Abstract
Leukocyte extravasation depends on various adhesion receptors at endothelial cell contacts. Here we have analyzed how mouse CD99 and CD99L2 cooperate with PECAM-1. We found that antibodies against mouse CD99 and PECAM-1 trap neutrophils between endothelial cells in in vitro transmigration assays. A sequential function, as has been suggested for human PECAM-1 and CD99, could not be demonstrated. In contrast to these in vitro results, blocking CD99 or CD99L2 or gene disruption of PECAM-1 trapped neutrophils in vivo between endothelial cells and the underlying basement membrane as revealed by electron microscopy and by 3-dimensional confocal fluorescence microscopy in the inflamed cremaster tissue. Leukocyte extravasation was inhibited in interleukin-1β-inflamed peritoneum and in the cremaster by PECAM-1 gene disruption and was further attenuated by blocking antibodies against CD99 and CD99L2. In addition, CD99 and CD99L2 were required for leukocyte extravasation in the cremaster after stimulation with tumor necrosis factor-α, where the need for PECAM-1 is known to be bypassed. We conclude that CD99 and CD99L2 act independently of PECAM-1 in leukocyte extravasation and cooperate in an independent way to help neutrophils overcome the endothelial basement membrane.
Introduction
When blood leukocytes emigrate from the circulation into the surrounding tissue to reach sites of injury or infection, they have to cross the vascular wall of postcapillary venules. Capturing and adhesion of leukocytes to the blood vessel wall rely on a series of molecular interactions mediated by selectins, chemokines, and integrins.1-3 Once firmly attached, leukocytes crawl on the apical surface of endothelial cells4 until they find suitable sites for transmigration through the vessel wall, a process described as diapedesis. The actual transmigration process can occur via 2 different pathways: paracellularly through the junctions between cells or transcellularly moving through the body of a single endothelial cell.5-11 The basement membrane is a second barrier that needs to be overcome.12-14
The majority, if not all, of the endothelial membrane and adhesion molecules mediating leukocyte diapedesis are found at endothelial junctions and probably participate in the paracellular (trans-junctional) route. PECAM-1 was the first endothelial adhesion receptor described to participate in this process.15,16 Others are the junctional adhesion molecules (JAM-A, -B, and -C),17-19 the related endothelial cell-selective adhesion molecule (ESAM),20 intercellular adhesion molecule-2 (ICAM-2),21 and the polio virus receptor,22 a member of the nectin family. All of these proteins belong to the large immunoglobulin supergene family. Three of them (ICAM-2, JAM-A, and PECAM-1) have recently been demonstrated to act sequentially during neutrophil diapedesis in the cremaster, based on confocal microscopy of whole-mount tissue stainings. According to these studies, ICAM-2 acts at a very early step when leukocytes probe endothelial contacts, JAM-A participates at a later step when leukocytes move through endothelial contacts, and PECAM-1 is required to overcome the basement membrane.23,24
CD99 is a glycoprotein that does not belong to any known protein family. It was found to participate in the in vitro diapedesis of monocytes through human endothelial cell monolayers.25 CD99 is found on most human leukocytes and at contacts between endothelial cells. Cloning of the mouse ortholog allowed us to generate antibodies against mouse CD99 with which we could show that CD99 indeed participates in leukocyte extravasation in vivo. We found that lymphocyte recruitment into inflamed skin was inhibited by antibodies against CD99.26 In addition, neutrophil recruitment to inflamed peritoneum required CD99, and it was also shown to participate in the extravasation of neutrophils in inflamed cremaster tissue.27 Intravital microscopy of the latter inflammation model demonstrated that CD99 indeed participated in the diapedesis process in vivo and not in capturing and adhesion of leukocytes at the blood vessel wall.
One of the most recently identified novel membrane proteins involved in leukocyte diapedesis is CD99L2.27 It is distantly related to CD99 with only 32% sequence identity and, like CD99, is a highly O-glycosylated membrane protein with a small extracellular domain of only little more than 100 amino acids. CD99L2 is widely distributed.28 It is found on most leukocytes and at cell contacts between endothelial cells. Although present on lymphocytes, it is not involved in lymphocyte extravasation in vivo, although it mediates neutrophil recruitment into inflamed peritoneum and into cremaster tissue. Like CD99, it participates in the diapedesis process but not in capturing and adhesion to the blood vessel wall as revealed by intravital microscopy.27
The impressive progress of recent years in identifying membrane proteins at endothelial contacts that participate in diapedesis has raised the question of how they act in concert. ICAM-2, JAM-A, and PECAM-1 are the only molecules that have been directly compared in this respect in vivo, and indeed act sequentially in the order listed here. Based on in vitro results, CD99 has been suggested to act subsequent to PECAM-1 during diapedesis. In contrast to in vivo results,23 in vitro anti–PECAM-1 antibodies have been reported to arrest leukocytes at the apical surface of endothelial cells,25 and anti-CD99 antibodies block leukocytes at a later step, halfway through endothelial cell contacts.25 The functional relationship of CD99 and PECAM-1 in vivo is unknown.
Here we have directly compared the role of PECAM-1 with the roles of CD99 and CD99L2 in vitro and in vivo in the mouse. We found that in vitro anti–PECAM-1 as well as anti-CD99 antibodies trapped leukocytes between endothelial cells cultured on transwell filters. By contrast, neither electron microscopy nor 3-dimensional (3D) confocal microscopy of stained inflamed cremaster whole mounts permitted detection of any leukocyte accumulation between endothelial cells on in vivo functional blocking of CD99 or CD99L2 with antibodies or by deleting the gene for PECAM-1, but rather lead to trapping of leukocytes in vivo between endothelial cells and basement membrane. In contrast to the sequentially acting ICAM-2, JAM-A, and PECAM-1, which are dependent on each other (ie, inhibitory antibodies have no additive effects24 ), blocking of CD99 or CD99L2 in combination with PECAM-1 deficiency did have additive inhibitory effects on leukocyte extravasation, as shown in 2 different inflammation models in vivo. We conclude that CD99 and CD99L2 act independently of PECAM-1 but at the same site during diapedesis: between endothelial cells and the basement membrane.
Methods
Cell culture
Animals
Ten- to 12-week-old C57BL/6 wild-type (WT) mice (Charles River Laboratories) and PECAM-1–deficient mice30 were used. All experiments were carried out under the German legislation for the protection of animals and were approved by the Landesamt fuer Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen.
Antibodies
Affinity-purified polyclonal rabbit antibodies against mouse CD99 and mouse CD99L2 and F(ab′)2-fragments were generated and purified as described.27 No endotoxin was detectable. Rabbit polyclonal ESAM antibodies have been described.31 Monoclonal antibodies (mAbs) were purified from hybridoma supernatants: anti–mouse ICAM-1 (YN1/1.7, rat IgG-2b, from ATCC), anti–mouse L-selectin (MEL14, from ATCC),32 anti–mouse P-selectin (RB40.34, rat IgG1), anti–mouse PECAM-1 (1G5.1, rat IgG2b; 5D2.6, rat IgG2a),20 and anti–mouse laminin-α5 (4G6, rat).33 Purchased antibodies include: anti–mouse PECAM-1 domain 1 mAb (2H8, IgG, AbD Serotec), anti–mouse CD11b (BD Biosciences), phycoerythrin-labeled goat anti–rabbit IgG, anti–rabbit IgG peroxidase-labeled polyclonal antibodies to rabbit IgG F(ab′)2 (Acris), and anti–mouse MRP-14 (goat; R&D Systems).
Flow cytometric analysis
For staining, 5 × 105 cells were incubated with 5 μg/mL of first antibody in flow cytometry buffer (phosphate-buffered saline [PBS], 1% fetal calf serum, 0.04% azide) at 4°C for 30 minutes. Cells were washed twice with the same buffer and stained with phycoerythrin-conjugated secondary antibodies. Cells were washed twice and analyzed by flow cytometry (FACSCanto; BD Biosciences).
Transmigration assay
Transendothelial migration of bone marrow–derived neutrophils through cultured endothelioma cells was performed as described.27 A total of 5 × 104 bEnd.5 cells/well of 6.5-mm transwells (Corning Life Sciences), coated with 50 μg/mL laminin (Boehringer Mannheim), were grown for 2 days and stimulated 16 hours before the assay either with 5nM tumor necrosis factor-α (TNF-α; R&D Systems) or with 10 ng/mL interleukin-1β (rmIL-1β; Abazyme). After washing away the cytokine, 5 × 105 polymorphonuclear leukocytes were added per transwell, and 40 ng/mL KC (Reprotech) was administered in the bottom chamber. Transmigration was allowed for 30 minutes. Antibodies against various antigens were preincubated with endothelial cells at 30 μg/mL 30 minutes before adding neutrophils. Migrated leukocytes were collected for cell counting (CASY, Schärfe-System). For assays that were analyzed by confocal microscopy, endothelial cells were labeled with cell tracker green before the assay. Nonadherent neutrophils were removed, and adherent and transmigrating neutrophils were fixed using 4% paraformaldehyde. Neutrophils were stained with an anti–CD11b-Alexa647 antibody, and nuclear staining was performed with Hoechst before embedding the filters in DAKO mount. Fluorescence signals were detected using a Zeiss LSM510 confocal laser scan microscope (Carl Zeiss).
Live imaging of neutrophil locomotion and transmigration
A total of 3.5 × 104 bEnd.5 cells were seeded on laminin-coated coverslips in 24-well culture dishes, cultivated for 2 days, and stimulated with TNF-α 16 hours before the experiment. Bone marrow–derived neutrophils (1 × 106/slide) were added on top of the bEnd.5 cell monolayer and were allowed to adhere for 10 minutes. Nonadherent cells were gently removed by replacing the medium. Lateral migration and transendothelial migration of neutrophils on endothelioma cells were visualized by time-lapse videomicroscopy using a Zeiss Axiovert 100 widefield microscope. Phase-contrast images were captured at 8 selected areas at 20× magnification every 20 seconds for a 20-minute period using a camera device connected to the microscope and controlled by ImagePro5.5 software. Lateral migration of individual neutrophils was determined using the ImagePro5.5 software. Seventy individual cell tracks were determined per antibody group to calculate the mean values of the accumulated distance. To determine the percentage of neutrophils that migrated across bEnd.5 cell monolayers, the number of phase-dark neutrophils (beneath the endothelial cell layer, n = 20) were manually counted and related to the number of phase-bright neutrophils on top of endothelial cells (n = 250-350) using ImageJ Version 1.43s software (National Institutes of Health).
IL-1β–induced peritonitis assay
Peritonitis assays were essentially performed as described,27 except for changing the stimulus to 10 ng IL-1β.
Intravital microscopy
Surgical preparation of cremaster muscles and intravital microscopy were essentially done as described.20,34,35 Inflammatory stimulation was achieved by intrascrotal injection of either 50 ng of IL-1β at 4 hours or 500 ng TNF-α at 2 hours before microscopic observation (n = 6 each group). At the same time, 50 μg of affinity-purified anti-CD99 or affinity-purified anti-CD99L2, or control IgG from the preimmune serum was injected intravenously. For each animal, 3 to 5 single unbranched postcapillary venules with diameters 17 to 35 μm were analyzed.
Electron microscopy
Four hours after inflammatory stimulation with IL-1β and antibody injection (as described in “Intravital microscopy”), the cremaster muscle was dissected and prepared for electron microscopy as described.27,36 For each vessel, the number of leukocytes in each of the following positions was determined: A, crossing the endothelium; B, between endothelium and basement membrane; and C, outside the vessel but within 50 μm of it. The fraction of leukocytes trapped between endothelial cells (A) or between endothelial cells and basement membrane (B) was calculated by the equations A/(A + B + C) or B/(A + B + C), respectively. For each antibody type, 32 to 52 nonoverlapping vessel sections of 2 or 3 mice were evaluated.
3D image analysis of cremaster by whole-mount confocal immunofluorescence
Whole-mount immunostained tissues were analyzed by confocal microscopy to localize the position of leukocytes during the extravasation process. After intrascrotal administration of 50 ng IL-1β, mice were given 50 μg of various antibodies intravenously. Three hours later, animals were killed, the cremaster muscle dissected and left in situ for an initial fixation with 4% paraformaldehyde in PBS for 10 minutes, and then it was removed from the animal and postfixed for 1 hour in the same fixative. After permeabilization with 0.1% Triton X-100 in PBS for 2 hours, tissues were blocked in 2% ovalbumin in PBS for 1 hour and stained with rabbit anti-ESAM polyclonal antibody, rat anti–laminin-α5 mAb, and goat anti–MRP-14 polyclonal antibody. First antibodies were detected by: Alexa Fluor 647-conjugated donkey anti–rabbit antibody, Alexa 488-conjugated donkey anti–rat antibody, and Alexa Fluor 568-conjugated donkey anti–goat antibody (Invitrogen). Z-stack images were acquired using a Zeiss LSM 510 confocal laser-scanning microscope. The exact localization of neutrophils within the venules (unbranched, 150 μm in length, 20-40 μm in diameter, and 50 μm around), the vessel segments were analyzed using the LSM Image Examiner 3D software (Carl Zeiss).
Statistics
Statistical analysis was done using the program Sigma Plot applying analysis of variance (ANOVA) multiple comparisons versus control group (Bonferroni t test). Statistical significance was determined by evaluating all available data (mean ± SD).
Results
Antibodies against CD99 and CD99L2 inhibit transmigration of PECAM-1–deficient neutrophils in vitro
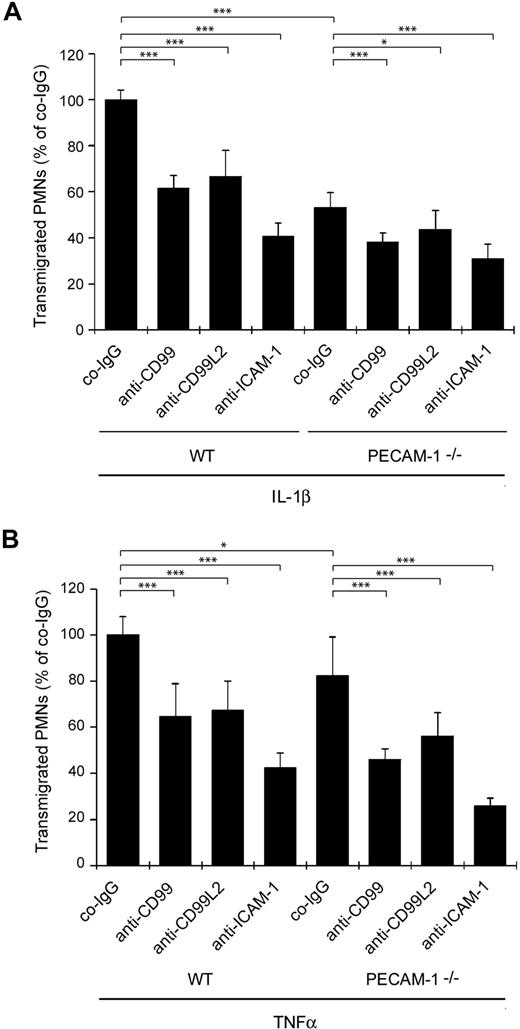
To elucidate the functional relationship between PECAM-1 and CD99 or CD99L2, we investigated whether the absence of PECAM-1 would affect the participation of CD99 or CD99L2 in transmigration of neutrophils across a monolayer of cultured bEnd.5 mouse endothelioma cells. We found that neutrophils isolated from PECAM-1−/− mice migrated less efficiently through the monolayer of IL-1β–stimulated bEnd.5 cells than WT neutrophils (Figure 1A). Antibodies against CD99 and against CD99L2 both inhibited transmigration of WT and PECAM-1−/− neutrophils (Figure 1A). Thus, blocking antibodies against CD99 and CD99L2 reduce neutrophil transmigration independent of the presence of PECAM-1 and have additive inhibitory effects to the lack of PECAM-1, demonstrating that CD99 and CD99L2 function separately and independently of PECAM-1. Similar results were found when bEnd.5 cells were stimulated with TNF-α instead of IL-1β (Figure 1B).
Antibodies against CD99 and CD99L2 block transendothelial migration of WT and PECAM-1–deficient neutrophils in vitro. Bone marrow–derived polymorphonuclear leukocytes from WT or PECAM-1–deficient mice were allowed to migrate for 30 minutes through a monolayer of bEnd.5 cells grown on transwell filters in the presence of 40 ng/mL KC in the lower chamber. Endothelial cells were stimulated with IL-1β (A) or TNF-α (B) 16 hours before the experiment. Thirty minutes before the start of the experiment, endothelial cells were incubated with 30 μg/mL of preimmune control IgG (co-IgG) or affinity-purified anti-CD99 IgG (anti-CD99) or affinity-purified anti-CD99L2 IgG (anti-CD99L2) or an mAb against ICAM-1 (anti–ICAM-1). ***P < .001. *P < .05. Statistical analysis was done by ANOVA evaluating all available data (n = 9) of 3 experiments.
Antibodies against CD99 and CD99L2 block transendothelial migration of WT and PECAM-1–deficient neutrophils in vitro. Bone marrow–derived polymorphonuclear leukocytes from WT or PECAM-1–deficient mice were allowed to migrate for 30 minutes through a monolayer of bEnd.5 cells grown on transwell filters in the presence of 40 ng/mL KC in the lower chamber. Endothelial cells were stimulated with IL-1β (A) or TNF-α (B) 16 hours before the experiment. Thirty minutes before the start of the experiment, endothelial cells were incubated with 30 μg/mL of preimmune control IgG (co-IgG) or affinity-purified anti-CD99 IgG (anti-CD99) or affinity-purified anti-CD99L2 IgG (anti-CD99L2) or an mAb against ICAM-1 (anti–ICAM-1). ***P < .001. *P < .05. Statistical analysis was done by ANOVA evaluating all available data (n = 9) of 3 experiments.
CD99 and CD99L2 act independent of PECAM-1 in neutrophil recruitment to inflamed peritoneum
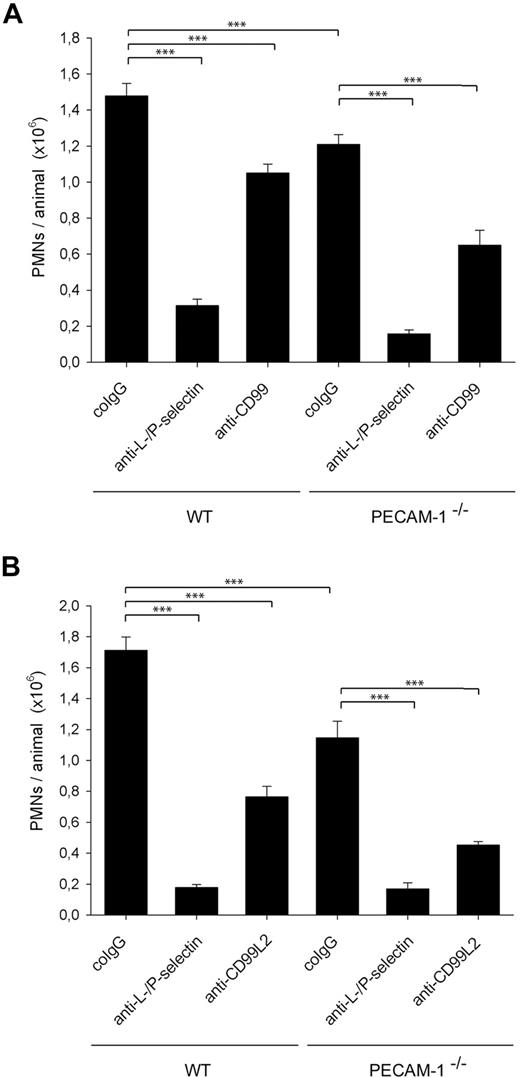
To address whether CD99 or CD99L2 has in vivo additional roles to PECAM-1 in mediating neutrophil recruitment into inflamed tissue, we investigated whether anti-CD99 and anti-CD99L2 antibodies would block neutrophil recruitment to inflamed peritoneum in PECAM-1–deficient mice. WT mice and PECAM-1−/− mice were stimulated intraperitoneally with IL-1β, and neutrophil recruitment was analyzed after 4 hours. As shown in Figure 2A, PECAM-1 deficiency reduced neutrophil recruitment by 18% (± 3%). Antibodies against CD99 reduced neutrophil accumulation in both WT and PECAM-1–deficient animals by 29% (± 3%) and 45% (± 5%), respectively, compared with control antibodies. Injection of PBS without antibodies gave similar results as negative control antibodies (not shown). As a positive inhibitory control, mice were treated with a combination of anti–L- and anti–P-selectin antibodies that blocked neutrophil recruitment by more than 80%. Similar results as for anti-CD99 antibodies were found with anti-CD99L2 antibodies that reduced neutrophil extravasation in WT and PECAM-1–deficient mice by 51% (± 4%) and 60% (± 2%), respectively (Figure 2B). Thus, reduced neutrophil recruitment in PECAM-1–deficient mice was further reduced by antibodies against CD99 and CD99L2, demonstrating that CD99 and CD99L2 act independently of PECAM-1 in neutrophil extravasation into inflamed peritoneum.
Antibodies against CD99 and CD99L2 inhibit IL-1β–elicited neutrophil extravasation into the peritoneal cavity of PECAM-1–deficient mice. Comparison of the function of (A) CD99 and PECAM-1 and (B) of CD99L2 and PECAM-1. Mice were intravenously injected with 50 μg of the following antibodies in PBS: preimmune co-IgG for negative controls, mAbs against P-selectin and L-selectin (anti–P-/L-selectin) for positive controls, or affinity-purified anti-CD99 IgG (anti-CD99, panel A), or affinity-purified anti-CD99L2 IgG (anti-CD99L2, panel B), immediately followed by intraperitoneal administration of IL-1β. Peritoneal leukocytes were removed 4 hours after stimulation, and neutrophil counts were determined. Results represent 3 independent experiments; for each determination of each experiment, 5 mice were analyzed. ***P < .001. Statistical analysis was done by ANOVA evaluating all available data.
Antibodies against CD99 and CD99L2 inhibit IL-1β–elicited neutrophil extravasation into the peritoneal cavity of PECAM-1–deficient mice. Comparison of the function of (A) CD99 and PECAM-1 and (B) of CD99L2 and PECAM-1. Mice were intravenously injected with 50 μg of the following antibodies in PBS: preimmune co-IgG for negative controls, mAbs against P-selectin and L-selectin (anti–P-/L-selectin) for positive controls, or affinity-purified anti-CD99 IgG (anti-CD99, panel A), or affinity-purified anti-CD99L2 IgG (anti-CD99L2, panel B), immediately followed by intraperitoneal administration of IL-1β. Peritoneal leukocytes were removed 4 hours after stimulation, and neutrophil counts were determined. Results represent 3 independent experiments; for each determination of each experiment, 5 mice were analyzed. ***P < .001. Statistical analysis was done by ANOVA evaluating all available data.
CD99 and CD99L2 act independently of PECAM-1 in the inflamed cremaster muscle
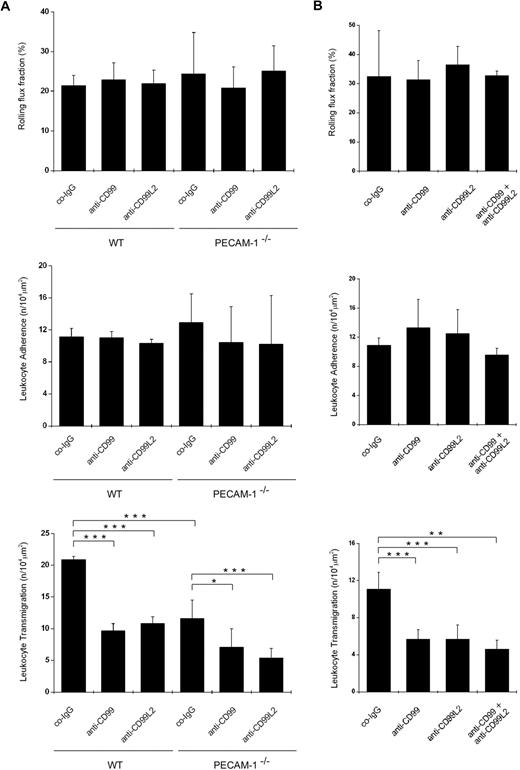
Using intravital microscopy, we analyzed whether antibodies against CD99 and CD99L2 would inhibit neutrophil extravasation in the IL-1β–stimulated cremaster muscle of PECAM-1–deficient mice. As expected, PECAM-1 deficiency reduced neutrophil extravasation considerably, whereas no significant effect was found on rolling flux or on leukocyte adherence to inflamed venules (Figure 3A; hemodynamic parameters in Tables 1 and 2). Antibodies against CD99 blocked neutrophil extravasation in WT as well as in PECAM-1−/− mice by 54% (± 5%) and 39% (± 11%), respectively (Figure 3A). Likewise, antibodies against CD99L2 inhibited neutrophil recruitment in WT and PECAM-1−/− mice by 48% (± 5%) and 55% (± 5%), respectively. Collectively, our results demonstrate that in vitro as well as in 2 in vivo inflammation models CD99 and CD99L2 participate in neutrophil diapedesis independent of the function of PECAM-1. In contrast, antibodies against CD99 and CD99L2 had no additive effects on leukocyte extravasation (Figure 3B).
Antibodies against CD99 and CD99L2 inhibit neutrophil extravasation in the cytokine-inflamed cremaster of WT and PECAM-1–deficient mice. Leukocyte rolling, adhesion, and extravasation in (A) 4-hour IL-1β or (B) 2-hour TNF-α inflamed cremaster were analyzed by intravital microscopy in WT (A-B) and PECAM-1–deficient mice (only panel A) intravenously injected with 50 μg antibody of preimmune co-IgG, affinity-purified anti-CD99 IgG (anti-CD99), or affinity-purified anti-CD99L2 IgG (anti-CD99L2; as indicated) or a combination of both antibodies (only panel B). Four or 2 hours later, respectively, the cremaster muscle was surgically prepared, and the number of rolling leukocytes (per second per millimeter), adherent leukocytes (per 104 μm2 of venule surface area), and extravasated leukocytes from cremasteric venules (per 104 μm2 tissue area) were determined by intravital near-infrared reflected light oblique transillumination microscopy. ***P < .001. **P < .01. *P < .05. Statistical analysis was done by ANOVA evaluating all available data (n = 3-6). Hemodynamic parameters are given in Tables 1 and 2.
Antibodies against CD99 and CD99L2 inhibit neutrophil extravasation in the cytokine-inflamed cremaster of WT and PECAM-1–deficient mice. Leukocyte rolling, adhesion, and extravasation in (A) 4-hour IL-1β or (B) 2-hour TNF-α inflamed cremaster were analyzed by intravital microscopy in WT (A-B) and PECAM-1–deficient mice (only panel A) intravenously injected with 50 μg antibody of preimmune co-IgG, affinity-purified anti-CD99 IgG (anti-CD99), or affinity-purified anti-CD99L2 IgG (anti-CD99L2; as indicated) or a combination of both antibodies (only panel B). Four or 2 hours later, respectively, the cremaster muscle was surgically prepared, and the number of rolling leukocytes (per second per millimeter), adherent leukocytes (per 104 μm2 of venule surface area), and extravasated leukocytes from cremasteric venules (per 104 μm2 tissue area) were determined by intravital near-infrared reflected light oblique transillumination microscopy. ***P < .001. **P < .01. *P < .05. Statistical analysis was done by ANOVA evaluating all available data (n = 3-6). Hemodynamic parameters are given in Tables 1 and 2.
Hemodynamic parameters of mice analyzed by intravital microscopy for Figure 3A
| . | WT + coIgG . | WT + anti-CD99 . | WT + anti-CD99L2 . | PECAM-1−/− + coIgG . | PECAM-1−/− + anti-CD99 . | PECAM-1−/− + anti-CD99L2 . |
|---|---|---|---|---|---|---|
| WBCs, ×106 cells/mL | 3.1 ± 0.4 | 3.5 ± 0.2 | 3.1 ± 0.4 | 4.9 ± 1.9 | 5.0 ± 1.3 | 3.7 ± 0.9 |
| Vessel diameter, μm | 28 ± 4 | 27 ± 2 | 26 ± 1 | 27 ± 1 | 27 ± 2 | 28 ± 3 |
| Wall shear rate, 1000 per second | 1.6 ± 0.3 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 |
| . | WT + coIgG . | WT + anti-CD99 . | WT + anti-CD99L2 . | PECAM-1−/− + coIgG . | PECAM-1−/− + anti-CD99 . | PECAM-1−/− + anti-CD99L2 . |
|---|---|---|---|---|---|---|
| WBCs, ×106 cells/mL | 3.1 ± 0.4 | 3.5 ± 0.2 | 3.1 ± 0.4 | 4.9 ± 1.9 | 5.0 ± 1.3 | 3.7 ± 0.9 |
| Vessel diameter, μm | 28 ± 4 | 27 ± 2 | 26 ± 1 | 27 ± 1 | 27 ± 2 | 28 ± 3 |
| Wall shear rate, 1000 per second | 1.6 ± 0.3 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 |
Data are mean ± SD (3-5 venules/mouse; n = 3-6).
WBCs indicates white blood cells.
Hemodynamic parameters of mice analyzed by intravital microscopy for Figure 3B
| . | WT + coIgG . | WT + anti-CD99 . | WT + anti-CD99L2 . | WT + anti-CD99/CD99L2 . |
|---|---|---|---|---|
| WBCs, ×106 cells/mL | 3.4 ± 0.2 | 3.4 ± 0.2 | 3.0 ± 0.2 | 2.2 ± 0.4 |
| Vessel diameter, μm | 31 ± 8 | 28 ± 6 | 33 ± 8 | 23 ± 8 |
| Wall shear rate, 1000 per second | 1.4 ± 0.4 | 1.6 ± 0.2 | 1.3 ± 0.4 | 1.8 ± 0.4 |
| . | WT + coIgG . | WT + anti-CD99 . | WT + anti-CD99L2 . | WT + anti-CD99/CD99L2 . |
|---|---|---|---|---|
| WBCs, ×106 cells/mL | 3.4 ± 0.2 | 3.4 ± 0.2 | 3.0 ± 0.2 | 2.2 ± 0.4 |
| Vessel diameter, μm | 31 ± 8 | 28 ± 6 | 33 ± 8 | 23 ± 8 |
| Wall shear rate, 1000 per second | 1.4 ± 0.4 | 1.6 ± 0.2 | 1.3 ± 0.4 | 1.8 ± 0.4 |
Data are mean ± SD (3-5 venules/mouse; n = 3-6).
WBCs indicates white blood cells.
In agreement with this, we found that CD99 and CD99L2, in contrast to PECAM-1, act independent of the type of inflammatory stimulus. The function of PECAM-1 is bypassed in TNF-α–induced inflammation in the cremaster or when the peritoneum is stimulated by thioglycollate.24 However, we found that anti-CD99 and anti-CD99L2 antibodies inhibit neutrophil recruitment in the TNF-α–stimulated cremaster (Figure 3B) and into thioglycollate-stimulated peritoneum.27
Blocking PECAM-1 and CD99 traps neutrophils between endothelial cells in vitro
Based on in vitro transmigration assays, human CD99 and PECAM-1 have been reported to act sequentially, with anti–PECAM-1 antibodies arresting leukocytes at the apical surface of endothelial cell contacts and anti-CD99 antibodies blocking leukocytes halfway through endothelial junctions.25 To determine where mouse PECAM-1 and CD99 act during diapedesis, we analyzed neutrophil transmigration through a monolayer of bEnd.5 endothelial cells grown on laminin-coated transwell filters using confocal fluorescence microscopy. For visualization, endothelial cells were labeled before the assay with cell tracker green. Neutrophils were allowed to transmigrate in the presence of either control antibodies or polyclonal antibodies against CD99 or the mAb 2H8 against PECAM-1. At the end of the assay, cells were fixed and neutrophils were visualized by staining for the integrin α-chain CD11b and by staining of nuclei with Hoechst dye.
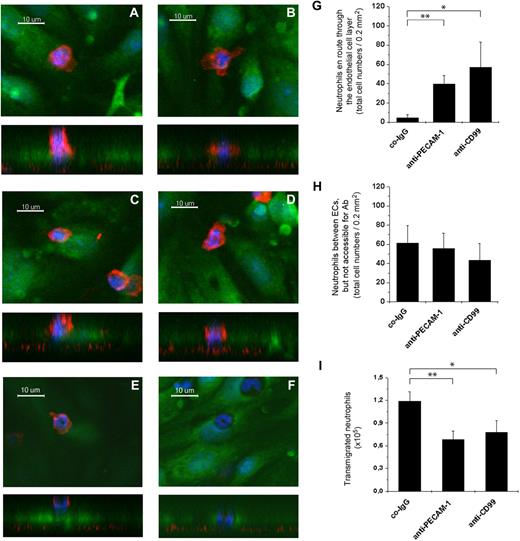
X-Z orthogonal sections allowed distinction between cells attached to the apical surface of endothelial cells (Figure 4E) and those that were engaged in the diapedesis process (Figure 4A-D). Neutrophils in the process of diapedesis were clearly embedded with at least parts of their nuclei within the endothelial cell layer but were still accessible for CD11b antibody staining (Figure 4A-D). Such cells were very rarely found in assays performed in the presence of control antibodies (2 or 3 cells per field) but were frequently detected if transmigration was inhibited with anti-CD99 (57 ± 28 cells per field) or anti–PECAM-1 antibodies (40 ± 10 cells per field; Figure 4G). A third population of leukocytes was identified in which the nuclei were completely embedded within the endothelial layer, but the cells were no longer accessible for staining with anti-CD11b antibodies (Figure 4F). We could not detect endothelial protrusions stained with cell tracker green covering these leukocytes, but assume that such structures are too thin to be detected by this method. Neutrophils trapped in this way and no longer accessible to anti-CD11b antibodies were found with similar frequencies in all assays independent of the antibodies used (Figure 4H), arguing that they represent a steady-state fraction of slow-migrating cells. A fourth population of cells were those that had reached the bottom chamber of the transwell filter assay. Counting them revealed that anti–PECAM-1 and anti-CD99 antibodies both successfully inhibited neutrophil transmigration in these assays (Figure 4I). We conclude that interference with the function of CD99 and PECAM-1 in these in vitro assays inhibits transmigration of neutrophils at a step where the cells move through the endothelial cell contacts and are still accessible from above. In contrast to results reported for human cells,25 we could not detect a difference in the position where anti–PECAM-1 or anti-CD99 antibodies trapped the leukocytes. Neutrophils detected at a slightly more apical position (Figure 4A,C) or a slightly more basal position (Figure 4B,D) between endothelial cells were found with similar frequencies on blocking CD99 or PECAM-1.
Antibodies against CD99 and PECAM-1 trap neutrophils between endothelial cells while they transmigrate through endothelial monolayers in vitro. Bone marrow–derived mouse neutrophils were allowed to transmigrate through monolayers of bEnd.5 cells on laminin-coated transwell filters in the presence of antibodies against PECAM-1 (A-B), CD99 (C-D), or control antibodies (E-F). Endothelial cells were stained with cell tracker green, cell nuclei with Hoechst dye (blue), and at the end of the assay cells were fixed and neutrophils were stained with antibodies against CD11b (red). Cells were visualized by confocal microscopy, and each example is depicted as X-Y and underneath as X-Z presentation. Examples for neutrophils are shown that were trapped at a more apical position (panels A,C) or a more basal position (panels B,D) during diapedesis (en route) through the endothelial cell layer. Neutrophils varied between these 2 positions with similar frequency independent of whether antibodies against CD99 or PECAM-1 were used. Examples for neutrophils on top of endothelial cells (panel E) and embedded between endothelial cells, but not accessible for anti-CD11b staining (panel F) are shown. Quantification of neutrophils in the positions “en route” through the endothelial cell monolayer (G), between endothelial cells and not accessible for antibody staining (H), and in the bottom chamber of the transwell filters (I). **P < .01. *P < .05. Statistical analysis was done by ANOVA evaluating all available data with n ≥ 60 for co-IgG and n ≥ 90 for anti–PECAM-1 and anti-CD99.
Antibodies against CD99 and PECAM-1 trap neutrophils between endothelial cells while they transmigrate through endothelial monolayers in vitro. Bone marrow–derived mouse neutrophils were allowed to transmigrate through monolayers of bEnd.5 cells on laminin-coated transwell filters in the presence of antibodies against PECAM-1 (A-B), CD99 (C-D), or control antibodies (E-F). Endothelial cells were stained with cell tracker green, cell nuclei with Hoechst dye (blue), and at the end of the assay cells were fixed and neutrophils were stained with antibodies against CD11b (red). Cells were visualized by confocal microscopy, and each example is depicted as X-Y and underneath as X-Z presentation. Examples for neutrophils are shown that were trapped at a more apical position (panels A,C) or a more basal position (panels B,D) during diapedesis (en route) through the endothelial cell layer. Neutrophils varied between these 2 positions with similar frequency independent of whether antibodies against CD99 or PECAM-1 were used. Examples for neutrophils on top of endothelial cells (panel E) and embedded between endothelial cells, but not accessible for anti-CD11b staining (panel F) are shown. Quantification of neutrophils in the positions “en route” through the endothelial cell monolayer (G), between endothelial cells and not accessible for antibody staining (H), and in the bottom chamber of the transwell filters (I). **P < .01. *P < .05. Statistical analysis was done by ANOVA evaluating all available data with n ≥ 60 for co-IgG and n ≥ 90 for anti–PECAM-1 and anti-CD99.
CD99 and CD99L2 are not involved in neutrophil motility on endothelial cells
To test whether blocking CD99 or CD99L2 might affect the lateral migration of neutrophils on endothelial cells, a step described as locomotion,37 we used time-lapse video microscopy. The mobility of bone marrow neutrophils added to monolayers of bEnd.5 cells was analyzed by determining the distance that neutrophils migrated randomly on the endothelial monolayer. Leukocytes on top of the endothelial cells were easily detectable as phase bright cells (Figure 5A). The mean distances that 70 neutrophils traveled in a given time in the presence of control, anti-CD99, anti-CD99L2, or anti–ICAM-1 antibodies are given in Figure 5C, and examples for 6 migration tracks are shown in Figure 5B. Neither anti-CD99 antibodies nor anti-CD99L2 antibodies affected the motility of neutrophils, whereas anti–ICAM-1 antibodies reduced the distance that neutrophils traveled by 38% (± 17%; Figure 5C). Likewise, PECAM-1 deficiency on neutrophils did not affect lateral neutrophil mobility (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In contrast to lateral mobility, the transmigration of neutrophils through the endothelial monolayer was inhibited by anti-CD99 and anti-CD99L2 antibodies by 56% (± 13%) and 42% (± 10%), respectively, and anti–ICAM-1 antibodies blocked transmigration almost completely (Figure 5D). We conclude that neither CD99 nor CD99L2 is required for normal motility of neutrophils on endothelial cells before transmigration.
Antibodies against CD99 and CD99L2 do not interfere with neutrophil lateral migration on endothelioma cells but selectively inhibit neutrophil transendothelial migration in vitro. bEnd.5 cells were cultured to confluence on laminin-coated coverslips and were incubated with 30 μg/mL of the indicated antibody for 15 minutes before the assay. Neutrophils were added on top of the bEnd.5 cell monolayer and were allowed to adhere for 10 minutes. Lateral migration (A-C) and transmigration of neutrophils (D) across the endothelial cell monolayer were visualized by time-lapse video microscopy. Phase-contrast images were captured every 20 seconds over a time period of 20 minutes. (A) Cell tracks of 4 individual neutrophils on a bEnd.5 cell monolayer in the presence of co-IgG. (B) Tracking data of 6 individual neutrophils (of 70 evaluated) for each antibody investigated. (C) Quantitative analysis of the distance that neutrophils migrated on endothelial cells during the 20-minute period in the presence of the indicated antibodies. (D) Percentage of transmigration of neutrophils across bEnd.5 monolayers during the time interval. ***P < .001. **P < .01. Statistical analysis was done by ANOVA evaluating all available data.
Antibodies against CD99 and CD99L2 do not interfere with neutrophil lateral migration on endothelioma cells but selectively inhibit neutrophil transendothelial migration in vitro. bEnd.5 cells were cultured to confluence on laminin-coated coverslips and were incubated with 30 μg/mL of the indicated antibody for 15 minutes before the assay. Neutrophils were added on top of the bEnd.5 cell monolayer and were allowed to adhere for 10 minutes. Lateral migration (A-C) and transmigration of neutrophils (D) across the endothelial cell monolayer were visualized by time-lapse video microscopy. Phase-contrast images were captured every 20 seconds over a time period of 20 minutes. (A) Cell tracks of 4 individual neutrophils on a bEnd.5 cell monolayer in the presence of co-IgG. (B) Tracking data of 6 individual neutrophils (of 70 evaluated) for each antibody investigated. (C) Quantitative analysis of the distance that neutrophils migrated on endothelial cells during the 20-minute period in the presence of the indicated antibodies. (D) Percentage of transmigration of neutrophils across bEnd.5 monolayers during the time interval. ***P < .001. **P < .01. Statistical analysis was done by ANOVA evaluating all available data.
Blocking CD99 and CD99L2 in vivo traps extravasating neutrophils at the level of the basement membrane
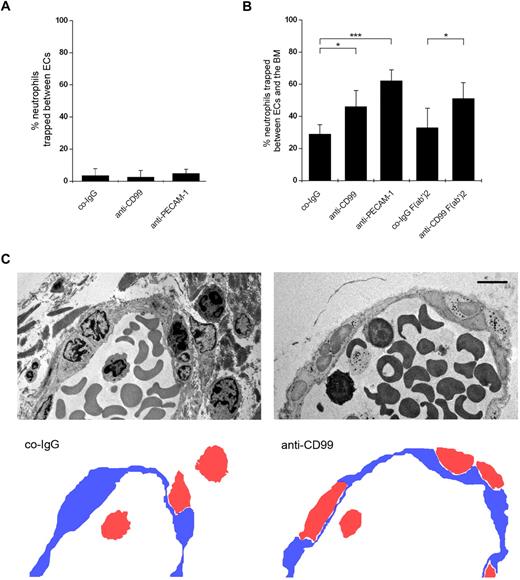
To determine in vivo at which step CD99 participates in the diapedesis process, we analyzed by electron microscopy where anti-CD99 antibodies trap neutrophils in the IL-1β-inflamed mouse cremaster. In Figure 6C, representative electron microscopy images of migrating neutrophils in the presence of control-IgG or anti-CD99 antibodies are shown. Numbers of neutrophils trapped during the diapedesis process between endothelial cells or arrested between the endothelium and the basement membrane were counted and given as a percentage of the sum of trapped and extravasated neutrophils (Figure 6A-B). Very few neutrophils were found trapped between endothelial cells, and numbers were similar for mice independent of the antibodies used (Figure 6A). In contrast, mice treated with anti-CD99 or anti–PECAM-1 antibodies showed significantly increased numbers of leukocytes trapped between the endothelium and the basement membrane, compared with control antibodies (Figure 6B). Anti-CD99 F(ab′)2 yielded similar inhibitory effects (Figure 6B). Thus, blocking CD99 and PECAM-1 both inhibit leukocyte extravasation at the level of the basement membrane. This is in agreement with previous results for antibodies against CD99L2.27
Localization of trapped neutrophils during diapedesis in cremaster venules by electron microscopy. (A-B) Proportion of neutrophils trapped between endothelial cells (A) and between endothelial cells and the basement membrane (B) in IL-1β-treated cremaster muscles. Analysis was done by transmission electron microscopy. Animals were treated with preimmune control antibodies (co-IgG), affinity-purified anti-CD99 antibodies (anti-CD99), a mixture of 2 mAb against mouse PECAM-1 (anti–PECAM-1), or control IgG F(ab′)2 or anti-CD99 F(ab′)2 (as indicated). Graphs represent the number of trapped neutrophils given as percentage of leukocytes that had passed the endothelial cell contacts. For each determination, 30 to 52 randomly selected vessel segments were analyzed. ***P < .001. *P < .05. Statistical analysis was done by ANOVA evaluating all available data. (C) Electron micrographs of vessel segments of IL-1β inflamed cremaster muscle of mice treated with control antibody from the preimmune serum (co-IgG) and affinity-purified anti-CD99 antibodies (anti-CD99). Positions of neutrophils (red) and endothelium (blue) are given in the cartoons at the bottom. Bar represents 5 μm.
Localization of trapped neutrophils during diapedesis in cremaster venules by electron microscopy. (A-B) Proportion of neutrophils trapped between endothelial cells (A) and between endothelial cells and the basement membrane (B) in IL-1β-treated cremaster muscles. Analysis was done by transmission electron microscopy. Animals were treated with preimmune control antibodies (co-IgG), affinity-purified anti-CD99 antibodies (anti-CD99), a mixture of 2 mAb against mouse PECAM-1 (anti–PECAM-1), or control IgG F(ab′)2 or anti-CD99 F(ab′)2 (as indicated). Graphs represent the number of trapped neutrophils given as percentage of leukocytes that had passed the endothelial cell contacts. For each determination, 30 to 52 randomly selected vessel segments were analyzed. ***P < .001. *P < .05. Statistical analysis was done by ANOVA evaluating all available data. (C) Electron micrographs of vessel segments of IL-1β inflamed cremaster muscle of mice treated with control antibody from the preimmune serum (co-IgG) and affinity-purified anti-CD99 antibodies (anti-CD99). Positions of neutrophils (red) and endothelium (blue) are given in the cartoons at the bottom. Bar represents 5 μm.
A limitation of the ultrastructural analysis arises from the fact that single sections are analyzed, leaving it unresolved whether in a plane above or below this section parts of the extravasated leukocyte may still be present between endothelial cells or even inside the vascular lumen. To resolve this, we turned to a 3D analysis based on whole-mount fluorescence stainings of cremaster tissue visualized by confocal microscopy. PECAM-1−/− mice or WT mice treated with control antibodies or antibodies against CD99 or CD99L2 were stimulated by intrascrotal injection of IL-1β, and 3 hours later the cremaster was isolated and fixed. Fixed cremaster was stained as a whole mount with antibodies against ESAM to stain endothelial cell contacts, with antibodies against laminin-α5 chain to label the basement membrane and with antibodies against the neutrophil antigen MRP14 (Figure 7A,C). Venules and extravasating neutrophils were analyzed by confocal microscopy, and neutrophils were classified in 3 categories: neutrophils embedded within the endothelial layer (A), neutrophils between endothelial cells and the basement membrane (B), and fully extravasated neutrophils (C; Figure 7B-C). The sum of neutrophils found in all 3 positions in a given vessel was set as 100%, and the percentage of neutrophils found at each of the 3 positions was given as percentage of this sum (Figure 7E). In addition, the total number of cells is given in Figure 7D. Neither antibodies against CD99 nor antibodies against CD99L2 increased the number of neutrophils embedded in the endothelial junctions (position A) compared with control antibodies. In contrast, the fraction of neutrophils trapped between endothelial cells and the basement membrane was increased 3.2-fold in anti–CD99-treated mice and 2.9-fold in anti–CD99L2-treated mice, clearly indicating that both membrane proteins participate in the migration of neutrophils through the basement membrane (Figure 7). In agreement with published data,23,24 we could confirm the accumulation of neutrophils between endothelial cells and basement membrane in PECAM-1 gene-deficient mice (not shown).
Localization of trapped neutrophils during diapedesis in cremaster venules by confocal fluorescence microscopy of stained tissue whole mounts. A 3D confocal image analysis of cremaster was used to determine sites of arrest of transmigrating leukocytes after the administration of blocking antibodies. Mice were intrascrotally stimulated with IL-1β and intravenously injected with anti-CD99 antibodies, anti-CD99L2 antibodies, or preimmune IgG as a control. Three hours later, whole mounts of cremaster muscles were immunostained for ESAM (green), laminin-α5 (red), and MRP-14 (blue) to visualize endothelial cell contacts, basement membrane, and neutrophils, respectively. Neutrophil localization relative to the endothelium and its underlying basement membrane was analyzed by confocal microscopy. (A) Fluorescent images showing a representative longitudinal vessel segment typically used for the 3D analysis and evaluation. The left panel shows an image of a venule stained for endothelial cell contacts (green) and neutrophils (blue) only. The image on the right depicts the same venule with the staining for the basement membrane (red) added. Projection images of only one-half of the venule have been used for better depiction. Bar represents 20 μm. (B) Schematic drawing illustrating the criteria used to define the positions of the neutrophils within the cremaster tissue samples. Position A indicates neutrophils embedded within the endothelial layer; position B, neutrophils located between endothelial cells and the basement membrane; and position C, fully emigrated neutrophils. (C) Representative images showing cross sections of venules with neutrophils located at the positions indicated in panel B (staining as in panel A). (D) The absolute numbers of neutrophils found at the positions indicated under the respective experimental conditions. Values are mean ± SD (on average, 20 of the most frequent vessel segments having 7-16 neutrophils in total analyzed). (E) Numbers of neutrophils found at the positions indicated expressed as percentage of total neutrophils. Here, all vessel segments having more than 5 neutrophils in total have been evaluated (on average, 30 vessel segments). co indicates control. ***P < .001. **P < .01. Statistical analysis was done by ANOVA evaluating all available data.
Localization of trapped neutrophils during diapedesis in cremaster venules by confocal fluorescence microscopy of stained tissue whole mounts. A 3D confocal image analysis of cremaster was used to determine sites of arrest of transmigrating leukocytes after the administration of blocking antibodies. Mice were intrascrotally stimulated with IL-1β and intravenously injected with anti-CD99 antibodies, anti-CD99L2 antibodies, or preimmune IgG as a control. Three hours later, whole mounts of cremaster muscles were immunostained for ESAM (green), laminin-α5 (red), and MRP-14 (blue) to visualize endothelial cell contacts, basement membrane, and neutrophils, respectively. Neutrophil localization relative to the endothelium and its underlying basement membrane was analyzed by confocal microscopy. (A) Fluorescent images showing a representative longitudinal vessel segment typically used for the 3D analysis and evaluation. The left panel shows an image of a venule stained for endothelial cell contacts (green) and neutrophils (blue) only. The image on the right depicts the same venule with the staining for the basement membrane (red) added. Projection images of only one-half of the venule have been used for better depiction. Bar represents 20 μm. (B) Schematic drawing illustrating the criteria used to define the positions of the neutrophils within the cremaster tissue samples. Position A indicates neutrophils embedded within the endothelial layer; position B, neutrophils located between endothelial cells and the basement membrane; and position C, fully emigrated neutrophils. (C) Representative images showing cross sections of venules with neutrophils located at the positions indicated in panel B (staining as in panel A). (D) The absolute numbers of neutrophils found at the positions indicated under the respective experimental conditions. Values are mean ± SD (on average, 20 of the most frequent vessel segments having 7-16 neutrophils in total analyzed). (E) Numbers of neutrophils found at the positions indicated expressed as percentage of total neutrophils. Here, all vessel segments having more than 5 neutrophils in total have been evaluated (on average, 30 vessel segments). co indicates control. ***P < .001. **P < .01. Statistical analysis was done by ANOVA evaluating all available data.
Discussion
Much progress has been made in recent years in identifying endothelial cell surface and adhesion molecules that participate in the transmigration of leukocytes through the blood vessel wall. However, very few studies have aimed at understanding in vivo how these proteins work in concert during diapedesis. So far, only ICAM-2, JAM-A, and PECAM-1 have been directly compared in their capacity to participate in leukocyte extravasation in vivo.24 They act sequentially at 3 different positions in the diapedesis route, and blocking or deleting any one of them leads to the same inhibitory effect as blocking all 3, suggesting that they all act in one and the same molecular pathway. Comparing CD99, CD99L2, and PECAM-1, we found here, using electron microscopy as well as confocal fluorescence microscopy, that all 3 proteins act in vivo at a late step during the diapedesis process, when neutrophils try to overcome the basement membrane. Importantly, in 2 different inflammation models, PECAM-1 gene ablation and antibodies against CD99 or CD99L2 had additive inhibitory effects on diapedesis, clearly indicating that CD99 and CD99L2 act independently of PECAM-1 defining 2 distinct, parallel mechanisms that are both required for leukocyte diapedesis.
In agreement with this interpretation, only PECAM-1 and not CD99 and CD99L2 support neutrophil extravasation in a stimulus-dependent way. In several reports, PECAM-1 was shown to be required for neutrophil extravasation in the cremaster model in response to IL-1β, whereas the need for PECAM-1 was bypassed if inflammation was triggered by TNF-α, fMLP, or LTB4.21,23,38 ICAM-2 and JAM-A showed a similar cytokine selectivity as PECAM-1.24 In contrast, we find that anti-CD99 and anti-CD99L2 antibodies inhibit leukocyte extravasation independent of whether TNF-α or IL-1β was used for stimulation. Similarly, PECAM-1 is bypassed in the peritonitis model if inflammation is triggered by thioglycollate, at least in the C57BL/6 background,39 whereas CD99 and CD99L2 are both required for neutrophil recruitment under these conditions.27 Thus, CD99 and CD99L2 mediate neutrophil diapedesis even under inflammatory conditions that bypass the need of PECAM-1, ICAM-2, and JAM-A, suggesting that the latter participate in a pathway that functions independent of the one supported by CD99 and CD99L2.
Despite supporting independent mechanisms, PECAM-1 seems to act at the same position during diapedesis through the vessel wall as CD99 and CD99L2: at the basal site of the endothelium, assisting in the migration through the basement membrane or the dissociation from the endothelial cells. Using electron microscopy, this is shown here, for the first time, for CD99. For CD99L2, we recently obtained similar results.27 A limitation of such electron microscopic studies is that single sections are analyzed and that it cannot be excluded that in a plane above or below the section, parts of the trapped leukocyte are still stuck between endothelial junctions or even inside the vascular lumen. Therefore, we have now extended our analysis to applying 3D confocal fluorescence microscopy that enabled us to analyze the 3D position of a large number of leukocytes over their entire cell body. By this technique, we could resolve that neutrophils indeed require CD99 and CD99L2, in addition to PECAM-1, to overcome the basement membrane.
The basement membrane is a major barrier in leukocyte extravasation,12-14 and very little is known about the mechanisms that leukocytes use to overcome this barrier. Likewise, it is not known in detail how PECAM-1, CD99, and CD99L2 participate in this process. It has been suggested that PECAM-1 is required to mobilize the integrin α6β1 to the surface of neutrophils, and it is conceivable that this laminin-binding integrin might be involved in migration on or through the basement membrane.14,40,41 Other possibilities by which CD99 and CD99L2 could support the migration of leukocytes through the basement membrane would be an activation of proteases or positive influences on the motility of neutrophils on the basement membrane. Indeed, we have recently shown that components of the basement membrane, such as laminin-α5, have a negative influence on the motility of leukocytes, and this leads to an inhibitory effect on the migration of leukocytes through the basement membrane.14 CD99 and CD99L2 could have opposite effects. Future studies will be necessary to clarify how endothelial cell contact proteins support leukocyte migration through the basement membrane.
In contrast to the situation in vivo, we found that in vitro antibodies against CD99 and PECAM-1 inhibit neutrophils while they are migrating through the endothelial junctions. This is largely in agreement with studies on the migration of human monocytes and human neutrophils through human umbilical vein endothelial cell monolayers,25,42 except for one important difference. Although these studies reported that an anti–human PECAM-1 antibody blocked neutrophils on top of the apical surface of endothelial cells, an anti-CD99 antibody blocked neutrophils “halfway through” the endothelial cell layer at a later step. By contrast, we found that neutrophils caught at a more apical or at a more basal site between endothelial cells were found with similar frequency on blocking with either anti-CD99 or anti–PECAM-1 antibodies, arguing against a sequential function of PECAM-1 and CD99. Although the reasons for these different results are unknown, it is possible that they are in part because of differences in the experimental setup, such as primary human endothelial cells grown on collagen-coated cover slips versus mouse endothelial cell lines grown on laminin-coated transwell filters. Despite these differences in experimental design, it is remarkable that our in vitro results do fully reproduce an essential function of PECAM-1 and CD99 for the migration of myeloid cells through junctions of cultured endothelial cells.
This is remarkable because our in vivo studies revealed no function for CD99, CD99L2, and PECAM-1 for the migration of neutrophils through endothelial junctions. For PECAM-1, these results are in full agreement with other in vivo studies that established the barrier of the basement membrane as the exclusive site of action for PECAM-1 based on electron microscopy27,30 and 3D confocal fluorescence microscopy.23,24 Analyzing FVB instead of C57Bl6 mice and using immunohistology, it was reported that antibodies against PECAM-1 trap leukocytes at the vessel wall,39 and similar results were shown for anti-CD99 antibodies.43 Thus, it is possible that, in mouse strains other than C57Bl6, PECAM-1 and CD99 may additionally act at other sites than the endothelial-basement membrane interface during diapedesis. Whether this is indeed the case would need to be analyzed further by electron microscopy, confocal microscopy, and intravital microscopy, as it has now been analyzed for C57Bl6 mice. Alternatively, it is possible that antibodies against different epitopes of PECAM-1 may trap leukocytes at different sites.44,45
At present, it is unclear why the sites of action of CD99 and PECAM-1 differ between in vitro and in vivo transmigration assays. It is possible that in vivo junctional mechanisms exist that bypass the function of PECAM-1 and CD99 during the migration of neutrophils through endothelial junctions, which are not active in vitro. As an example, we found, by analyzing ESAM gene-deficient mice, that ESAM, a tight junction associated endothelial membrane protein, is necessary in vivo to support neutrophil extravasation,20 whereas ESAM-deficient endothelioma cells show no defect in neutrophil transmigration in vitro (S.B. and D.V., unpublished data, July 2005).
In conclusion, we found that blocking mouse CD99 and PECAM-1 in in vitro transmigration assays traps neutrophils between endothelial cells, confirming previous results with human cells, whereas a sequential action of PECAM-1 at a more apical position than CD99 could not be detected. In contrast to the in vitro results, CD99, CD99L2, and PECAM-1 were found to act in vivo at a later stage of the diapedesis process, the penetration through the basement membrane. Although all 3 membrane proteins acted at the same stage of the diapedesis process, our results suggest that CD99 and CD99L2 act independently from PECAM-1, based on 2 findings. First, CD99 and CD99L2 on the one hand and PECAM-1 on the other hand contribute to the diapedesis process in an additive, independent manner. Second, CD99 and CD99L2 are required for neutrophil extravasation under conditions (eg, stimulation with TNF-α or thioglycollate) that bypass the need for PECAM-1. Because ICAM-2, JAM-A, and PECAM-1 were recently found to function at sequential steps during diapedesis, which depend on each other and share a similar stimulus dependence, we conclude that CD99 and CD99L2 both support a different, independent mechanism than the one defined by the ICAM-2, JAM-A, PECAM-1 pathway.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Max-Planck-Gesellschaft, the Interdisziplinäres Klinisches Forschungszentrum Münster, Deutsche Forschungsgemeinschaft (SFB 492, D.V.; and Emmy-Noether Program, A.Z.), and the European Community (NoE MAIN 502935; F.K., D.V.).
Authorship
Contribution: M.G.B., H.L., B.P., A.G.K., A.K., A.Z., K.W.-B., H.W., D.Z., S.M., and S.B. performed, designed, and analyzed experiments; L.S. provided valuable reagents; F.K., and D.V. initiated the study and designed and supervised the research project; and M.G.B. and D.V. contributed to data analysis and interpretation and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.G.K. is German Heart Centre Munich, Technical University Munich, Munich, Germany. The current affiliation for B.P. is Calvin, Phoebe & Joan Snyder Institute for Infection, Immunity & Inflammation, University of Calgary, Calgary, AB.
Correspondence: Dietmar Vestweber, Max-Planck-Institute of Molecular Biomedicine, Roentgenstrasse 20, D-48149 Münster, Germany; e-mail: vestweb@mpi-muenster.mpg.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal