In this issue of Blood, Highfill and colleagues introduce a novel procedure to generate arginase-producing MDSCs that can prevent GVHD while maintaining antileukemic responses. An alternative non-cell–based approach using pegylated arginase-1 is also introduced.
Allogeneic hematopoietic stem cell transplantation (HSCT) can be an effective treatment for certain hematologic malignancies due to the graft-versus-tumor (GVT) response mediated by donor T cells. However, this procedure has significant hurdles including graft-versus-host-disease (GVHD), infectious complications, and leukemia resistance/relapse. GVHD is a complex disease that occurs after HSCT when donor T cells recognize foreign histocompatibility antigens on recipient tissues. The resulting donor T-cell reactivity can facilitate the destruction of several host tissues including skin, lung, liver, gut, and those of hematopoietic origin.1 Preserving GVT responses while eliminating GVHD has been a long-standing goal of both basic and clinical researchers.
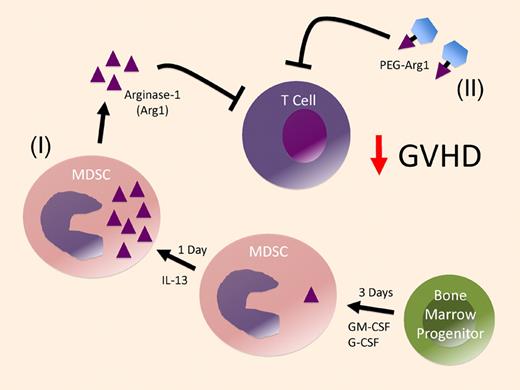
Arginase-1–producing MDSC derived from bone marrow progenitors prevent GVHD. Nonseparated bone marrow cells are cultured with GM-CSF and G-CSF for 3 days to generate MDSC and then an additional day with IL-13 to produce high levels of arginase-1 in the MDSC (I). These MDSC then suppress alloreactive T cells primarily through arginase-1 production. As an alternative pathway, pegylated-arginase-1 (PEG-Arg1) can be used to inhibit T-cell alloreactivity (II).
Arginase-1–producing MDSC derived from bone marrow progenitors prevent GVHD. Nonseparated bone marrow cells are cultured with GM-CSF and G-CSF for 3 days to generate MDSC and then an additional day with IL-13 to produce high levels of arginase-1 in the MDSC (I). These MDSC then suppress alloreactive T cells primarily through arginase-1 production. As an alternative pathway, pegylated-arginase-1 (PEG-Arg1) can be used to inhibit T-cell alloreactivity (II).
Myeloid-derived suppressor cells (MDSCs) comprise a heterogeneous population of cells known to suppress T-cell activation and the functions of other immune cells.2 These cells arise from bone marrow progenitors and form distinct lineages of cells based on the combination of factors that influence their growth, including vascular endothelial growth factor (VEGF), granulocyte-macrophage colony stimulating factor (GM-CSF), granulocyte-colony stimulating factor (G-CSF), and other immunomodulatory cytokines.3 Murine MDSCs are CD11b+ and Gr-1+ (Ly6C/Ly6G) and are further delineated based on the selective expression of Ly6G (granulocytic MDSCs) or Ly6C (monocytic MDSCs).4 MDSCs suppress T cells through a variety of mechanisms including the production of arginase-1, an enzyme that depletes arginine from the local microenvironment. In this issue, Highfill and colleagues report a new method for generating MDSCs from nonseparated bone marrow cells.5 A single infusion of these MDSCs at the time of transplantation suppressed the proliferation of donor T cells, decreased expression of the CD3ζ chain, and reduced production of interferon-γ. Importantly, these effects resulted in decreased GVHD-related mortality without eliminating the GVT effect. These MDSCs express arginase-1, and the investigators clearly demonstrate that arginase-1 is primarily responsible for their ability to inhibit alloresponses. Importantly, these MDSCs migrated to tissues where donor T-cell priming occurs.
Recently, Zhou and colleagues also reported on the generation of MDSCs from stem cells.6 Those MDSCs were also able to inhibit GVHD, but it is worth highlighting several key differences between their report and the current study. First, while Zhou et al demonstrated that MDSCs could be generated from bone marrow progenitors, their GVHD experiments focused on the use of MDSCs derived from embryonic stem cells. The second major difference between these studies is the culture conditions used to generate MDSCs. The MDSCs generated by Zhou et al were derived from purified progenitor cell populations; they required 8-17 days of culture in rather complex mixtures of 7 different cytokines. In contrast, MDSCs generated in the current study involved the culture of nonseparated bone marrow cells for only 4 days in the presence of 3 cytokines: GM-CSF, G-CSF, and interleukin-13 (IL-13). The presence of IL-13 (during the last day of culture) is the most likely factor responsible for the high levels of arginase-1 (please refer to “(I)” in the figure), because IL-13 has been shown to induce production of arginase-1 by macrophages.7 A third major difference between these studies is the mechanism of inhibition: Zhou et al identified inducible NO synthase, IL-10, and regulatory T-cell induction as the primary suppressive mediators, while the current study by Highfill et al found that arginase-1 was primarily responsible for the suppressive activity. Although the different suppressive mechanisms observed in these 2 studies may be due to the different culture conditions used to generate the MDSCs, the progenitor cell sources could partly account for the differences. Finally, the current study involved only a single infusion of MDSCs (at the time of transplantation), while Zhou and colleagues administered a total of 3 MDSC infusions in their GVHD studies.
As an interesting alternative to the use of arginase-1+ MDSC cellular therapy, Highfill and colleagues also demonstrate the ability of pegylated-arginase-1 (PEG-arg1) to inhibit GVHD. PEG-arg1 was tested based on the reported ability of this compound to target malignant cells dependent upon arginine for their proliferation.8,9 Remarkably, the GVHD inhibitory effects mediated by PEG-arg1 were quite similar to those induced by the arginase-1+ MDSCs. Although the ability of PEG-arg1 to inhibit GVHD needs to be investigated further, based on the challenges associated with getting cell-based therapies in the clinic, administration of PEG-arg1 to inhibit GVHD may prove to be a more readily translatable approach (refer to “(II)” in the figure).
In conclusion, the report by Highfill et al provides a novel process for generating highly suppressive MDSCs that are dependent upon arginase-1 activity for their suppressive function. These cells inhibit GVHD while maintaining beneficial GVT effects. In the future, it will be interesting to determine whether additional MDSC infusions can be more efficacious, and a more detailed study of the impact on GVT reactivity is warranted because it is possible that arginine starvation could negatively impact the tumor-reactive donor T cells. It is also conceivable that GVT reactivity would be more negatively impacted by systemic PEG-arg1 administration than infusion of arginase-1+ MDSCs, which may primarily act in localized tissue microenvironments.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal