In this issue of Blood, Martinelli and colleagues describe that certain single-nucleotide polymorphisms in the LDL receptor gene independently associate with high plasma levels of coagulation factor VIII in patients with coronary artery disease.1
In 1985, Drs M. S. Brown and J. L. Goldstein received the Nobel Prize for their discoveries concerning the regulation of cholesterol homeostasis. They identified the low-density lipoprotein receptor (LDLR) as a pivotal player in cholesterol metabolism through binding to apolipoproteins E and B-100.2 The importance of the LDLR is demonstrated by the fact that genetic defects within the LDLR gene are the underlying cause of familial hypercholesterolemia.2 These patients display elevated plasma LDL cholesterol concentrations and have a concomitant increased risk for atherosclerosis and coronary artery disease (CAD). Cholesterol-containing apolipoproteins were the only identified LDLR ligands for many years. In 2005, we demonstrated that LDLR also binds to coagulation factor VIII (FVIII) in vitro and contributes to the catabolism of FVIII in mice.3 In this issue of Blood, Martinelli and colleagues build further on these studies by convincingly showing for the first time an independent association between certain LDLR single-nucleotide polymorphisms (SNPs) and increased plasma levels of FVIII in human subjects with CAD.1 Their interesting findings support the hypothesis that, in addition to cholesterol metabolism, LDLR also plays a critical role in regulating plasma FVIII levels in humans.
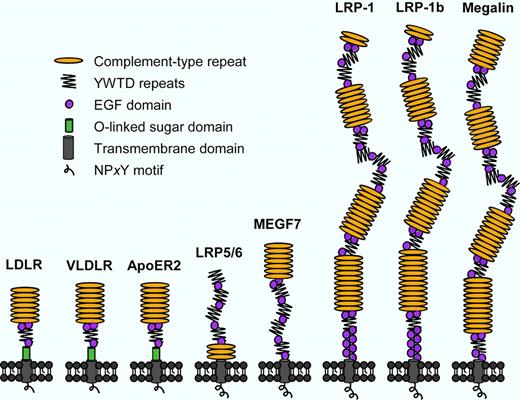
Structural representation of mammalian LDLR family members. The LDLR family consists of at least 8 homologous transmembrane receptors in mammals, which are composed of the same protein domains with similar topological organizations. Coagulation factor VIII directly binds to LDLR, LRP-1, VLDLR, and megalin (LRP-2) via their clusters of complement-type repeats.3,5-8 ApoER2 indicates apolipoprotein E receptor 2; and MEGF7, multiple EGF repeat-containing protein 7.
Structural representation of mammalian LDLR family members. The LDLR family consists of at least 8 homologous transmembrane receptors in mammals, which are composed of the same protein domains with similar topological organizations. Coagulation factor VIII directly binds to LDLR, LRP-1, VLDLR, and megalin (LRP-2) via their clusters of complement-type repeats.3,5-8 ApoER2 indicates apolipoprotein E receptor 2; and MEGF7, multiple EGF repeat-containing protein 7.
In their study, Martinelli et al measure FVIII coagulant activity in human subjects with or without CAD and confirm that high FVIII levels are an independent risk factor for CAD.1 Interestingly, high levels of FVIII in plasma positively associate with the occurrence of LDLR T-allele SNPs rs688 and rs2228671.1 Whereas the LDLR rs688 polymorphism is an independent predictor of plasma FVIII and increased risk of CAD, it does not significantly associate with an altered lipid profile in plasma.1 This indicates that this particular LDLR polymorphism is associated with cardiovascular disease independent of plasma lipids in these patients, probably through a FVIII-related mechanism. The molecular mechanism by which these LDLR SNPs may regulate plasma FVIII remains an intriguing question that deserves further study. Both SNPs do not result in an amino acid change, but rs688 has been postulated to increase transmembrane-less soluble LDLR in plasma that could bind to FVIII and may interfere with its uptake by LDLR on cells.
LDLR is a member of the LDLR gene family that includes at least 8 homologous receptors in mammals, which play partially overlapping roles in many aspects of cell physiology (see figure).4 Our previous mouse studies have shown that LDLR cooperates with LDLR-related protein-1 (LRP-1) in regulating plasma FVIII levels in vivo.3,5 In in vitro–binding assays, FVIII directly binds to 4 members of the LDLR family, including LDLR, LRP-1, megalin (LRP-2), and very-low-density lipoprotein receptor (VLDLR; see figure).3,5-8 Interestingly, 2 polymorphisms in the LRP-1 gene have previously been proposed as independent predictors of plasma FVIII activity that increase the risk of venous thrombotic events.9,10 Although Martinelli and colleagues are unable to link plasma FVIII with 1 known LRP-1 polymorphism (−25C/G) in their study group,1 it would be exciting to further investigate SNPs in LDLR family genes in general as determinants of FVIII levels in plasma, either alone or in combination.
Understanding the catabolism of FVIII in vivo is of particular interest because it could provide novel therapeutic targets for CAD, hemophilia, and/or thrombosis. High levels of plasma FVIII (> 150%) are a major risk factor for CAD and both arterial and venous thrombosis in humans. Up-regulation of LDLR protein expression may be of therapeutic interest for patients who have elevated plasma FVIII levels. Enhancement of LDLR protein expression is achieved by treatment with 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (also called statins). Statins are widely recognized in the treatment of hypercholesterolemia in humans. It would be appealing to study whether statins have the potential to lower elevated levels of plasma FVIII in humans, with the ultimate goal of reducing the risk of atherosclerotic and thrombotic events.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal