroxidase-conjugated anti–mouse/anti–rabbit secondary antibodies (Roche Applied Biosystem), and visualized using Chemiluminescence Western Blotting Substrate (Roche Applied Biosystem).
Confocal microscopy
Raji cells were left to adhere for 1 hour at 37°C on poly-L-lysine-coated glass slides (Biocoat, BD Biosciences). Cells were washed and fixed (4% paraformaldehyde for 20 minutes at room temperature) and preincubated for 15 minutes with human serum. After addition of primary mAbs, mAb 256 (ascites diluted at 1/1000) or purified mouse IgG1 (10 μg/mL) and incubation for 15 minutes at 4°C, samples were washed and stained with goat anti–mouse Ig-Alexa 488. After washing, slides were dried, mounted using Fluoromount G solution (Southern Biotechnology), and analyzed using a Leica SP1 confocal microscope (63 × 1.4 NA objective).
Data analysis
The significance of differences between 2 means was assessed with a paired Student t test. Differences were considered statistically significant with P less than .05.
Results
Human Vδ2− γδ T-cell subsets display cytolytic and proliferative responses against transformed B lymphoid target cells
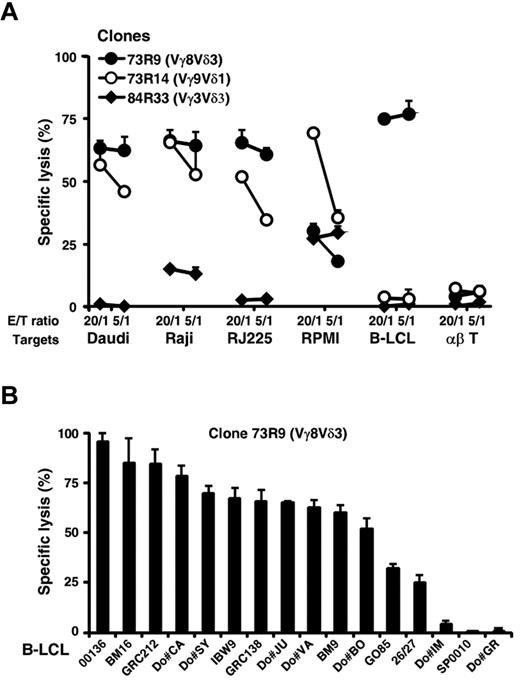
In an attempt to study the activation requirements of human Vδ2− γδ T cells, we first isolated in vitro tumor-specific polyclonal and clonal γδ T-cell populations derived from mixed lymphocyte-tumor cultures using a Burkitt lymphoma line (Raji) not recognized by Vδ2+ γδ T cells. After a 2-week coculture with irradiated Raji cells in IL-2-supplemented medium, γδ T cells were purified by immunomagnetic sorting using a TCR Cδ-specific mAb, and their phenotype was analyzed. The majority (> 75%) of γδ T cells expressed Vδ2− TCRs, including Vγ8Vδ3, Vγ9Vδ1, or Vγ8Vδ1 T-cell populations (data not shown). γδ T cells were next cloned by limiting dilution, and their reactivity against various target cells was measured. Strikingly, several Vδ2− γδ T-cell clones (8/16 and 14/30 from, respectively, donor (Do)#73 and Do#84), such as Vγ8Vδ3 T cells, strongly reacted in both cytotoxicity and proliferation assays against several B lymphoid target cells, such as Raji and Daudi Burkitt lymphoma cells and B-LCLs, in contrast to Vγ9Vδ2 T cells, which were stimulated for their cytolytic activity by Daudi cells only (Table 1). As shown in Figure 1A, the activation process of Vδ2− γδ T-cell clones was independent of MHC molecule expression as Daudi and RJ225 cells, which are, respectively, MHC class I− and II− targets, were lysed by 2 Vδ2− T-cell clones (73R9, Vγ8Vδ3 and 73R14, Vγ9Vδ1). Importantly, the ability of Vδ3+ clonal and polyclonal T-cell lines derived from PBLs or thymocytes of different donors to react against B lymphoid cells was further confirmed and extended using a large panel of EBV+ and transformed B-cell lines (Figure 1B; supplemental Figure 1).
Cytolytic and proliferative activity of human Vδ2neg γδ T-cell clones against B lymphoid target cells
| Clones* . | TCR† . | Daudi . | Raji . | RPMI 8226 . | B-LCL (Do#DA) . | T αβ§ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysis‡ . | Proliferation . | Lysis . | Proliferation . | Lysis . | Proliferation . | Lysis . | Proliferation . | Lysis . | Proliferation . | ||
| 73R5 | Vγ8Vδ3 | 72 ± 1‖ | 1669 ± 6 | 52 ± 3‖ | 3453 ± 1325 | 7 ± 0 | 0 | 67 ± 12‖ | 29 389 ± 2479¶ | 14 ± 1 | 5887 ± 174 |
| 73R9 | Vγ8Vδ3 | 74 ± 2‖ | 18 889 ± 3156¶ | 50 ± 3‖ | 12 103 ± 824¶ | 3 ± 0 | 3317 ± 1081 | 80 ± 6‖ | 35 686 ± 2899¶ | 4 ± 6 | 825 ± 274 |
| 73R11 | Vγ8Vδ3 | 74 ± 0‖ | 12 815 ± 1598¶ | 50 ± 1‖ | 10 906 ± 436¶ | 7 ± 2 | 4324 ± 1300 | 86 ± 1‖ | 29 981 ± 902¶ | 7 ± 2 | 1894 ± 468 |
| 73R13 | Vγ8Vδ3 | 50 ± 8‖ | 192 ± 100 | 27 ± 1 | 4206 ± 1525 | 4 ± 2 | 3276 ± 1528 | 62 ± 3‖ | 2649 ± 768 | 7 ± 7 | 8551 ± 1173 |
| 73R14 | Vγ9Vδ1 | 73 ± 5‖ | 25 711 ± 274¶ | 35 ± 5‖ | 9963 ± 269 | 63 ± 5‖ | 3913 ± 200 | 12 ± 0 | 22 108 ± 395¶ | 12 ± 1 | 10 019 ± 730¶ |
| 84R11 | Vγ8Vδ1 | 1 ± 0 | 0 | 6 ± 2 | 0 | 15 ± 3 | 0 | 13 ± 3 | 12 102¶ | 6 ± 10 | 0 |
| 84R33 | Vγ3Vδ3 | 1 ± 0 | 0 | 2 ± 0 | 2905 ± 199 | 23 ± 5 | 2501 ± 656 | 1 ± 1 | 0 | 15 ± 6 | 3919 ± 50 |
| 84R45 | Vγ2-3Vδx | 0 | 0 | 3 ± 1 | 0 | 29 ± 1 | 0 | 4 ± 1 | 0 | 9 ± 10 | 0 |
| 84R50 | Vγ2-3Vδx | 1 ± 0 | 0 | 2 ± 0 | 3604 ± 74 | 39 ± 4‖ | 1312 ± 849 | 1 ± 3 | 0 | 7 ± 2 | 282 ± 174 |
| 84R51 | Vγ9Vδ2 | 64 ± 4‖ | ND | 9 ± 2 | ND | ND | ND | 5 ± 1 | ND | ND | ND |
| 84R48 | Vγ9Vδ2 | 63 ± 10‖ | ND | 4 ± 8 | ND | ND | ND | 15 ± 3 | ND | ND | ND |
| Clones* . | TCR† . | Daudi . | Raji . | RPMI 8226 . | B-LCL (Do#DA) . | T αβ§ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysis‡ . | Proliferation . | Lysis . | Proliferation . | Lysis . | Proliferation . | Lysis . | Proliferation . | Lysis . | Proliferation . | ||
| 73R5 | Vγ8Vδ3 | 72 ± 1‖ | 1669 ± 6 | 52 ± 3‖ | 3453 ± 1325 | 7 ± 0 | 0 | 67 ± 12‖ | 29 389 ± 2479¶ | 14 ± 1 | 5887 ± 174 |
| 73R9 | Vγ8Vδ3 | 74 ± 2‖ | 18 889 ± 3156¶ | 50 ± 3‖ | 12 103 ± 824¶ | 3 ± 0 | 3317 ± 1081 | 80 ± 6‖ | 35 686 ± 2899¶ | 4 ± 6 | 825 ± 274 |
| 73R11 | Vγ8Vδ3 | 74 ± 0‖ | 12 815 ± 1598¶ | 50 ± 1‖ | 10 906 ± 436¶ | 7 ± 2 | 4324 ± 1300 | 86 ± 1‖ | 29 981 ± 902¶ | 7 ± 2 | 1894 ± 468 |
| 73R13 | Vγ8Vδ3 | 50 ± 8‖ | 192 ± 100 | 27 ± 1 | 4206 ± 1525 | 4 ± 2 | 3276 ± 1528 | 62 ± 3‖ | 2649 ± 768 | 7 ± 7 | 8551 ± 1173 |
| 73R14 | Vγ9Vδ1 | 73 ± 5‖ | 25 711 ± 274¶ | 35 ± 5‖ | 9963 ± 269 | 63 ± 5‖ | 3913 ± 200 | 12 ± 0 | 22 108 ± 395¶ | 12 ± 1 | 10 019 ± 730¶ |
| 84R11 | Vγ8Vδ1 | 1 ± 0 | 0 | 6 ± 2 | 0 | 15 ± 3 | 0 | 13 ± 3 | 12 102¶ | 6 ± 10 | 0 |
| 84R33 | Vγ3Vδ3 | 1 ± 0 | 0 | 2 ± 0 | 2905 ± 199 | 23 ± 5 | 2501 ± 656 | 1 ± 1 | 0 | 15 ± 6 | 3919 ± 50 |
| 84R45 | Vγ2-3Vδx | 0 | 0 | 3 ± 1 | 0 | 29 ± 1 | 0 | 4 ± 1 | 0 | 9 ± 10 | 0 |
| 84R50 | Vγ2-3Vδx | 1 ± 0 | 0 | 2 ± 0 | 3604 ± 74 | 39 ± 4‖ | 1312 ± 849 | 1 ± 3 | 0 | 7 ± 2 | 282 ± 174 |
| 84R51 | Vγ9Vδ2 | 64 ± 4‖ | ND | 9 ± 2 | ND | ND | ND | 5 ± 1 | ND | ND | ND |
| 84R48 | Vγ9Vδ2 | 63 ± 10‖ | ND | 4 ± 8 | ND | ND | ND | 15 ± 3 | ND | ND | ND |
ND indicates not determined.
γδ T-cell clones were derived from MLTC performed with PBL of 2 healthy human donors (Do#73 and Do#84), as described in “Cells.”
Expression of Vγ and Vδ TCR chains was determined by flow cytometry using specific mAbs.
Cytolytic activity (% of specific lysis) and γδ T-cell proliferation (cpm) were measured, respectively, in a standard 51Cr release (30:1 effector-to-target cell ratio) and 3H thymidine incorporation assays, as described in “Proliferation.” Data are shown as mean ± SD of triplicate (n = 3).
Control human CD8+ αβ T-cell line.
Cytolysis value > 30%.
Proliferation value > 10 000 cpm.
Human Vδ2− γδ T-cell subsets display strong cytolytic responses against transformed B lymphoid target cells. (A) Cytolytic activity of 3 distinct mixed lymphocyte-tumor culture-derived human Vδ2− γδ T-cell clones (73R9, 73R14, and 84R33) was measured by 51Cr release assay after coculture together with Burkitt lymphoma (Daudi, Raji, and RJ225), multiple myeloma (RPMI226), B-LCL (Do#AD), and αβ T cells at both 20:1 and 5:1 γδ T cell-to-target ratios. (B) Cytolytic activity of a Vγ8Vδ3 T-cell clone (73R9) was measured by 51Cr release assay after coculture together with B cells from a panel of 7 B-LCLs generated from PBMCs of healthy donors (Do#) or 9 homozygous typing cells at a 20:1 γδ T cell-to-target ratio. (A–B) Data are the percentage of specific lysis in triplicate assays (mean ± SD), calculated as described in “Cytolytic activity.” Data are representative of at least 3 independent experiments performed with γδ T-cell clones from different healthy donors.
Human Vδ2− γδ T-cell subsets display strong cytolytic responses against transformed B lymphoid target cells. (A) Cytolytic activity of 3 distinct mixed lymphocyte-tumor culture-derived human Vδ2− γδ T-cell clones (73R9, 73R14, and 84R33) was measured by 51Cr release assay after coculture together with Burkitt lymphoma (Daudi, Raji, and RJ225), multiple myeloma (RPMI226), B-LCL (Do#AD), and αβ T cells at both 20:1 and 5:1 γδ T cell-to-target ratios. (B) Cytolytic activity of a Vγ8Vδ3 T-cell clone (73R9) was measured by 51Cr release assay after coculture together with B cells from a panel of 7 B-LCLs generated from PBMCs of healthy donors (Do#) or 9 homozygous typing cells at a 20:1 γδ T cell-to-target ratio. (A–B) Data are the percentage of specific lysis in triplicate assays (mean ± SD), calculated as described in “Cytolytic activity.” Data are representative of at least 3 independent experiments performed with γδ T-cell clones from different healthy donors.
Taken together, these findings indicate that a wide range of B lymphoid cells, such as Burkitt lymphoma cells and EBV+ B-LCLs, strongly activate particular human Vδ2− γδ T-cell subsets, in an MHC class I- and II-independent manner.
mAb 256 specifically inhibits cytolytic responses of Vδ2− γδ T cells induced by human B lymphoid target cells
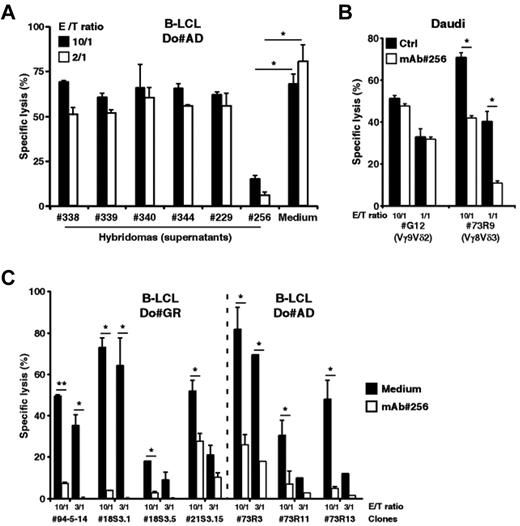
We next sought to identify key molecular partners that, on expression by B lymphoid cells, could contribute to their recognition by human Vδ2− γδ T cells. To this end, we performed intraperitoneal immunization of mice against human EBV+ B-LCLs, generated and cloned B hybridomas, which were screened for the production of mAbs able to stain B-LCLs and inhibit B-LCL killing by Vδ2− γδ T cells. For this initial screening and several forthcoming experiments described in this study, we used as responder T cells the 73R9 (Vγ8Vδ3) T-cell clone because of its strong cytolytic (CTL) and proliferative activity against B-LCL. The hybridoma clone 256 (murine IgG1) was further selected as it dramatically reduced the 73R9 T-cell clone cytolytic responses against B-LCL, compared with other B-LCL-reactive mAbs (Figure 2A). Importantly, although purified mAb 256 strongly inhibited the lysis of Daudi cells by Vγ8Vδ3 T cells, it had no effect on the reactivity of Vγ9Vδ2 T cells against the same target cells (clone G12, Figure 2B). Finally, the biologic effect of mAb 256 was confirmed and extended by analyzing its ability to block the reactivity of various clonal and polyclonal lines of human Vδ2− γδ T cells derived from different donors (Figure 2C; supplemental Figure 2).
mAb 256 specifically inhibits cytolytic responses of Vδ2− γδ T cells induced by human B lymphoid target cells. (A) Mice were immunized against human EBV+ B-LCLs. Cytolytic activity of the 73R9 γδ T-cell clone (Vγ8Vδ3) was measured by 51Cr release assay after a 4-hour coculture together with B cells from human B-LCL (Do#AD) at 10:1 and 2:1 γδ T cell-to-target ratios, in the presence of supernatants (dilution, 1:5) collected from 6 distinct cloned hybridomas. (B) Cytolytic activity of the G12 (Vγ9Vδ2) and 73R9 (Vγ8Vδ3) γδ T-cell clones was measured by 51Cr release assay after a 4-hour coculture together with Burkitt lymphoma cells (Daudi) at 10:1 and 1:1 γδ T cell-to-target ratios in the absence (Ctrl, filled bars) or in the presence of mAb 256 (ascites 1:1000 dilution, open bars; n > 3). Similar data were obtained using different Vγ9Vδ2 T-cell clones/lines from distinct donors. (C) Cytolytic activity of 7 human Vδ2− γδ T-cell clones was measured by 51Cr release assay after a 4-hour coculture together with target cells from 2 different B-LCLs (Do#GR, left and Do#AD, right) at 10:1 and 3:1 γδ T cell-to-target ratios in the presence of mAb 256 (ascites 1:500, open bars). γδ T-cell clones 94–5-14, 73R3, 73R11, and 73R13 were derived from PBL of 2 distinct donors. Clones 18S3.1, 18S3.5, and 21S3.15 were derived from human fetal thymi of 2 distinct donors. (A-C) Data are the percentage of specific lysis (mean ± SD) and are representative of at least 3 independent experiments. *P < .05. **P < .005.
mAb 256 specifically inhibits cytolytic responses of Vδ2− γδ T cells induced by human B lymphoid target cells. (A) Mice were immunized against human EBV+ B-LCLs. Cytolytic activity of the 73R9 γδ T-cell clone (Vγ8Vδ3) was measured by 51Cr release assay after a 4-hour coculture together with B cells from human B-LCL (Do#AD) at 10:1 and 2:1 γδ T cell-to-target ratios, in the presence of supernatants (dilution, 1:5) collected from 6 distinct cloned hybridomas. (B) Cytolytic activity of the G12 (Vγ9Vδ2) and 73R9 (Vγ8Vδ3) γδ T-cell clones was measured by 51Cr release assay after a 4-hour coculture together with Burkitt lymphoma cells (Daudi) at 10:1 and 1:1 γδ T cell-to-target ratios in the absence (Ctrl, filled bars) or in the presence of mAb 256 (ascites 1:1000 dilution, open bars; n > 3). Similar data were obtained using different Vγ9Vδ2 T-cell clones/lines from distinct donors. (C) Cytolytic activity of 7 human Vδ2− γδ T-cell clones was measured by 51Cr release assay after a 4-hour coculture together with target cells from 2 different B-LCLs (Do#GR, left and Do#AD, right) at 10:1 and 3:1 γδ T cell-to-target ratios in the presence of mAb 256 (ascites 1:500, open bars). γδ T-cell clones 94–5-14, 73R3, 73R11, and 73R13 were derived from PBL of 2 distinct donors. Clones 18S3.1, 18S3.5, and 21S3.15 were derived from human fetal thymi of 2 distinct donors. (A-C) Data are the percentage of specific lysis (mean ± SD) and are representative of at least 3 independent experiments. *P < .05. **P < .005.
Altogether, these results suggest that mAb 256 binds to surface molecules, which probably play a specific and critical role in the recognition by several human Vδ2− γδ T-cell subsets of a wide range of B lymphoid target cells.
mAb 256 binds to approximately 110 kDa cell surface proteins widely expressed on human B cells and PBMCs
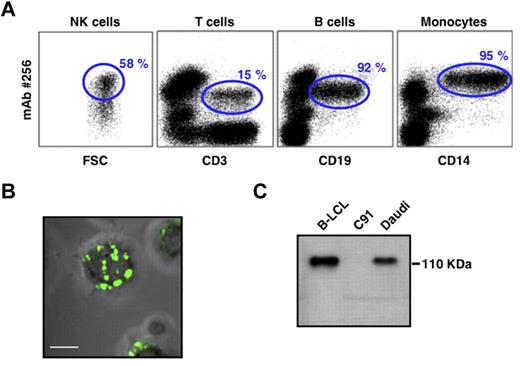
To better characterize the nature of the molecule(s) bound by mAb 256, we first analyzed their cellular distribution. mAb 256 stained almost all types of transformed B cells tested (LCLs and lymphomas; data not shown). Within ex vivo human PBMCs, mAb 256 bound to a fraction of T cells, most NK cells (although at heterogeneous levels) and brightly stained almost all CD19+ B cells and CD14+ monocytes (Figure 3A). Confocal microscopy analysis of Raji cells confirmed that the Ag 256 was mainly expressed at the cell surface and showed a distribution within numerous patches on B-cell membranes (Figure 3B). Finally, the Ag#256 was susceptible to proteolytic digestion by pronase (data not shown), which supported the protein nature of these molecules that were detected by Western blot as a single band of approximately 110 kDa in B-LCL and Daudi cell lysates (Figure 3C).
PBMC expression and biochemical features of mAb 256 ligand(s). (A) Surface expression of Ag#256 on NK cells (CD3−, CD56+), T cells (CD3+), B cells (CD19+), and monocytes (CD14+) within ex vivo human PBMCs was analyzed by flow cytometry (mAb 256, 10 μg/mL). Values for the frequency of Ag#256+ cells within each total cell subset are indicated in the quadrants (gated events). Results are representative of at least 3 independent experiments performed with PBMCs of different donors. (B) Surface expression of Ag#256 (green staining) was analyzed on Raji cells by confocal microscopy. Scale bar represents 5 μm. No significant staining was detected when purified mouse IgG1 (isotype control) was used as the primary mAb. (C) Cell lysates of human B-LCL (Do#AD), C91 T2.2, and Daudi cell lines were analyzed by immunoblotting using mAb 256 as primary mAb.
PBMC expression and biochemical features of mAb 256 ligand(s). (A) Surface expression of Ag#256 on NK cells (CD3−, CD56+), T cells (CD3+), B cells (CD19+), and monocytes (CD14+) within ex vivo human PBMCs was analyzed by flow cytometry (mAb 256, 10 μg/mL). Values for the frequency of Ag#256+ cells within each total cell subset are indicated in the quadrants (gated events). Results are representative of at least 3 independent experiments performed with PBMCs of different donors. (B) Surface expression of Ag#256 (green staining) was analyzed on Raji cells by confocal microscopy. Scale bar represents 5 μm. No significant staining was detected when purified mouse IgG1 (isotype control) was used as the primary mAb. (C) Cell lysates of human B-LCL (Do#AD), C91 T2.2, and Daudi cell lines were analyzed by immunoblotting using mAb 256 as primary mAb.
These results indicated that mAb 256 specifically recognizes membrane-bound protein(s) of approximately 110 kDa, which are highly expressed on B cell-related targets, such as Daudi, Raji, and B-LCLs, as well as on main subsets of human PBMCs.
mAb 256 recognizes the ILT2 (LILRB1/CD85j) receptor
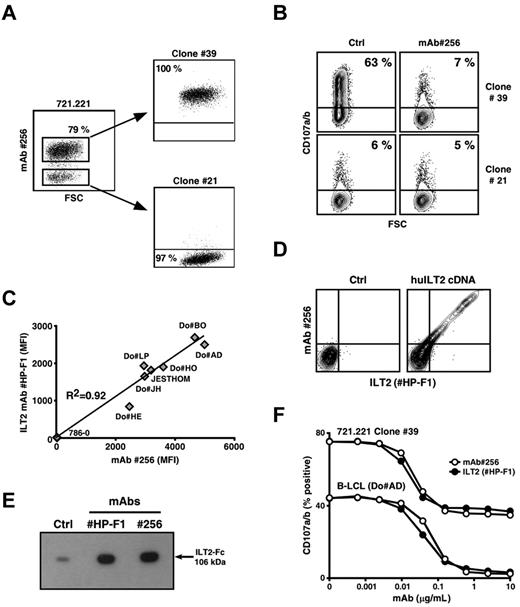
Staining of various human cell lines by mAb 256 revealed the presence of 2 clearly distinct cell subsets within a batch of the 721.221 cell line, an MHC class I− B-LCL variant generated by EMS-induced mutagenesis.26 The lack of Ag#256 surface expression by a substantial fraction of 721.221 B cells (∼ 20%) was stable and not the result of the presence of cell contaminants (data not shown). Both subsets were next sorted, and several clonal populations, expressing or not Ag#256 molecules, were isolated (Figure 4A). Strong up-regulation of CD107a/b occurred in Vγ8Vδ3 T cells after short-term incubation with Ag#256+ 721.221 B cells (clone 39; Figure 4B upper left dot plot). This reactivity was drastically reduced in the presence of the mAb 256 (Figure 4B upper right dot plot), that is, to background levels observed after coincubation of Vγ8Vδ3 T cells with Ag#256-negative B-LCL variants (Figure 4B lower dot plots).
mAb 256 specifically recognizes the LILRB1/CD85j/ILT2 receptor. (A) Expression of Ag#256 was analyzed on 721.221 human B cells by flow cytometry. Ag#256+ and Ag#256− B cells were next isolated by cell sorting and cloned. Values for the frequency of Ag#256 + and Ag#256− cells within each cell subset are indicated in the quadrants. (B) Vγ8Vδ3 T cells (clone 73R9) were incubated together with Ag#256+ (39) and Ag#256− (21) 721.221 B-cell clones (ratio: 1/1) in the absence (Ctrl, left) or presence (right) of mAb 256 (10 μg/mL). CD107a/b mobilization was measured by flow cytometry within activated γδ T cells. Values for the percentage of CD107a/b+ T cells are indicated in the quadrants. (C) Seven human B-cell lines and a negative control human renal carcinoma line (786–0) were stained with either α-ILT2 specific mAb (HP-F1, 10 μg/mL) or mAb 256 (10 μg/mL). Correlation coefficient (R2) calculation between ILT2 and Ag#256 mean fluorescence intensities (MFIs) obtained after analysis by flow cytometry is indicated on the graph. (D) Human osteosarcoma cells (U2OS) were stably transfected with either empty (left) or human ILT2 cDNA (right) plasmids, costained with α-ILT2 specific mAb (HP-F1, 10 μg/mL) and mAb 256 (10 μg/mL), and analyzed by flow cytometry. (E) The binding of both α-ILT2 specific mAb (HP-F1) and mAb 256 to recombinant human ILT2-Fc molecule (106 kDa) was analyzed by immunoblotting. Ctrl indicates secondary mAb alone. (F) Human B-LCL (Do#AD) and Ag#256+ 721.221 B cells (clone 39) were preincubated for 15 minutes with either α-ILT2 mAb (HP-F1, ●) or mAb 256 (○) at the indicated increasing concentrations. Vγ8Vδ3 T cells (clone 73R9) were next incubated for 4 hours together with B cells (ratio = 1:1). Values for the percentage of CD107a/b+ γδ T cells are indicated in the graph. Data are representative of at least 3 independent experiments.
mAb 256 specifically recognizes the LILRB1/CD85j/ILT2 receptor. (A) Expression of Ag#256 was analyzed on 721.221 human B cells by flow cytometry. Ag#256+ and Ag#256− B cells were next isolated by cell sorting and cloned. Values for the frequency of Ag#256 + and Ag#256− cells within each cell subset are indicated in the quadrants. (B) Vγ8Vδ3 T cells (clone 73R9) were incubated together with Ag#256+ (39) and Ag#256− (21) 721.221 B-cell clones (ratio: 1/1) in the absence (Ctrl, left) or presence (right) of mAb 256 (10 μg/mL). CD107a/b mobilization was measured by flow cytometry within activated γδ T cells. Values for the percentage of CD107a/b+ T cells are indicated in the quadrants. (C) Seven human B-cell lines and a negative control human renal carcinoma line (786–0) were stained with either α-ILT2 specific mAb (HP-F1, 10 μg/mL) or mAb 256 (10 μg/mL). Correlation coefficient (R2) calculation between ILT2 and Ag#256 mean fluorescence intensities (MFIs) obtained after analysis by flow cytometry is indicated on the graph. (D) Human osteosarcoma cells (U2OS) were stably transfected with either empty (left) or human ILT2 cDNA (right) plasmids, costained with α-ILT2 specific mAb (HP-F1, 10 μg/mL) and mAb 256 (10 μg/mL), and analyzed by flow cytometry. (E) The binding of both α-ILT2 specific mAb (HP-F1) and mAb 256 to recombinant human ILT2-Fc molecule (106 kDa) was analyzed by immunoblotting. Ctrl indicates secondary mAb alone. (F) Human B-LCL (Do#AD) and Ag#256+ 721.221 B cells (clone 39) were preincubated for 15 minutes with either α-ILT2 mAb (HP-F1, ●) or mAb 256 (○) at the indicated increasing concentrations. Vγ8Vδ3 T cells (clone 73R9) were next incubated for 4 hours together with B cells (ratio = 1:1). Values for the percentage of CD107a/b+ γδ T cells are indicated in the graph. Data are representative of at least 3 independent experiments.
Having established a tight correlation between Ag#256 expression by 721.221 B cells and their ability to activate Vγ8Vδ3 T cells, we selected 721.221 B-cell variants expressing or not Ag#256 (clones 39 and 21) for a comparative microarray analysis of gene expression. Among differentially expressed genes, increased levels of mRNA coding for LILRB1 (for leukocyte Ig-like receptor, subfamily B, member 1) were detected within the Ag#256+ clone, compared with its negative counterpart (data not shown). In line with previous studies suggesting similar cell expression and distribution patterns between ILT2 and Ag#256 in PBMCs,20,27 additional experiments relying on the use of specific anti–human ILT2 mAbs unambiguously identified ILT2 as the surface molecule specifically recognized by mAb 256. This assumption was supported by: (1) the tight correlation between the mean fluorescence intensity values obtained after staining of various cell lines with either ILT2-specific or 256 mAbs (Figure 4C), (2) costaining of human ILT2 transfectants by ILT2-specific and 256 mAbs (Figure 4D), (3) Western blot analysis of recombinant human ILT2-Fc molecules (Figure 4E) and, finally (4) comparative titrations of the inhibitory effect of anti-ILT2 and 256 mAbs on Vγ8Vδ3 T-cell reactivity, after preincubation of B cells with these mAbs (Figure 4F). Importantly, no expression of ILT2 was detected on clone 73R9 used in these experiments (data not shown).
Taken together, these results indicate that ILT2, initially described as an inhibitory receptor expressed by NK and T cells, plays a mandatory role in the antigenic activation of particular subsets of human Vδ2− γδ T cells, when expressed on B lymphoid target cells.
B cell-expressed ILT2 surface molecules are necessary for the antigenic activation of the Vγ8Vδ3 T-cell clone 73R9 but do not interact with the γδ TCR
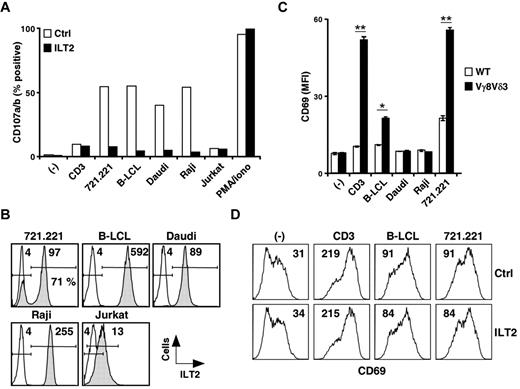
We next assessed induction of CD107a/b up-regulation on Vγ8Vδ3 T cells (73R9) after incubation with different ILT2bright target cells (eg, 721.221, B-LCLs, Daudi, Raji cells) or ILT2−/dim cells (eg, Jurkat T cells). Whereas saturating doses of anti-TCR/CD3 mAbs and ILT2dim cells had a weak stimulatory effect, strong CD107 up-regulation was induced by all ILT2bright target cells tested and efficiently abrogated by anti-ILT2 mAbs (Figure 5A; and data not shown). Strikingly, similar levels of γδ T-cell activation (% of CD107a/b) were observed in the presence of these various ILT2bright B cells (Figure 5A), despite major differences in terms of ILT2 expression intensities (Figure 5B). We next tested whether the necessary contribution of ILT2 for γδ T-cell activation involved direct ILT2 recognition by γδ TCRs (eg, Vγ8Vδ3). As shown in Figure 5C, a significant TCR-mediated activation of Jurkat J.RT3-T3.5 T-cell transfectants, expressing Vγ8 and Vδ3 TCR chains from clone 73R9 (supplemental Figure 3), was detected after coculture with both B-LCL and 721.221 cells. Surprisingly, Daudi and Raji target cells, which both strongly expressed ILT2 and were previously shown to induce strong degranulation of activated Vγ8Vδ3 T cells, were unable to stimulate Jurkat Vγ8Vδ3 TCR transductants. Moreover, we failed to detect any inhibitory effect of anti-ILT2 mAbs on the activation of Vγ8Vδ3 TCR Jurkat transductants induced by B-LCLs and 721.221 cells (Figure 5D). Finally, we noticed that numerous ILT2− cell lines, including the 721.221 B-cell clone 21, were able to strongly activate Vγ8Vδ3 TCR Jurkat transductants, despite their weak ability to stimulate the Vγ8Vδ3 T-cell clone 73R9 (data not shown).
B cell-expressed ILT2 acts as a ligand for a costimulatory receptor for the antigenic activation of Vγ8Vδ3 T cells. (A) The 73R9 γδ T-cell clone (Vγ8Vδ3) was incubated for 4 hours together with different target cells (721.221, B-LCL, Do#AD; Daudi, Raji, and Jurkat) in the absence (open bars) or presence (filled bars) of α-ILT2 mAb (256, 10 μg/mL). CD107a/b mobilization was measured by flow cytometry, and the values for the percentage of CD107a/b+ γδ T cells are indicated. − indicates no stimulation; CD3, α-CD3 mAb (UCHT1, 5 μg/mL); and PMA/Iono, phorbol myristate acetate and ionomycin control. (B) Target cells described in panel A were stained with α-ILT2 mAb (256, shaded histograms) or control mAb (IgG1, open histograms) and analyzed by flow cytometry. Values for MFI of ILT2+ cells are indicated. The frequency of ILT2+ cells within the 721.221 B-cell line is indicated. (C) J.RT3-T3.5 Jurkat T cells expressing the 73R9 TCR (Vγ8Vδ3, filled bars) or not (WT, open bars) were incubated for 4 hours with different human B-cell lines (B-LCL, Do#AD; Daudi, Raji, and 721.221). Surface expression of CD69 on Jurkat T cells was measured by flow cytometry. − indicates no stimulation; and CD3, α-CD3 mAb (UCHT1, 5 μg/mL). Data are MFI of triplicate samples (mean ± SD) and are representative of at least 3 independent experiments. *P < .05. **P < .005. (D) Vγ8Vδ3 (73R9) Jurkat T cells were incubated for 4 hours together with human B cells (721.221 or B-LCL, Do#AD) in the absence (Ctrl) or presence of α-ILT2 mAb (256, 10 μg/mL, ILT2). − indicates no stimulation; and CD3, α-CD3 mAb (UCHT1, 5 μg/mL). Surface expression of CD69 on Jurkat T cells was measured by flow cytometry. Values for the MFI of CD69 stainings are indicated. Data are representative of at least 3 independent experiments.
B cell-expressed ILT2 acts as a ligand for a costimulatory receptor for the antigenic activation of Vγ8Vδ3 T cells. (A) The 73R9 γδ T-cell clone (Vγ8Vδ3) was incubated for 4 hours together with different target cells (721.221, B-LCL, Do#AD; Daudi, Raji, and Jurkat) in the absence (open bars) or presence (filled bars) of α-ILT2 mAb (256, 10 μg/mL). CD107a/b mobilization was measured by flow cytometry, and the values for the percentage of CD107a/b+ γδ T cells are indicated. − indicates no stimulation; CD3, α-CD3 mAb (UCHT1, 5 μg/mL); and PMA/Iono, phorbol myristate acetate and ionomycin control. (B) Target cells described in panel A were stained with α-ILT2 mAb (256, shaded histograms) or control mAb (IgG1, open histograms) and analyzed by flow cytometry. Values for MFI of ILT2+ cells are indicated. The frequency of ILT2+ cells within the 721.221 B-cell line is indicated. (C) J.RT3-T3.5 Jurkat T cells expressing the 73R9 TCR (Vγ8Vδ3, filled bars) or not (WT, open bars) were incubated for 4 hours with different human B-cell lines (B-LCL, Do#AD; Daudi, Raji, and 721.221). Surface expression of CD69 on Jurkat T cells was measured by flow cytometry. − indicates no stimulation; and CD3, α-CD3 mAb (UCHT1, 5 μg/mL). Data are MFI of triplicate samples (mean ± SD) and are representative of at least 3 independent experiments. *P < .05. **P < .005. (D) Vγ8Vδ3 (73R9) Jurkat T cells were incubated for 4 hours together with human B cells (721.221 or B-LCL, Do#AD) in the absence (Ctrl) or presence of α-ILT2 mAb (256, 10 μg/mL, ILT2). − indicates no stimulation; and CD3, α-CD3 mAb (UCHT1, 5 μg/mL). Surface expression of CD69 on Jurkat T cells was measured by flow cytometry. Values for the MFI of CD69 stainings are indicated. Data are representative of at least 3 independent experiments.
Altogether, these results strongly suggest that ILT2 is not a direct ligand of the Vγ8Vδ3 TCR but, instead, probably delivers critical and necessary costimulatory signals during antigenic activation of particular subsets of human Vδ2− γδ T cells.
ILT2 costimulates the antigenic activation of some Vδ2− γδ T-cell subsets through engagement of MHC class I molecules
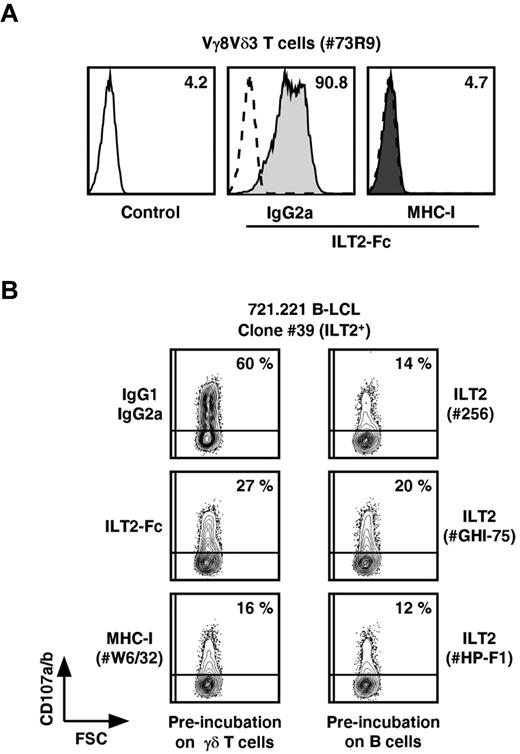
We next sought whether interactions between ILT2 and MHC class I membrane molecules, the only ILT2 ligands so far described, could deliver a mandatory costimulatory signal for productive γδ T-cell activation when expressed by B and T cells, respectively. The establishment of specific interactions between recombinant human ILT2-Fc and MHC class I molecules expressed on Vγ8Vδ3 T cells was first assessed by cytometric analysis (Figure 6A). These interactions were abrogated on preincubation of T cells with anti–MHC class I mAbs. Vγ8Vδ3 T-cell degranulation induced by ILT2+ 721.221 B cells (clone 39) was inhibited by different anti-ILT2 mAbs. Similarly, preincubation of effector Vγ8Vδ3 T cells with ILT2-Fc molecules or anti–MHC class I mAbs reduced their reactivity against ILT2+ B-cell targets (Figure 6B). Of note, the specificity of blocking mAbs and ILT2-Fc molecules was assessed by the lack of MHC class I and ILT2 molecules expression on, respectively, 721.221 B cells and γδ T-cell effectors.
Inhibition of B-LCL-induced activation of Vγ8Vδ3 T cells by ILT2-Fc molecules and mAbs specific for ILT2 or MHC class I molecules. (A) The 73R9 γδ T-cell clone (Vγ8Vδ3) was preincubated for 15 minutes with either isotypic control mAb (IgG2a) or α-MHC-I mAb (W6/32, 10 μg/mL, MHC-I) and next stained with soluble human recombinant ILT2-Fc molecules (30 μg/mL). Values for MFI of ILT2 staining measured by flow cytometry are indicated. Control indicates secondary mAb alone. (B) The 73R9 γδ T-cell clone (Vγ8Vδ3) was incubated for 4 hours with an ILT2+ B-cell line (721.221, clone 39). Control mAb (IgG1, IgG2a), ILT2-Fc molecules (30 μg/mL), α-MHC-I mAb (W6/32, 10 μg/mL), or α-ILT2 mAbs (256, GHI-75, HP-F1, 10 μg/mL) were preincubated with either effector (left) or target cells (right), as indicated. CD107a/b mobilization was measured by flow cytometry, and values for the percentage of CD107a/b+ γδ T cells are indicated in the quadrants. Data are representative of at least 3 independent experiments.
Inhibition of B-LCL-induced activation of Vγ8Vδ3 T cells by ILT2-Fc molecules and mAbs specific for ILT2 or MHC class I molecules. (A) The 73R9 γδ T-cell clone (Vγ8Vδ3) was preincubated for 15 minutes with either isotypic control mAb (IgG2a) or α-MHC-I mAb (W6/32, 10 μg/mL, MHC-I) and next stained with soluble human recombinant ILT2-Fc molecules (30 μg/mL). Values for MFI of ILT2 staining measured by flow cytometry are indicated. Control indicates secondary mAb alone. (B) The 73R9 γδ T-cell clone (Vγ8Vδ3) was incubated for 4 hours with an ILT2+ B-cell line (721.221, clone 39). Control mAb (IgG1, IgG2a), ILT2-Fc molecules (30 μg/mL), α-MHC-I mAb (W6/32, 10 μg/mL), or α-ILT2 mAbs (256, GHI-75, HP-F1, 10 μg/mL) were preincubated with either effector (left) or target cells (right), as indicated. CD107a/b mobilization was measured by flow cytometry, and values for the percentage of CD107a/b+ γδ T cells are indicated in the quadrants. Data are representative of at least 3 independent experiments.
These observations indicate that the ILT2 receptor, when expressed by B lymphoid cells, provides critical and mandatory costimulation anti–transformed B-cell responses mediated by a subset of human Vδ2− γδ T cells, through interactions with their MHC class I surface molecules.
Discussion
Most of our understanding of human γδ T-cell immunobiology has been drawn from analyses of the Vγ9Vδ2 T-cell subset, which represents the major γδ PBL population in adults. However, the localization, functions, and antigenic/costimulation requirements of human Vδ2− T-cell subsets remain ill defined. To further address these issues and identify key molecules implicated in the activation process of human Vδ2− γδ T-cell subsets, we took advantage of mixed lymphocyte-tumor culture assays to first isolate tumor reactive subsets among human Vδ2− γδ T cells. We showed that PBL-derived Vδ2− γδ T cells, although initially detected at very low frequencies in adult PBMCs, predominated after 2 weeks of culture in the presence of Raji tumor cells, a Burkitt lymphoma cell line. After cell sorting and cloning, we further demonstrated that several polyclonal and clonal Vδ2− γδ T-cell populations reacted against transformed human B cells, such as the Raji and Daudi Burkitt lymphoma cells and EBV+ B-LCLs, as evidenced in cytolysis and proliferation assays. In contrast, Vγ9Vδ2 T cells did not react to any B-cell targets, with the exception of Daudi cells, in line with previous observations showing the absence of cytolytic and proliferative responses of Vγ9Vδ2 T cells against human B-LCLs.28
Interestingly, an important fraction of PBL-γδ T-cell lines and clones that reacted against B cells expressed a Vδ3+ γδ TCR, frequently associated with Vγ8 chains. Several other Vδ3+ T-cell lines and clones were next isolated from PBLs of different donors and displayed similar B-cell reactivity patterns. Moreover, our data further showed that various Vγ8Vδ3 T-cell polyclonal and clonal populations of thymic origin exhibited similar reactivities against B cells, thus indicating that these intrinsic functional responses did not reflect a bias response of peripheral populations. Human Vδ3+ γδ T cells, rarely detected in PBLs of healthy adults, are generally located in liver and gut epithelia. It will be interesting to determine whether similar B-cell reactivity is observed among mucosal human Vδ3+ γδ T cells. Our knowledge about human Vδ3+ γδ T-cell subsets remains very limited as these cells have been primarily studied under pathologic circumstances. These subsets were identified within lymphoid infiltrates during local tissue inflammation, such as synovial fluids in rheumatoid arthritis or epithelia in celiac disease, where expression of the Vδ3 segment was frequently associated with the Vγ8 chain.29,30 Increased frequency of Vδ3+ subsets was also detected within PBLs and skin of lupus patients31,32 or after viral infections by HIV33 or cytomegalovirus (CMV), particularly in an immunodepressed environment.34-36 In these latter studies, dramatic expansion of Vδ1, Vδ3, and Vδ5 γδ T-cell subsets expressing diverse Vγ chains was detected in the PBLs of patients receiving renal, lung, or stem cell allografts. Importantly, Vδ1 and Vδ3 T cells from CMV-infected transplanted patients specifically proliferated in response to CMV-infected fibroblast lysates through a TCR-dependent mechanism. CMV infection in transplanted patients was accompanied by a striking restriction of CDR3 size distribution in the Vδ3 segment. In a recent study, Vermijlen et al also detected oligoclonal expansions of Vδ2− subsets of γδ T cells, including Vδ3+ T cells, in the cord blood of CMV-infected newborns.37 Altogether, these studies showing important peripheral expansions of rare γδ T-cell subsets, including Vδ3+ T cells, suggest that most of these T cells probably originate from tissues, where they are activated after CMV or HIV infection and redistributed into the blood.38 Interestingly, our results are fully in line with 2 previous studies describing peripheral expansions of Vδ1+ and Vδ3+ γδ T cells in PBLs of patients with EBV-infected Burkitt lymphoma cells or B-cell leukemia.39,40 Accordingly, we showed that several subsets of human Vδ2− γδ T cells, originally present at low frequencies in PBLs of healthy donors, are able to recognize a variety of B-cell lymphomas and EBV+ lymphoid B cells, in an MHC-independent manner. Importantly, B-LCLs or B-cell tumors induced strong in vitro proliferative and cytolytic γδ T-cell functional responses. Antitumor reactivities of Vδ3+ T cells have been further confirmed in experiments showing their ability to efficiently lyse a variety of human myeloma cell lines (data not shown). Altogether, these results suggest that peripheral Vδ2− subsets of γδ T cells, as well as the major Vγ9Vδ2 T-cell population, play complementary roles in immunosurveillance against lymphoid and myeloid tumor cells.
Comparative analysis of γδ T-cell reactivities and tumor-binding patterns of multimerized recombinant γδ TCRs (data not shown) suggested that several subsets of human Vδ2− γδ T-cell populations, which express different TCR V segments (eg, Vγ8, Vγ9, Vδ1, and Vδ3), show antigenic reactivity patterns clearly distinct from that of Vγ9Vδ2 T cells. In an attempt to identify new Vδ2− γδ TCR ligand(s), we immunized mice against a B-LCL target and selected mAbs that specifically inhibited Vδ2− γδ CTL responses to B lymphoma lines. Surprisingly, although mAb 256 did not affect the activation of Vγ9Vδ2 T cells induced by the same Daudi target cells, it had also no effect on the reactivity of Vγ8Vδ3 TCR Jurkat transductants. This suggested that Ag#256 molecules, which play a key role in the activation process of Vδ2− T cells by B-LCLs and tumors B cells, was not a Vδ2− TCR ligand. Nevertheless, our results clearly indicated that Ag#256, further characterized for its protein nature and cell surface expression, was expressed not only by B lymphoid cells but also by many PBMC subsets and plays a key role as a costimulatory molecule of antitumor responses mediated by Vδ2− T cells.
Although we failed to isolate and identify the cell membrane molecules bound to mAb 256 by biochemistry approaches, RNA profiling of B-LCL variants that differed by their expression of the 256 epitope allowed us to identify the ILT2 receptor as the Ag of mAb 256. Leukocyte immunoglobulin-like receptors are encoded by a set of genes within the leukocyte receptor cluster on chromosome 19q13.4, adjacent to the killer Ig-like receptor genes that are responsible for controlling NK- and CD8 T-cell survival and effector functions.41,42 The ILT2 inhibitory receptor, also known as LILRB1/leukocyte immunoglobulin-like receptor-1/CD85j, is the only leukocyte immunoglobulin-like receptor expressed by human T cells, including γδ T cells, and has been shown to down-regulate their effector functions.19,23,43 ILT2 is an immunoreceptor tyrosine-based inhibitory motif-bearing receptor for most classic and nonclassic MHC class I molecules, and directly interacts with the monomorphic α3 domain of MHC class I and the associated β2-microglobulin molecules. Interestingly, ILT2 is expressed by a variety of myeloid and lymphoid cell subsets, such as dendritic cells, but its functions in the latter cell subsets remain ill defined.24,44 In B cells, cross-linking of ILT2 has been shown to inhibit both IgG and IgE production and to down-regulate cytokine production.45
To our knowledge, this study describes a new role played by ILT2 as a critical costimulatory molecule of Vδ2− γδ T-cell activation, when expressed by B lymphoid cell targets. Moreover, our results suggest that this effect is probably not the result of target cell alterations induced by ILT2 signaling, but rather by ILT2-mediated engagement of MHC class I molecules on effector γδ T cells. These findings extend previous studies showing that engagement of MHC class I molecules by natural ligands, such as CD8 or CD160, or anti–MHC class I mAbs can provide a signal to T cells that may either amplify functional responses induced by TCR/CD3 crosslinking or induce cell death by apoptosis.46-49 Whether MHC class I costimulation is induced in γδ T cells through specific signal pathways or is consecutive of a particular remodeling of the immunologic synapse between effector and target cells, eventually associated with a particular kinetic of activation, remains to be determined. However, a comparative preliminary analysis by confocal microscopy revealed no differences of ILT2, TCR/CD3, or MHC class I molecule expression or localization on conjugates established between either ILT2+ or ILT2− B-LCLs and Vγ8Vδ3 T cells (data not shown). Thus, in addition to their well-described ability to present peptides to TCRs, MHC class I molecules might be directly involved in T-cell costimulation, especially for T-cell subsets expressing low levels of “classic” costimulatory molecules, such as γδ T cells. Importantly, such a costimulatory process is probably not restricted to either particular MHC class I molecules, as indicated by the broad range of specificities described for ILT2 binding (HLA-A, B, C, E, F, and G alleles) as well as the extended reactivities of a variety of Vδ2− γδ T-cell populations from different donors, or ILT2, as many other MHC class I-ligands, such as the CD166 natural killer receptor have also been identified.41
In conclusion, this study provides new insights into the activation modalities of ill-defined human γδ T-cell subsets against stressed/transformed lymphoid or myeloid cells. The fact that ILT2 is strongly up-regulated along several physiopathologic processes, associated with expansion of Vδ2− γδ T-cell subsets (eg, HIV and CMV infections)50 would suggest a more prominent costimulatory function played by this receptor in this particular pathologic circumstances, an issue that remains to be formally tested. On a more general standpoint, our results provide new evidence for the importance of costimulatory signals triggered in some particular T-cell subsets by “nonprofessional” surface partners, such as MHC class I molecules.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Philippe Hulin and the PICell plateform (IFR26, Nantes, France) for providing expert technical assistance in videomicroscopy, Michelle Heslan (Inserm U643, Nantes, France) for her help with cell sorting on the FACSAria, Dr Miguel Lopez-Botet (IMIM, Barcelona, Spain) for providing the ILT2 plasmid, and M. J. Loirat (EFS, Nantes, France) for providing blood samples.
This work was supported by Inserm, Université de Nantes, Agence Nationale de la Recherche (no. ANR A05118GS), Association pour la Recherche sur le Cancer (no. 4953), and the Commission of the European Union Program (Cancer Immunotherapy; no. E06005NP/EEA06004GNP).
Authorship
Contribution: C.H., M.-A.P., and S.N. performed experiments; C.H. and E.S. analyzed results and made the figures; J.D.-M., M.B., and E.S. designed the research; C.H., M.B., and E.S. wrote the paper; and all authors read and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emmanuel Scotet, Inserm UMR 892, CRCNA, IRT UN, 8 quai Moncousu, 44007 Nantes cedex, France; e-mail: Emmanuel.Scotet@inserm.fr; and Marc Bonneville, Inserm UMR 892, CRCNA, IRT UN, 8 quai Moncousu, 44007 Nantes cedex, France; e-mail: bonnevil@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal