Abstract
Platelet transfusion is currently the primary medical treatment for reducing thrombocytopenia in patients with inherited thrombocytopenias. To evaluate whether stimulating megakaryopoiesis could increase platelet count in these conditions, we treated patients with a severe thrombocytopenia induced by MYH9 mutations (MYH9-related disease) with a nonpeptide thrombopoietin receptor agonist, eltrombopag. Twelve adult patients with MYH9-RD and platelet counts of less than 50 × 109/L received 50 mg of eltrombopag orally per day for 3 weeks. Patients who achieved a platelet count higher than 150 × 109/L stopped therapy, those with 100 to 150 platelets × 109/L continued treatment at the same eltrombopag dose for 3 additional weeks, while those with less than 100 platelets × 109/L increased the eltrombopag dose to 75 mg for 3 weeks. Major responses (platelet count of at least 100 × 109/L or 3 times the baseline value) were obtained in 8 patients, minor responses (platelet counts at least twice the baseline value) in 3. One patient did not respond. Bleeding tendency disappeared in 8 of 10 patients with bleeding symptoms at baseline. Mild adverse events were reported in 2 patients. The availability of thrombopoietin mimetics opened new prospects in the treatment of inherited thrombocytopenias. This study is registered at www.clinicaltrials.gov as NCT01133860 (European Union Drug Regulating Authorities Clinical Trials number 2008-001903-42).
Introduction
Inherited thrombocytopenias are a heterogeneous group of diseases characterized by a reduced number of blood platelets and a bleeding tendency that ranges from life-threatening to very mild.
Some of these disorders only affect megakaryocytes and platelets, while others involve different cell types and result in additional phenotypic abnormalities.1
MYH9-related disease (MYH9-RD) is one of the less rare forms of inherited thrombocytopenia.2 It derives from mutations of the gene MYH9 for the heavy chain of nonmuscle myosin IIA and is characterized by large platelets and thrombocytopenia, both of which are congenital. Moreover, MYH9-RD is variably associated with young-adult onset of progressive high-frequency sensorineural hearing loss, presenile cataract, and a proteinuric nephropathy often evolving into end-stage renal failure.3,4
Only 2 therapeutic options are available for increasing platelet counts in inherited thrombocytopenias, and have either little transient efficacy or carry the risk of life-threatening side effects. Platelet transfusions transiently improve thrombocytopenia, but expose to the risks of acute reactions, transmission of infectious diseases, and refractoriness to subsequent platelet transfusions. Allogeneic hematopoietic stem cell transplantation can cure inherited thrombocytopenias, but it is a risky procedure and only a few forms with severe prognosis are currently considered for this treatment.5-7 Moreover, 1-deamino-8-D-arginine vasopressin8 and activated factor VII9 have been reported to transiently shorten bleeding time in few cases, but they have side effects and are still regarded as experimental in inherited thrombocytopenias.
Novel thrombopoiesis-stimulating agents have been developed and 2 of them have been shown to increase platelet count in a few forms of acquired thrombocytopenia. Eltrombopag, an orally available nonpeptide agonist of the thrombopoietin (TPO) receptor, ameliorated thrombocytopenia in patients with chronic immune thrombocytopenia (ITP)10,11 and thrombocytopenia associated with hepatitis C virus-related cirrhosis,12 while romiplostim, an injectable TPO peptide agonist, increased platelet count in patients with low-risk myelodysplastic syndrome,13 as well as in chronic ITP.14-16 Eltrombopag and romiplostim are now approved by the Food and Drug Administration in the United States and by European Medicines Agency in Europe for the second-line treatment of patients with chronic ITP.
Based on these findings, the reasoning stood that TPO mimetics could be effective also in inherited thrombocytopenias. Since it has been recently shown that megakaryocytes of patients with MYH9-RD respond in vitro to TPO stimulation,17 we decided to test the hypothesis that eltrombopag ameliorates thrombocytopenia in this condition.
Methods
Patients
Patients were enrolled at the Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo Foundation of Pavia, Italy, and the Policlinico S Maria della Misericordia of Perugia, Italy. The institutional review boards of the participating centers approved the protocol, and all patients gave written informed consent in accordance with the Declaration of Helsinki. Inclusion criteria included patients to be at least 16 years of age, a diagnosis of MYH9-RD confirmed by identification of the causative MYH9 mutations, and a platelet count lower than 50 × 109/L. Exclusion criteria included patients with conditions known to involve the risk of thromboembolic events (eg, atrial fibrillation), history of thrombosis within 1 year, use of drugs that affect platelet function (including, but not limited to, aspirin, clopidogrel or nonsteroidal anti-inflammatory drugs) or anticoagulants, females who were nursing or pregnant, alcohol or drug addiction, and altered renal function as defined by creatinine ≥ 20 mg/L.
Study design
This was a phase II, multicentre, open-label, dose escalation trial. Enrolled patients received orally eltrombopag 50 mg daily for 21 days (Revolade, GlaxoSmithKline). Patients with platelet counts lower than 100 × 109/L at day 21 increased their dose of eltrombopag to 75 mg daily for 21 additional days. Patients with platelet counts between 100 and 150 × 109/L at day 21 continued eltrombopag 50 mg daily for the following 21 days, while patients with more than 150 × 109 platelets/L stopped therapy.
The primary endpoints were the achievement of a platelet count over 100 × 109/L or at least 3 times the baseline value (major response), or at least twice the baseline value but less than major response (minor response).
Secondary end points included safety and tolerability, and the reduction of bleeding tendency as measured by the World Health Organization (WHO) bleeding scale (grade 0, no bleeding; grade 1, petechiae; grade 2, mild blood loss; grade 3, gross blood loss; and grade 4, debilitating blood loss).
Assessments and outcome measures
Baseline evaluations included physical examination, assessment of bleeding tendency in the previous 7 days according to the WHO bleeding scale, ophthalmic evaluation, aspartate aminotransferase, alanine aminotranferease, bilirubin, creatinine, urine analysis, serum TPO level, and blood counts by cell counter. Since electronic counters greatly underestimate both platelet count and volume in subjects with MYH9-RD,18 platelet counts were also evaluated by phase-contrast microscopy in a counting chamber, and platelet size was measured by software-assisted microscopy examination of peripheral blood films.19 Only platelet counts measured by microscopy were used for the purposes of this study. Routine blood chemistry and urine analysis were performed by standard methods. Serum TPO was measured using the commercial enzyme-linked immunosorbent assay kit, Quantikine Human TPO Immunoassay, from R&D Systems.
All examinations performed at enrollment were repeated at days 21 and 42 (in patients receiving eltrombopag for 6 weeks), as well as 15 and 30 days after the end of therapy, with the exception of ophthalmic assessment, which has been performed only at the end of therapy and 30 days later.
In patients with more than 100 × 109 platelets/L at the end of therapy, we evaluated also the in vitro platelet aggregation after stimulation with adenosine diphosphate (5 and 20μM), collagen (5 and 20 mg/mL) and ristocetin (3 mg/mL) by the densitometric method of Born in native platelet-rich plasma. The extent of platelet aggregation was measured 5 minutes after the addition of stimulating agents and results obtained in patients were compared with the normal ranges in the laboratories where the assay was performed.
Statistical analysis
Sample size and power.
We used the Fleming method for phase II studies to compute the power of our study. Based on this method, we stated the following hypotheses: the treatment would deserve further evaluation if we reached a proportion of success of at least 50% (Pa); the proportion of success deserving no further investigation was fixed at 20% (Po). With 16 patients enrolled, a 1-sided type I error of 5%, the power of the study would be 86%. An alternative hypotheses statement considered Pa = 60% and Po = 30%. In this case the power was computed to 82%.
The sample size of 16 was determined by the feasibility to enroll patients with this rare condition presenting the inclusion criteria for the study.
Data analysis.
The proportion of patients responding at the end of treatment was computed together with its 95% exact binomial confidence interval. Changes from baseline to the end of treatment were assessed with the Wilcoxon sign rank test for continuous variables and the McNemar exact test for categorical variables. The change from baseline and its 95% confidence interval was reported. Changes over time from baseline to the end of follow-up were assessed by general linear models, with calculation of Huber-White robust standard errors to account for intra-patient correlation of measures. Comparison between response groups of baseline characteristics were performed with the Fisher exact test and the Mann-Whitney U test, for categorical and continuous variables, respectively. All tests were 2-sided. Stata 11 (StataCorp) was used for computation.
Results
Study population
From the 120 patients enrolled in the Italian Registry for MYH9-RD (http://www.registromyh9.org) since its institution in 2006, 23 patients matched the requirements for this clinical trial, while 22 were excluded because they had less than 16 years, 60 because their platelet counts were higher than 50 × 109/L, and 15 for other reasons (eg, kidney failure, previous thrombosis, or pregnancy). All subjects matching the inclusion criteria were invited to participate in the study whereby only 12 patients accepted. Since no other patients with MYH9-RD were available, the study included 12 subjects instead of the 16 planned. Patients were enrolled between January 26, 2009, and January 26, 2010. Their baseline clinical and laboratory features at enrolment are reported in Table 1 and in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Six patients had previously received both platelets and red blood cell transfusions for treatment of major bleeding episodes. For the control of menorrhagia, 5 females required hormonal treatments, 1 endoscopic endometrial ablation, and 1 hysterectomy. Before recognition of MYH9-RD, 7 patients had been misdiagnosed with autoimmune thrombocytopenia and received immunosuppressive treatments, and 4 of them had undergone splenectomy. No significant increases in platelet count had been reported after these treatments.
All patients completed 3 to 6 weeks of the study treatment and underwent the planned examinations. In vitro platelet aggregation was measured at the end of treatment in the 7 patients with platelet counts higher than 100 × 109/L. All patients were evaluated 15 days after the end of treatment, and 10 patients also after 30 days, while 2 patients refused the last visit.
Platelet count
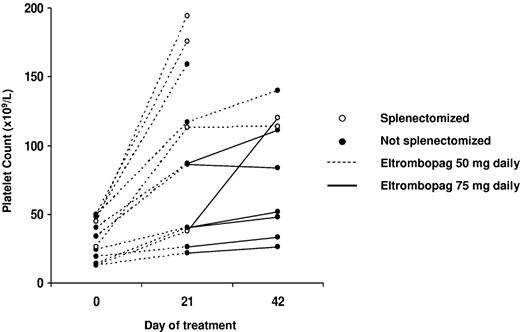
The time course of platelet count in each patient is shown in Table 2 and Figure 1. As expected, platelet count evaluated microscopically was nearly always higher than that measured by the cell counter and, as defined by the study protocol, values obtained by the microscope method were used for the purposes of this trial.
Time course of platelet count evaluated microscopically in the 12 MYH9-RD patients treated with eltrombopag. The dosage of eltrombopag is described, as well the splenectomized or not splenectomized state.
Time course of platelet count evaluated microscopically in the 12 MYH9-RD patients treated with eltrombopag. The dosage of eltrombopag is described, as well the splenectomized or not splenectomized state.
After 3 weeks at the eltrombopag dose of 50 mg daily, 3 patients achieved platelet counts of 150 × 109/L or more and stopped therapy. Two patients had a platelet counts between 100 and 150 × 109/L and continued treatment at the same dosage for 3 additional weeks, while 7 patients had less than 100 × 109 platelets/L and received eltrombopag 75 mg daily for 3 weeks.
A major response was obtained in 8 patients (67%), in 5 of them after 3 weeks of eltrombopag 50 mg daily, and in 3 cases after 3 additional weeks at the dose of 75 mg. Three patients (25%) achieved a minor response, 1 after 3 weeks at 50 mg daily, and 2 after 3 additional weeks at 75 mg. In 1 patient the treatment resulted in no response (Table 3).
Given the lower than expected number of patients accepting to participate in the study (12 vs 16 in the power calculation) and the prevalence of response, the power of the study to detect a 5% significant difference, when the proportions deserving no further investigation were fixed by design at 20% and 30%, was recalculated. It was above 90% for the overall response and above 80% for the major response.
Mean platelet count at the end of treatment was significantly higher than at baseline (104 × 109/L vs 31 × 109/L, P =.0022; Table 3). In the 11 patients who achieved major or minor responses, mean platelet count was still higher than baseline 15 days after discontinuation of the drug (66 vs 32 × 109 platelets/L, P = .005), while it returned to levels near base-line 15 days later (in the 9 responders who underwent the last scheduled visit).
Further explorative analyses showed no significant association between platelet response and patients' baseline characteristics (platelet count, TPO levels, and mutations in the motor domain or the tail of MYH9 protein; data not shown), with the only exception of previous splenectomy, in that all the 4 asplenic patients achieved major responses with platelet counts higher than 100 × 109/L. The mean platelet count of splenectomized patients at the end of therapy was 151 × 109/L with respect to 81 × 109 of nonsplenectomized subjects (P = .04). However, due to the little number of investigated patients and the confounding effect of splenectomy, we cannot exclude that we missed actual correlations between some patients' features (as genetic defect) and treatment response.
In vitro platelet aggregation
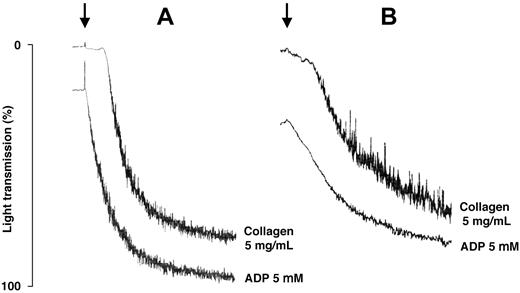
The extent of platelet aggregation was within the normal range after all tested agonists in 5 of the 7 patients who achieved platelet counts higher than 100 × 109/L, while it was slightly reduced after adenosine diphosphate and collagen in 2 patients. Of note, the 5 patients with normal platelet aggregation had mutations in the tail domain of MYH9 protein, while those with mild defects had mutations in the motor domain (Figure 2). These results have to be interpreted with caution, because no appropriate control exists for comparing the in vitro aggregation of subjects with very large platelets.
Tracings of in vitro platelet aggregation obtained at the end of eltrombopag treatment in patients with mutations of MYH9 protein affecting the tail or the motor domain of the molecule. (A) Mutations of MYH9 protein affecting the tail (patient no. 5 in Table 2). (B) Mutations of MYH9 protein affecting the motor domain of the molecule (patient no. 1). Arrows indicate the addition of the stimulating agents reported beside the tracings.
Tracings of in vitro platelet aggregation obtained at the end of eltrombopag treatment in patients with mutations of MYH9 protein affecting the tail or the motor domain of the molecule. (A) Mutations of MYH9 protein affecting the tail (patient no. 5 in Table 2). (B) Mutations of MYH9 protein affecting the motor domain of the molecule (patient no. 1). Arrows indicate the addition of the stimulating agents reported beside the tracings.
Platelet size and thrombopoietin levels
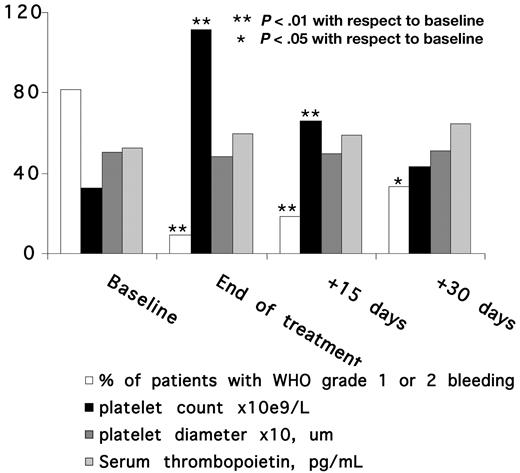
As expected, platelet diameters at baseline were much higher than normal range. Platelet size did not change either during treatment or after its cessation (Figure 3), and this indicates that the observed increases in platelet count were paralleled by corresponding increases in total platelet mass.
Time course of bleeding tendency, platelet count, platelet diameter, and serum thrombopoietin level in the 11 patients who achieved major or minor responses by eltrombopag treatment.
Time course of bleeding tendency, platelet count, platelet diameter, and serum thrombopoietin level in the 11 patients who achieved major or minor responses by eltrombopag treatment.
Mean serum TPO level was higher in patients (median, 43.7 pg/mL) than in 40 healthy subjects (14.5 pg/mL; P < .01) and this figure did not change neither during treatment with eltrombopag nor after its discontinuation (Figure 3).
Bleeding
Of 12 patients, 10 had grade 1 or 2 bleeding symptoms, as measured by the WHO bleeding scale, during the week preceding the start of eltrombopag, while 2 were asymptomatic (Table 2). Upon treatment, bleeding diathesis quickly ameliorated and disappeared in 8 cases, while it remained unchanged in the only patient with no response to eltrombopag (peak platelet count 33 × 109/L) and in another who achieved a minor response (peak platelet count 26 × 109/L).
The benefit in terms of bleeding diathesis lasted well beyond treatment discontinuation, in that the percentage of subjects with bleedings was still lower 15 and 30 days after stopping therapy than at baseline (Figure 2).
Safety
Treatment was well tolerated in all cases, with only 2 patients reporting mild and transient headache and 1 patient suffering from transient dry mouth at the beginning of treatment. The only blood parameter that changed during treatment was platelet count. Results of ophthalmic assessment at the end of therapy were unchanged, including the only patient presenting with cataract at baseline. This finding is relevant, since eltrombopag has been suspected of increased risk of cataracts in mice.20 Furthermore, posttreatment assessments did not identify any adverse event.
Discussion
The recently introduced TPO mimetics represent an appealing therapeutic hypothesis for increasing platelet count in MYH9-RD, since in vitro studies suggested that differentiation and maturation of patients' megakaryocytes upon TPO stimulation occur normally and thrombocytopenia derives from complex defects of platelet release.17 Thus, increasing the number of megakaryocytes could remedy for the reduced efficiency of thrombopoiesis. Since in vitro studies revealed that platelet aggregation is normal or only slightly defective in MYH9-RD,21,22 increasing platelet count is expected to reduce bleeding tendency.
This study evaluated the effect of eltrombopag in 12 MYH9-RD patients and confirmed these hypotheses. Mean platelet count at the end of treatment was significantly higher than at baseline, and 7 of 12 patients had their platelet counts over 100 × 109/L. Importantly, bleeding symptoms completely disappeared in all them, and in vitro platelet aggregation was completely normal in 5 of these cases and only slightly reduced in 2. On this basis we believe that eltrombopag made these patients eligible for invasive procedures. In fact, 1 patient (patient no. 1), who had previously required platelet transfusion to prevent serious and prolonged gum bleeding upon tartar removal, received this procedure at the end of therapy without any significant blood loss. Eltrombopag showed a benefit also in some patients with milder responses, in that bleeding tendency disappeared in 1 of the 3 previously symptomatic patients who achieved a platelet count below 100 × 109/L. On the whole, eltrombopag improved hemostasis in 8 of 10 patients with spontaneous bleeding at baseline.
Concerning the dosage of eltrombopag, 5 of the 7 patients achieving platelet counts higher than 100 × 109/L obtained this result after 3 weeks at 50 mg daily, while 2 required 3 additional weeks at 75 mg daily. In 4 of the 5 remaining patients, the dose of 75 mg obtained slightly higher platelet counts than the lower dose, while similar results have been obtained in 1 case by the 2 dosages.
Of the parameters recorded at baseline, a previous splenectomy seemed to predict treatment outcome, in that all the asplenic subjects achieved platelet counts higher than 100 × 109/L. The absence of spleen seems to have favored response to eltrombopag particularly in patients with mutations in the head domain of MYH9 protein. This hypothesis is supported also by the observation that, although starting from the same degree of thrombocytopenia, a nonsplenectomized adolescent with this type of mutation (patient no. 12) achieved 33 × 109 platelets/L at the end of treatment with the higher eltrombopag dose, while his splenectomized mother (patient no. 11) obtained 113 × 109 platelets/L receiving the lower dose.
Treatment with eltrombopag was well tolerated in all cases, with only very mild side effects in 2 patients. In particular, the drug was not reported to cause development or worsening of cataract. This is an important finding, since MYH9 mutations predispose to this defect, which was described in animals treated with eltrombopag.20 Moreover, no patient had worsening of the bleeding tendency with respect to baseline after stopping the drug, a problem that has been described in some ITP patients.20 Although it is possible that we didn't observe this phenomenon because of the small number of investigated patients, it may also be that the high TPO levels of MYH9-RD patients prevented it.
In conclusion, 50-75 mg of eltrombopag per day increased platelet count and reduced bleeding tendency in most patients with MYH9-RD. Eltrombopag, therefore, represents a new therapeutic option for MYH9-RD patients with severe thrombocytopenia and recurrent episodes of spontaneous bleeding, as well as for patients with milder degree of thrombocytopenia that need to transiently improve hemostasis in preparation for elective surgery or invasive procedure. Although short term treatment with eltrombopag was safe in our experience, we have no information on the side effects of long-term treatment in MYH9-RD, and, therefore, a careful follow-up is required for patients receiving this drug for a long time. Particular attention should be devoted to examining whether the long term administration of eltrombopag in subjects with MYH9 mutations results in cataract or produces bone marrow fibrosis, a side effect that has been observed in patients receiving this drug for other disorders.20 Moreover, attention should be devoted also to the possibility that increasing platelet count in subjects with very large platelets induces thrombotic risk.
Further research is required to ascertain whether TPO mimetics are effective also in other forms of inherited thrombocytopenia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all our patients and their families. We also thank Gaetano Bonifacio (GlaxoSmithKline) for critically revising the study proposal.
This study was supported by grants from the Telethon Foundation (GGP06177) and the IRCCS Policlinico San Matteo Foundation. GlaxoSmithKline made eletrombopag available for the study and partially supported the clinical and laboratory analyses required by the trial.
Authorship
Contribution: C.L.B., A.P., and P.G. designed the research, interpreted results, and wrote the manuscript; A.P., P.G., A.S., P.N., T.F., V.B., A.M.M., and F.M. acquired data; C.K. performed statistical analysis; and all authors had access to primary clinical trial data and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlo L. Balduini, Clinica Medica III, IRCCS Policlinico San Matteo Foundation–University of Pavia, Piazzale Golgi, 27100 Pavia, Italy; e-mail: c.balduini@smatteo.pv.it.