To the editor:
We read with interest the paper in Blood from Asselta et al1 describing a form of human factor XI (fXI) encoded by an mRNA splice variant lacking exons 6 and 7 (fXI-Δ6/7). We observe 3 bands when plasma fXI purified by IgG affinity chromatography is size fractionated by nonreducing gel electrophoresis (Figure 1A). The dominant 160 kDa form contains 2 identical subunits connected by a disulfide bond.2-4 The fainter band (70-80 kDa) migrating with reduced fXI represents single subunits. The third band, marked X in Figure 1A, is likely the approximately 105-kDa species observed in plasma immunoprecipitates by Asselta et al.1 FXI subunits contain 4 apple domains (A1-A4, exons 3-10) and a protease domain (exons 11-15).2,3 A fXI-Δ6/7 subunit would lack parts of A2 and A3 (amino acids 145-234). However, modeling (Figure 1B) indicates a complete apple domain could form from the N- and C-terminal portions of A2 and A3, respectively, encoded by exons 5 and 8.5 Asselta et al showed that fibroblasts transfected with fXI-Δ6/7 cDNA synthesize a protein that may have this hybrid domain,1 and postulated that fXI-Δ6/7 and the 105-kDa form in plasma are identical based on electrophoretic mobility. Here we show that the 105-kDa plasma form is not likely to be fXI-Δ6/7.
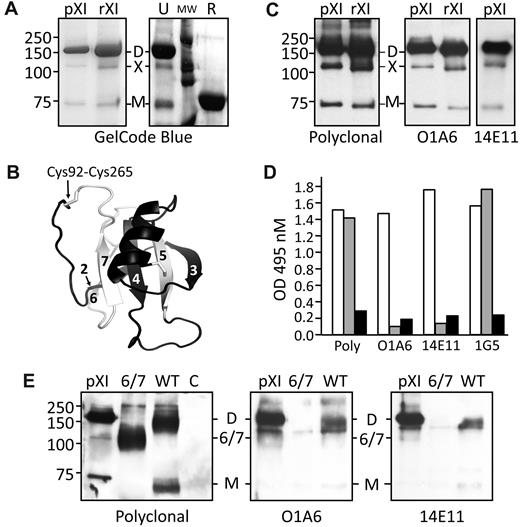
SDS-polyacrylamide gel electrophoresis, western immunoblots, and ELISA of human factor XI. (A left panel) GelCode blue–stained 10% polyacrylamide–sodium dodecyl sulfate gel of plasma (pXI) or recombinant (rXI) human fXI (2-4 μg) purified using a monoclonal IgG against the A3 domain (pXI) or protease domain (rXI). (A right panel) Recombinant fXI (4 μg) run unreduced (U) or reduced (R). MW indicates molecular weight markers. (B) Ribbon model of a hypothetical hybrid apple domain encoded by the fXI Δ6/7 mRNA, based on the primary amino acid sequence encoded by exons 5 (black ribbon) and 8 (white ribbon) and the crystal structure of human fXI.5 The domain is bounded by a disulfide bond between Cys92 and Cys265. Strands 2-7 of the β-sheet are numbered. (C) Chemiluminescent Western blots of plasma (pXI) or recombinant (rXI) fXI using goat polyclonal anti–factor XI IgG (left panel), monoclonal IgG O1A6 (recognizes A3 domain; center panel), or monoclonal IgG 14E11 (recognizes A2 domain; right panel) as the primary antibody. (D) ELISA of cell lysates from HEK293 fibroblasts transiently transfected with expression constructs for WT-fXI (□), fXI-Δ6/7 ( ), or empty vector (pJVCMV6,7 ; ■). The ELISA capture antibodies were a goat polyclonal anti–human fXI IgG (Poly), O1A6, 14E11, or a monoclonal antibody against the protease domain (1G5). The detection antibody was a goat polyclonal anti–human fXI IgG conjugated to horseradish peroxidase. (E) Chemiluminescent Western blots of immunoprecipitates of lysates of HEK293 cells transfected with WT-fXI (WT), fXI-Δ6/7 (Δ6/7), or empty vector (C). pXI is a plasma fXI control. Immunoprecipitation was done with IgG 1G5.6,7 Primary antibodies are indicated below each blot. For panels A, C, and E, positions of molecular mass standards (in kDa) are indicated on the left. D indicates fXI dimer; M, fXI monomer; X, putative fXI Δ6/7 splice variant; and 6/7, fXI-6/7 splice variant.
), or empty vector (pJVCMV6,7 ; ■). The ELISA capture antibodies were a goat polyclonal anti–human fXI IgG (Poly), O1A6, 14E11, or a monoclonal antibody against the protease domain (1G5). The detection antibody was a goat polyclonal anti–human fXI IgG conjugated to horseradish peroxidase. (E) Chemiluminescent Western blots of immunoprecipitates of lysates of HEK293 cells transfected with WT-fXI (WT), fXI-Δ6/7 (Δ6/7), or empty vector (C). pXI is a plasma fXI control. Immunoprecipitation was done with IgG 1G5.6,7 Primary antibodies are indicated below each blot. For panels A, C, and E, positions of molecular mass standards (in kDa) are indicated on the left. D indicates fXI dimer; M, fXI monomer; X, putative fXI Δ6/7 splice variant; and 6/7, fXI-6/7 splice variant.
SDS-polyacrylamide gel electrophoresis, western immunoblots, and ELISA of human factor XI. (A left panel) GelCode blue–stained 10% polyacrylamide–sodium dodecyl sulfate gel of plasma (pXI) or recombinant (rXI) human fXI (2-4 μg) purified using a monoclonal IgG against the A3 domain (pXI) or protease domain (rXI). (A right panel) Recombinant fXI (4 μg) run unreduced (U) or reduced (R). MW indicates molecular weight markers. (B) Ribbon model of a hypothetical hybrid apple domain encoded by the fXI Δ6/7 mRNA, based on the primary amino acid sequence encoded by exons 5 (black ribbon) and 8 (white ribbon) and the crystal structure of human fXI.5 The domain is bounded by a disulfide bond between Cys92 and Cys265. Strands 2-7 of the β-sheet are numbered. (C) Chemiluminescent Western blots of plasma (pXI) or recombinant (rXI) fXI using goat polyclonal anti–factor XI IgG (left panel), monoclonal IgG O1A6 (recognizes A3 domain; center panel), or monoclonal IgG 14E11 (recognizes A2 domain; right panel) as the primary antibody. (D) ELISA of cell lysates from HEK293 fibroblasts transiently transfected with expression constructs for WT-fXI (□), fXI-Δ6/7 ( ), or empty vector (pJVCMV6,7 ; ■). The ELISA capture antibodies were a goat polyclonal anti–human fXI IgG (Poly), O1A6, 14E11, or a monoclonal antibody against the protease domain (1G5). The detection antibody was a goat polyclonal anti–human fXI IgG conjugated to horseradish peroxidase. (E) Chemiluminescent Western blots of immunoprecipitates of lysates of HEK293 cells transfected with WT-fXI (WT), fXI-Δ6/7 (Δ6/7), or empty vector (C). pXI is a plasma fXI control. Immunoprecipitation was done with IgG 1G5.6,7 Primary antibodies are indicated below each blot. For panels A, C, and E, positions of molecular mass standards (in kDa) are indicated on the left. D indicates fXI dimer; M, fXI monomer; X, putative fXI Δ6/7 splice variant; and 6/7, fXI-6/7 splice variant.
), or empty vector (pJVCMV6,7 ; ■). The ELISA capture antibodies were a goat polyclonal anti–human fXI IgG (Poly), O1A6, 14E11, or a monoclonal antibody against the protease domain (1G5). The detection antibody was a goat polyclonal anti–human fXI IgG conjugated to horseradish peroxidase. (E) Chemiluminescent Western blots of immunoprecipitates of lysates of HEK293 cells transfected with WT-fXI (WT), fXI-Δ6/7 (Δ6/7), or empty vector (C). pXI is a plasma fXI control. Immunoprecipitation was done with IgG 1G5.6,7 Primary antibodies are indicated below each blot. For panels A, C, and E, positions of molecular mass standards (in kDa) are indicated on the left. D indicates fXI dimer; M, fXI monomer; X, putative fXI Δ6/7 splice variant; and 6/7, fXI-6/7 splice variant.
First, the 160- and 105-kDa species are both secreted by fibroblasts transfected with full-length factor XI cDNA (Fig 1A).6,7 Second, monoclonal antibodies that bind to the A3 (O1A6)8,9 or A2 (14E11)9 domains both recognize all 3 fXI forms on Western blots (Figure 1C). We transfected HEK293 fibroblasts with expression constructs for wild type fXI (WT-fXI) and fXI-Δ6/7. In an enzyme-linked immunosorbent assay (ELISA), cell lysates for WT-fXI and fXI-Δ6/7 both gave strong signals when polyclonal anti–human fXI IgG or a monoclonal IgG against the protease domain (1G5)6,7 is the capture antibody (Figure 1D). In contrast, only WT-fXI is captured by O1A6 or 14E11. Immunoprecipitates of cell lysates were studied by Western blotting. Again, polyclonal IgG recognized both WT-fXI and fXI-Δ6/7 (Figure 1E left panel), whereas O1A6 and 14E11 recognized only WT-fXI (Figure 1E center and right panels). These data clearly show that the 105-kDa plasma protein is not identical to the protein from cells transfected with the fXI-Δ6/7 cDNA.
The nature of the 105-kDa plasma species is not clear. It probably is a dimer, as it is not observed in reduced fXI (Figure 1A). Because both 160- and 105-kDa forms are secreted together by cells in serum-free media, they probably vary due to differences in posttranslational modifications. The apple domains of fXI form a tightly packed platform with extensive interdomain interfaces.5,10 Our initial evaluation indicates the hybrid domain in fXI-Δ6/7 would not form interfaces properly, possibly resulting in an arrangement where the apple domains are like beads on a string. The disruption of the platform would likely interfere with binding to high-molecular-weight kininogen,10 a protein that forms a complex with fXI in plasma. This, and the absence of fXI coagulant activity,1 raise questions about the function of such a protein, if it was expressed.
Authorship
Acknowledgment: This work was supported by grants HL58837 and HL81326 from the National Heart, Lung, and Blood Institute.
Contribution: D.G. wrote the initial draft of the letter; M.-f.S. and Q.C. prepared fXI and performed Western blots; A.M. characterized IgG O1A6 and 14E11; A.G. and E.I.T. generated and characterized IgG O1A6 and 14E11; and J.E. modeled fXI apple domains. All authors contributed to writing the final version of the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Gailani, MD, Division of Hematology/Oncology, Vanderbilt University, 777 Preston Research Bldg, 2220 Pierce Ave, Nashville, TN 37232-6305; e-mail: dave.gailani@vanderbilt.edu.
References
National Institutes of Health