Abstract
High levels of coagulation factor VIII (FVIII) have been associated with cardiovascular disease. Low-density lipoprotein receptor (LDLR) has been recently demonstrated to contribute to FVIII clearance from plasma. The aim of this study was to evaluate 3 single nucleotide polymorphisms in SMARCA4-LDLR gene locus (rs1122608, rs2228671, and rs688) and FVIII coagulant activity (FVIII:c) in subjects with (n = 692) or without (n = 291) angiographically confirmed coronary artery disease (CAD). High FVIII:c levels were an independent risk factor for CAD. The rs688 and rs2228671 genotypes were predictors of FVIII:c with T alleles associated with higher FVIII:c levels. The rs2228671T allele was associated also with reduced total and LDL-cholesterol levels. With respect to the risk of CAD, no association was found for rs2228671. Consistently with higher FVIII:c levels, the rs688T allele was associated with CAD, whereas, consistently with a favorable lipid profile, the rs1122608T allele was associated with a decreased CAD prevalence. After adjustment for classic cardiovascular risk factors, including plasma lipids, rs688 remained associated with CAD (OR for T carriers: 1.67 with 95% confidence interval, 1.10-2.54). Haplotype analysis confirmed such results. Our data suggest that polymorphisms at LDLR locus modulate FVIII:c levels and may be associated with CAD risk independently from plasma lipids.
Introduction
Coagulation factor VIII (FVIII) plays a fundamental role in the amplification and propagation of the coagulation cascade, enabling the thrombin burst during hemostasis. High levels of FVIII are well known to be an important risk factor for venous thrombosis1-3 and have been associated also with an increased risk of arterial thrombosis and coronary artery disease (CAD).4-6 As a consistent counterpart, hemophilia patients with low levels of FVIII present a lower mortality resulting from ischemic heart disease than the general population.7-9
Although the heritability of FVIII plasma levels is high (near 40%–60%),10,11 variations of FVIII gene explain only a minor proportion of this broad variance.12-14 Up to now, single nucleotide polymorphisms (SNPs) at only 2 other genes, the ABO blood-group locus and the low density lipoprotein receptor-related protein 1 (LRP1), have been demonstrated to contribute to the modulation of FVIII plasma levels.10,15,16 The ABO blood-group determinants have been proposed to affect, through different glycosylation patterns, the processing and/or the clearance of von Willebrand factor (VWF), which in turn binds and stabilizes FVIII.17,18 The multifunctional endocytic receptor LRP1 mediates cellular uptake and subsequent degradation of FVIII,19-21 and SNPs at this locus have been associated with FVIII activity (FVIII:c) and the risk of venous thromboembolism.15,16 Recently, also the low-density lipoprotein receptor (LDLR) has been proposed to contribute to the regulation of FVIII plasma levels. Indeed, FVIII has been demonstrated to be a ligand of LDLR, and in mice models LDLR cooperates with LRP1 in regulating plasma levels of FVIII in vivo, suggesting a previously unrecognized link between LDLR and hemostasis.22
Few molecules have been recognized to play a pivotal role in atherosclerotic disease, such as LDLR. The cornerstone work of the Nobel prize winners Goldstein and Brown has clearly demonstrated the importance of LDLR in cholesterol homeostasis and, consequently, in the processes that led to atherosclerotic cardiovascular disease.23 This is further emphasized by the LDLR mutations leading to familial hypercholesterolemia, a well-known cause of accelerated atherosclerosis.24 Moreover, recent genome-wide association studies have highlighted that common variations at the LDLR locus are strongly associated with proatherogenic lipid profile and with CAD.25,26
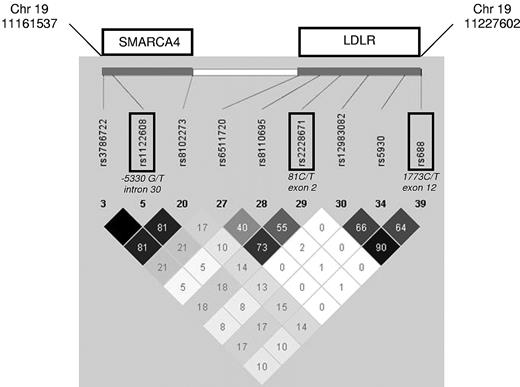
Among the several common genetic variants described at LDLR locus, the rs2228671 C/T appears to have the strongest association with LDL-cholesterol levels across multiple European populations, with the T allele being associated with decreased LDL-cholesterol levels and, consistently, to a decreased risk of CAD.27 The rs688 C/T in exon 12 has been recently shown to alter splicing efficiency, with the T allele being associated with increased total and LDL-cholesterol levels in premenopausal women.28 Finally, the rs1122608 G/T at the 19p13 locus within SMARC4A intron 30 and adjacent to LDLR gene has been reproducibly associated with high LDL-cholesterol levels (G allele) and with an increased risk of myocardial infarction (MI) by genome-wide association studies.25,26 Remarkably, these 3 SNPs have been shown not to be in significant linkage disequilibrium (Figure 1), being thus highly informative for SMARCA4-LDLR gene locus.
Linkage disequilibrium structure of the genomic region surrounding SNPs rs1122608, rs2228671, and rs688. Displayed are the HapMap data (r2)33 of the SMARCA4-LDLR locus. Part of SNPs, included in high linkage disequilibrium blocks, have been omitted.
Linkage disequilibrium structure of the genomic region surrounding SNPs rs1122608, rs2228671, and rs688. Displayed are the HapMap data (r2)33 of the SMARCA4-LDLR locus. Part of SNPs, included in high linkage disequilibrium blocks, have been omitted.
Methods
Study population
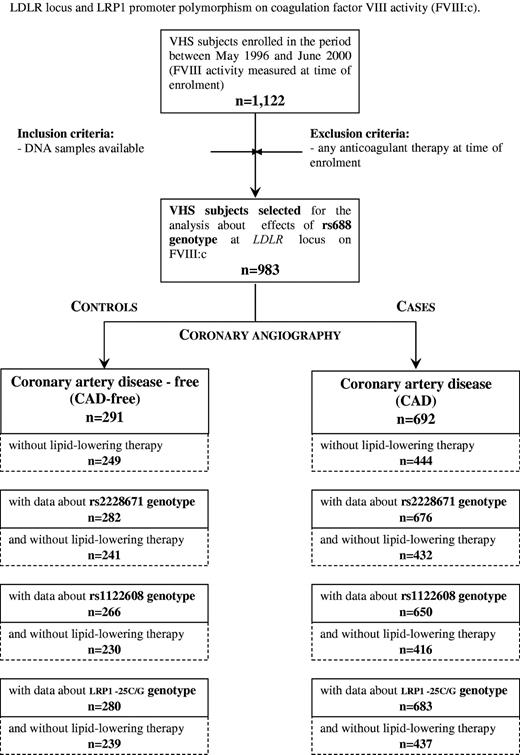
This study was performed within the framework of the Verona Heart Study, a regional survey aimed to search for new risk factors for CAD in subjects with angiographic documentation of their coronary vessels. Details about enrollment criteria have been described elsewhere.29,30 In brief, adult patients of both sexes who were recruited consecutively from those referred to the Institutes of Internal Medicine and to the Institute of Cardiovascular Surgery of the University of Verona in Italy between May 1996 and June 2000 and undergoing a coronary angiography examination (n = 1122). A total of 983 subjects, who were not taking anticoagulant drugs and for whom rs688 polymorphisms and FVIII:c data were available, were included in the present study. FVIII:c was measured at time of enrollment (details about study design are summarized in Figure 2). A total of 291 subjects had completely normal coronary arteries, being submitted to coronary angiography for reasons other than CAD, mainly valvular heart disease (CAD-free group), and served as controls. These subjects were also required to have neither history nor clinical or instrumental evidence of atherosclerosis in vascular districts beyond the coronary bed, and none was on antiplatelet drugs. A total of 692 subjects had angiographically proven CAD (the majority of them being candidates for coronary artery bypass grafting) with at least one of major epicardial coronary arteries (left anterior descending, circumflex, and right) affected with more than or equal to 1 significant stenosis (≥ 50% lumen reduction). Most CAD patients were taking antiplatelet drugs (95.2%). According to the hypothesis to be tested, subjects with nonadvanced CAD (ie, coronary stenosis < 50%) were not included in the study. The angiograms were assessed by cardiologists who were unaware that the patients were to be included in the study.
Verona Heart Study (VHS) design for the analysis of the effects of polymorphisms at LDLR locus and LRP1 promoter polymorphism on FVIII:c.
Verona Heart Study (VHS) design for the analysis of the effects of polymorphisms at LDLR locus and LRP1 promoter polymorphism on FVIII:c.
All participants came from the same geographic area (Northern Italy), with a similar socioeconomic background. At the time of blood sampling, a complete clinical history was collected, including the assessment of cardiovascular risk factors, such as obesity, smoking, hypertension, and diabetes, as well as data about drug therapies (eg, lipid-lowering therapies). The study was approved by the Ethic Committee of our institution (Azienda Ospedaliera, Verona). An informed consent was obtained from all the participants after a full explanation of the study in accordance with the Declaration of Helsinki.
Biochemical analysis and coagulation factor activity assays
Samples of venous blood were drawn from each subject, after an overnight fast, at time of enrollment before coronary angiography. Serum lipids, as well as other CAD risk factors, including high-sensitivity C-reactive protein (hs-CRP), were determined as previously described.30 The 4-variables version of the Modification of Diet in Renal Disease equation was used to estimate the glomerular filtration rate (GFR) from serum creatinine levels. Plasma factor II activity (FII:c), factor V activity (FV:c), and FVIII:c were measured on a Behring Coagulation Timer (Dade-Behring) by modification of the one-stage clotting method with the use of relative deficient plasma (Dade Behring). Coagulation time by Behring Coagulation Timer was calibrated with standard human plasma (Dade-Behring). The intra-assay and inter-assay coefficients of variations were less than 5%. Results of factors activities were expressed in terms of international units per decaliter.
SNPs and genotyping
Three SNPs tagging the SMARCA4-LDLR gene locus were selected (ie, rs688, rs2228671, and rs1122608). The 3 selected SNPs tag most variability of the SMARCA4-LDLR region from the HapMap project (Figure 1). Genomic DNA was prepared from whole blood samples by phenol-chloroform extraction. For rs688 and rs2228671 polymorphisms, genotyping was performed as follows: briefly, the rs688 polymorphism (LDLR 1773C/T) was detected by polymerase chain reaction (PCR) using the following primers: forward 5′-CGCCTCTACTGGGTTGACT-3′ and reverse 5′-CATCTTGGCTTGAGTGATCT-3′. The PCR fragment (200 bp) was digested with the HincII restriction enzyme (T allele, 15 and 185 bp; C allele, 15, 36, and 149 bp). The rs2228671 polymorphism (LDLR 81C/T) was detected by PCR using the following primers: forward, 5′-CTCTCAGTGGGCGACAGACG-3′; and reverse, 5′-CAACATGGCGAGACCCTGTC-3′. The PCR fragment (194 bp) was digested with the BstUI restriction enzyme (C allele, 20 and 174 bp; T allele, 194 bp). As regards rs1122608 polymorphism (SMARCA4 5330G/T), genotyping was attempted using the iPLEX MassARRAY platform (Mass Array, Sequenom). PCR primers were designed by Sequenom Mass-Array-Assay-Design program, as previously described.26
Considering that the original studies in mice supported a cooperation of LDLR with LRP1 in modulating plasma levels of FVIII,22 we explored the possibility of an interaction between genetic variants within the 2 genes. Thus, our study population was genotyped also for the LRP1-25C/G promoter polymorphism, which has been previously associated with different levels of FVIII:c.15 This SNP analysis was performed using an Assay-by-Design (Applied Biosystems) on a Chromo 4 (Bio-Rad).
Statistics
Calculations were performed with SPSS, Version 17.0 statistical package (SPSS). Distributions of continuous variables in groups were expressed as mean plus or minus SD. Skewed variables, such as hs-CRP, were logarithmically transformed, and geometric means with 95% confidence interval (95% CI) were reported. Quantitative data were assessed using the Student t test or by analysis of variance. Correlations between quantitative variables were assessed using Pearson correlation test. Data about lipid variables were assessed both in the whole study population and also after excluding subjects taking lipid-lowering therapies. Qualitative data were analyzed with the χ2 test and with χ2 for linear trend analysis when indicated. A value of P less than .05 was considered statistically significant. Within each group examined, the frequencies of the SMARCA4, LDLR, and LRP1 genotypes associated with each of the polymorphisms were compared by the χ2 test, with the values predicted on the basis of the Hardy-Weinberg equilibrium. Pairwise linkage disequilibrium was examined as described by Devlin and Risch.31
To assess the possible genetic predictors of FVIII:c levels, LDLR polymorphisms were included in a linear regression model adjusted for the main, known determinants of FVIII:c (ie, gender, age, blood group, and inflammation status) as well as for CAD status and renal function. Potential interactions among genotypes in determining FVIII:c levels were investigated by F test in general linear models.
To assess associations with CAD, odds ratios (ORs) with 95% CI were estimated by logistic regression models adjusted for classic risk factors (sex, age, body mass index, smoking, hypertension, diabetes, LDL- and high-density lipoprotein [HDL]-cholesterol, triglycerides, estimated GFR, and hs-CRP). Statistical power was calculated by Altman nomogram.
Haplotype frequencies were estimated using R software with haplo.stats package (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). Haplotypes present in less than 10 persons were excluded from the analyses. The relationships between haplotypes and laboratory and clinical outcomes were examined using a generalized linear model regression of a trait on haplotype effects, allowing for ambiguous haplotypes (haplo.glm function).32 Statistical significance of associations was ascertained by randomly permuting the disease status of the sample persons in 1000 replicates by Monte Carlo method.
Results
FVIII:c levels and risk of CAD
The general characteristics of the study population, divided into CAD-free and CAD groups, are summarized in Table 1. As expected, the classic risk factors for atherosclerosis were more frequent in subjects with CAD than in those without CAD.
CAD patients had higher FVIII:c levels than CAD-free subjects (172 ± 55 vs 149 ± 47 IU/dL; P < .001). According to the tertile distribution of FVIII:c in the whole population, the proportion of CAD patients increased progressively from the lowest to the highest tertile (59.9%, 69.2%, and 81.9%, respectively; P < .001 by χ2 for trend analysis). Considering subjects with FVIII:c in the lowest tertile as the reference group, a progressive greater risk of CAD was observed across the other tertiles also after adjustment for all the traditional cardiovascular risk factors (ie, sex, age, body mass index, smoking, hypertension, diabetes, LDL- and HDL-cholesterol, triglycerides, estimated GFR, and hs-CRP; Figure 3).
Risk of CAD according to FVIII:c tertile distribution (the lowest tertile was considered the reference group).
Risk of CAD according to FVIII:c tertile distribution (the lowest tertile was considered the reference group).
Noteworthy, no significant correlation was found between FVIII:c levels and any plasma lipid concentration (data not shown). On the other hand, FVIII:c levels were higher in females than in males (181 ± 55 vs 161 ± 52 IU/dL; P < .001) and in no-O blood group carriers than in 0 blood group carriers (175 ± 53 vs 149 ± 51 IU/dL; P < .001). Finally, FVIII:c was directly correlated with age (Pearson coefficient = .239; P < .001) and hs-CRP (Pearson coefficient = .210; P < .001), and inversely with estimated GFR (Pearson coefficient = −0.193; P < .001).
Genotype/fenotype correlations for SNPs at LDLR/SMARCA4 loci
The genotype distribution for each SNP was consistent with Hardy-Weinberg equilibrium both in cases and in controls (Table 1). Moreover, the 3 SNPs tested were not in linkage disequilibrium in HapMap data (Figure 1),33 and a similar linkage disequilibrium pattern was also observed in our study population (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
rs688-LDLR exon 12.
The carriers of the rs688 T allele had higher FVIII:c levels than CC-homozygotes (Table 2). In a regression model adjusted for the main determinants of FVIII:c variability, the carriership of T allele remained a significant predictor of FVIII:c (β-coefficient = 7.49; P = .037; Table 3). On the other hand, no significant association was found with lipid variables, both in the whole study population and in subjects without lipid-lowering therapies (Table 4). Consistent with the high FVIII:c levels, the carriers of rs688 T allele were more represented among CAD patients (Table 1), with an increased OR for CAD (1.39 with 95% CI, 1.05-1.86 by univariate analysis). Remarkably, the carriership of T allele remained an independent risk of CAD also after adjustment for all the traditional cardiovascular risk factors, including lipid profile (OR = 1.48 with 95% CI, 1.01-2.16, by multiple logistic regression analysis adjusted for sex, age, body mass index, smoking, hypertension, diabetes, LDL- and HDL-cholesterol, triglycerides, estimated GFR, and hs-CRP).
rs2228671-LDLR exon 2.
The carriers of the rs2228671 T allele presented also higher FVIII:c levels (Table 2), and in a regression model adjusted for the main determinants of FVIII:c variability, the carriership of T allele remained a significant predictor of FVIII activity (β-coefficient = 7.43; P = .048; Table 3). On the other hand, carriership of the T allele was associated with a more favorable lipid profile, particularly evident in subjects without lipid-lowering therapies, characterized by lower plasma concentrations of total and LDL-cholesterol, and a lower total to HDL-cholesterol ratio (Table 4). Carriers of the rs2228671 T allele did not present a different risk of CAD (Table 1).
rs1122608-SMARCA4 intron 30.
The rs1122608 genotype was not associated with FVIII:c levels (Table 2), whereas the carriers of the rs1122608 T allele were characterized by a favorable lipid profile, particularly evident in subjects without lipid-lowering therapies, with lower triglyceride plasma concentrations and lower total to HDL-cholesterol ratio (Table 4). Consistent with the favorable lipid profile, the carriers of rs1122608 T allele were less represented among CAD patients (Table 1), with a reduced OR for CAD (0.64 with 95% CI, 0.48-0.85 by univariate analysis).
As regards the association with CAD, the LDLR and SMARCA4 SNPs were included in a multiple logistic regression model adjusted for all the traditional cardiovascular risk factors, including lipid profile. Only the carriership of rs688 T allele remained an independent risk of CAD (OR = 1.67 with 95% CI, 1.10-2.54), whereas the rs1122608 genotype lost its statistically significant association (OR = 0.82 with 95% CI, 0.54-1.25).
Analysis of single nucleotide polymorphisms in the LDLR locus (rs688, rs2228671) for FII:c and FV:c levels
To evaluate whether the genotype effect of rs688 and rs2228671 was specific for FVIII or more broad on other coagulation factors, also FII:c and FV:c levels were assessed. Remarkably, no association was found between the aforementioned polymorphisms and levels of FII:c or FV:c (supplemental Table 2).
Analysis of LRP1-25C/G polymorphism
The LRP1-25C/G polymorphism was not associated with either CAD (Table 5) or FVIII:c levels or lipid parameters (Table 5 in the whole study population and supplemental Table 3 in subjects without lipid-lowering therapy). The homozygotes for the G allele showed a trend toward low concentrations of total and LDL-cholesterol and high levels of FVIII:c (Table 5). However, the low number of the GG-homozygotes (n = 16) does not allow to draw any firm conclusion. Remarkably, both LDLR SNPs (rs688 and rs2228671) remained significant predictors of FVIII:c variability after the inclusion of LRP1-25 C/G polymorphism in a sex- and age-adjusted regression model (for rs688 carriership: β-coefficient = 7.81; P = .029; for rs2228671 carriership: β-coefficient = 9.38; P = .027). Moreover, no significant interaction was found among LRP1 and LDLR polymorphisms (LRP1-25C/G vs LDLR rs688: F = 1.662, P = .198; LRP1-25C/G vs LDLR rs2228671: F = 1.249, P = .287).
Haplotype analysis
We then performed a haplotype analysis considering the 3 SMARCA4-LDLR SNPs (rs1122608, rs2228671, and rs688). The 2 most frequent haplotypes in the whole population were G-C-C and G-C-T (Table 6). In sex- and age-adjusted generalized model regressions of traits, the haplotypes were significantly associated with both FVIII:c levels and lipid plasma concentrations (ie, total and LDL-cholesterol) and total to HDL-cholesterol ratio. Consistent with such results, haplotypes were also associated with a different prevalence of CAD (Table 6). In particular, the G-C-T haplotype, characterized by the high FVIII:c-associated rs688 T allele, was more frequent in CAD than in CAD-free subjects (36.6% vs 31.6%, respectively), whereas the T-C-C haplotype, characterized by the favorable lipid profile-associated rs1122608 T allele, was less frequent in CAD than in CAD-free subjects (14.1% vs 18.5%, respectively). Subjects with G-C-T haplotype carried a greater risk of CAD than subjects with T-C-C haplotype (OR = 1.59 with 95% CI, 1.17-2.18; P = .003 in a sex- and age-adjusted model and P = .021 after 1000 permutations by Monte Carlo method; Table 6).
Discussion
In this study, we demonstrate, for the first time, that SNPs at the LDLR locus may contribute to the variability of FVIII:c. Moreover, these SNPs were differently associated with CAD, consistent with FVIII:c levels and independent from lipid profile. Thus, the LDLR locus may have pleiotropic influences on either plasma lipid or coagulation factor concentrations, which in turn may modulate the risk of CAD.
To put our results into perspective, we propose the following considerations.
FVIII:c and CAD risk
FVIII is well known to play a pivotal role in the coagulation cascade. Elevated FVIII levels are established important risk factor for venous thrombosis1-3,34 and have been associated also with an increased risk of CAD and MI by previous epidemiologic studies.4-6,35-37 Concordantly, in our angiographically studied population, high levels of FVIII:c were associated with a progressive increase of CAD risk, regardless of all traditional risk factors, including those known to be correlated with FVIII:c, such as hs-CRP, and GFR. The mechanisms linking FVIII with atherosclerosis need still to be completely elucidated. Nevertheless, it is of interest to note that immunohistochemical studies on human atherectomy specimens have revealed the presence of FVIII near macrophages and smooth muscle cells, suggesting that FVIII may enhance the thrombogenicity of atherosclerotic plaques.38
rs688 and FVIII-mediated modulation of CAD risk
The rs688 is a synonymous SNP located within LDLR exon 12 and has been originally discovered in 1988.39 This genetic variant is quite common among white populations, being the frequency of T allele carriers near 65%. Previous studies have related rs688 with lipid profile,28,40 but to the best of our knowledge, no association with cardiovascular disease has been described. Zhu et al proposed a functional role for this SNP through neutralization of an exon splicing enhancer,28 and independently from the splicing factor SFRS13A.41 In this way, the T allele has been associated with an increased proportion of LDLR isoforms lacking the transmembrane domain. The “truncated” (soluble) LDLR has been proposed to act by binding LDL and inhibiting its uptake by the normal, transmembrane LDLRs. According to this hypothesis, the T allele was associated with increased total and LDL-cholesterol in premenopausal females of the Framingham Offspring Study, but not in males or postmenopausal females, suggesting a possible sex- and age-specific effect.28 In our study, we did not detect any significant association between rs688 and lipid profile. Our study population was represented mainly by males, and almost all the females were postmenopausal. Thus, our results are substantially in agreement with those by Zhu et al.28 On the other hand, we found that rs688 was an independent predictor of FVIII:c; and consistent with the high FVIII:c levels, the T allele was associated with an increased risk of CAD. Moreover, the association of rs688 with CAD risk remained unchanged after adjustment for plasma lipids by logistic regression but was no longer statistically significant after inclusion of FVIII:c levels. Our results strongly support the hypothesis of an alternative pathway linking LDLR to the cardiovascular risk regardless of the lipid profile (ie, through modulation of FVIII:c levels; supplemental Figure 1A). In a previous work, Bovenschen et al22 suggested, for the first time, this potential link showing that FVIII is a ligand for LDLR, which in turn cooperates with LRP in regulating FVIII plasma levels in mice models. Indeed, the adenovirus-mediated overexpression of human LDLR in mice accelerated FVIII clearance, further supporting the role of LDLR in FVIII metabolism. On the other hand, the same authors also demonstrated the selective interaction of LDLR with FVIII but not with VWF, the FVIII carrier protein.22 This observation is particularly relevant to our study, in view of the lack of available data on VWF levels (“Study limitations and strengths”). The specificity of the LDLR-FVIII is further indirectly supported by the lack of any association between LDLR SNPs and other coagulation factor activities, such as FII:c and FV:c.
rs1122608 and plasma lipid-mediated modulation of CAD risk
The rs1122608 polymorphism is adjacent to the LDLR gene (within SMARCA4 intron 30) and is also common, being the frequency of the minor allele (T) near 30% in the white populations. The role of this SNP has been recently highlighted by recent genome-wide association studies showing an association of this SNP with MI.26 The functional meaning of this marker of the 19p13 locus remains unknown, but it could be in linkage disequilibrium with pathogenic SNP within the adjacent LDLR, being associated with LDL-cholesterol levels.25 Our results support this view because the carriers of T allele presented a favorable lipid profile and a reduced risk of CAD, whereas the adjustment for plasma lipids by logistic regression abolished the significant association between rs1122608 and CAD. Again, this suggests that the link between rs1122608 and CAD is through changes in plasma lipids (supplemental Figure 1B) and that, unlike the rs688, there is no additional pathway contributing to the risk profile.
rs2228671 and the balance of FVIII and lipid phenotypes on CAD risk
The rs2228671 polymorphism is located within LDLR exon 2 in the third position of a triplet coding for cysteine. Similar to the other LDLR intragenic SNP rs688, it does not result in an amino acid change. Up to now, there is no evidence of a putative intrinsic functional role.27 The rs2228671 is common, with the minor T allele representing approximately 10% in the white populations, and has been associated with lipid profile in genome-wide association studies, particularly with LDL concentration.25 A study in European populations performed by Wellcome Trust Case Control (WTCC) and the Cardiogenics Consortia has related the minor T allele with a decrease in both LDL-cholesterol level and CAD risk. The present study confirms the association of this SNP with LDL-cholesterol, but not with CAD. On the other hand, rs2228671 was an independent predictor of FVIII:c, thus representing an appealing example of a genetic variant associated with 2 different biochemical phenotypes. Intriguingly, the rs2228671-related effects on the lipid and coagulation phenotypes were theoretically contrasting as regards to the susceptibility to CAD. Indeed, the minor T allele was associated with both high FVIII:c and low LDL-cholesterol; thus, we were tempted to speculate that the proatherogenic action of high FVIII:c could be counterbalanced by the atheroprotective role of low LDL-cholesterol. According to the putative net sum of effects on the final phenotype, the risk of CAD was unchanged in our population (supplemental Figure 1C).
Haplotype analysis: SMARCA4-LDLR haplotypes as determinant of plasma lipids, FVIII:c, and CAD risk
The 3 SNPs at LDRL locus were not in linkage disequilibrium, allowing us to perform an informative haplotype analysis of this locus. Consistent with the SNPs analyses, the SMARCA4-LDLR haplotypes were associated with both plasma lipids, FVIII:c levels, and CAD. In this sense, the difference in risk of CAD between the haplotypes G-C-T and T-C-C (rs1122608-rs2228671-rs688) is paradigmatic. The G-C-T, including the rs688 T allele, showed consistently high FVIII:c levels and was more represented in CAD patients. The T-C-C, including the rs1122608 T allele, showed consistently low LDL-cholesterol levels and was more represented in CAD-free subjects. The low frequencies of the other haplotypes (T-T-T and T-T-C), characterized by alleles with potentially either harmful or favorable effects, do not allow to draw any further conclusion.
LRP1-25C/G polymorphism did not significantly interact with LDLR SNPs in determining FVIII:c
Because previous work on LDLR knockout mice suggested a cooperation between LDLR and LRP1 in modulating FVIII:c levels,22 we also genotyped our study population for the known −25C/G polymorphism in the LRP1 promoter.15 However, this SNP was not significantly associated with either FVIII:c levels or CAD and did not show any significant interactions with LDLR polymorphisms. After inclusion of the −25C/G LRP1 polymorphism in the regression model, both rs688 and rs2228671 remained significant predictors of FVIII:c variability, further supporting an independent association of these LDLR polymorphisms with FVIII:c in humans.
Study limitations and strengths
Our study has some limitations that need to be taken in account. These are mainly related to the case-control design, as well as the relatively low and unbalanced sample size. Nevertheless, it was sufficiently powered for both FVIII:c and genotype analysis (> 85% by Altman nomogram). Similarly, the angiographic definition of cases and controls represents a strength of our work, preventing from enrolling in control group subjects with asymptomatic, but still significant CAD.
With respect to the association between FVIII:c and CAD, the lack of available data on VWF represents a potential limitation. VWF carries and stabilizes FVIII in the circulation and plays an important role in hemostasis by facilitating platelet activation and thrombus formation. Therefore, it has been argued that FVIII involvement in arterial thrombosis may reflect the interaction with VWF.34 On the other hand, epidemiologic studies have shown that the risk of venous thrombosis in persons with elevated FVIII:C levels is independent of VWF levels,1,42 and the Atherosclerosis Risk and Communities investigators recently reported an almost identical association of FVIII:c and VWF with CAD events during long-term follow-up (average, 12.4 years).43 Because the molecular interaction between LDLR appears limited to FVIII and not to VWF,22 it is plausible that the lack of VWF data did not weaken the cornerstone concept of our hypothesis (ie, a FVIII-related pathway linking LDLR and CAD beyond lipoprotein metabolism). Moreover, it is noteworthy that our analysis about FVIII:c determinants has been adjusted also for ABO blood group, which in turn has been proposed to affect FVIII:c levels by differential glycosylation and thus metabolism/catabolism of VWF.17,18
Finally, it should be emphasized that none of the 3 SNPs at the 19p3 locus, including the rs688,28 has been definitively proven to be functional per se; thus, further studies are needed to identify the true functional variants. Thus, biologic studies investigating the potential physiologic function of LDLR in FVIII catabolism in humans as well as exploring whether LDLR SNPs actually affect FVIII binding/uptake are particularly warranted. On the other hand, convincing proofs have been recently provided about interaction of FVIII with receptors of LDLR family,44-46 suggesting that a prolongation of FVIII lifetime may indeed result from mutations of residues critical for the interaction of FVIII with both LRP and LDLR.44
In conclusion, in this study 3 SNPs at the 19p3 locus (SMARCA4-LDLR) were associated with both plasma lipid concentrations and FVIII:c levels and, consistent with these intermediate phenotypes, with CAD risk. In particular, the results on rs688 suggest that an LDLR polymorphism may be associated with cardiovascular disease beyond the lipoprotein metabolism pathway, probably through a FVIII-related mechanism. To the best of our knowledge, our results are the first supporting the hypothesis of a role of LDLR in regulating FVIII plasma levels in humans, accordingly with the original data by Bovenschen et al in vitro and in mice models.22
Our results also provide light in the still obscure field of the genetic determinants of FVIII and further emphasize the potential importance of FVIII-related pathways in atherosclerotic disease.
The functional dynamism and the potential adaptability to new biochemical properties are increasingly recognized as a pivotal capacity of several molecules acting in biologic pathways,47 and receptors are particularly suitable to multiple binding functions. Our results suggest that the biochemical properties and abilities of LDLR are not limited to lipoprotein metabolism but may be extended to hemostatic balance. Remarkably, patients with familial hypercholesterolemia have been shown to present a net procoagulant pattern also independent of plasma LDL-cholesterol level48 and could be an adequate population model addressing the role of an extremely low expression of LDLR in modulating FVIII:c levels in further studies. Moreover, by proposing new functional capacities of LDLR activity, our results may offer new interpretation for therapeutic implications. Interestingly, a recent clinical trial has shown that the lipid-lowering therapy with rosuvastatin was associated with a decreased risk of venous thrombosis.49 This effect was not completely explained by the well-known actions of statins (eg, lipid-lowering and anti-inflammatory). On the other hand, considering that statins are known to increase the expression of LDLR, the possible ensuing increased clearance of FVIII:c may represent an adjunctive mechanism for decreasing the thrombotic risk. However, it should be noted that no clear statement of causality can be drawn from data reflecting a risk association, as well as that our results ask for validation in other population studies, particularly with prospective design. Further studies are needed in this direction addressing the intriguing prospect of alternative pathways linking LDLR and cardiovascular risk beyond lipoprotein metabolism.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Sekar Kathiresan (Cardiovascular Research Center and Cardiology Division and Center for Human Genetic Research, Massachusetts General Hospital, Boston, MA; Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, MA) for the ongoing cooperation and for providing the data about rs1122608 genotype, Mrs Maria Zoppi for invaluable secretarial help, and Dr Patrizia Guarini, Diego Minguzzi, and Patrizia Pattini for excellent technical help.
This work was supported by the Italian Ministry of University and Research, the Veneto Region, the Cariverona Foundation, Verona, Italy, and the Carife Foundation, Ferrara, Italy.
Authorship
Contribution: N.M. designed research, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; D.G. and B.L. designed research, collected data, analyzed and interpreted data, and wrote the manuscript; M.P. and G. Marchetti performed research and contributed to subsequent manuscript discussion; G. Malerba and P.F.P. performed statistical analysis and contributed to subsequent manuscript discussion; R.C. and O.O. performed research and contributed to coordination and critical review of the paper; and F.B. designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicola Martinelli, Department of Medicine, University of Verona, Policlinico G.B. Rossi, 37134 Verona, Italy; e-mail: nicola.martinelli@univr.it.