Abstract
Previous studies have indicated that blockade of signaling through the T-cell receptor (TCR)/calcineurin/nuclear factor of activated T cells (NFAT) pathway impairs transplantation tolerance induced with anti-CD154 antibody. By using an allogeneic bone marrow transplantation model, we examined the role of the TCR/calcineurin/NFAT pathway for tolerance induction with anti-CD154. Calcineurin blockade by cyclosporine A led to a failure of CD8 but not CD4 tolerance, and experiments in NFAT1−/− mice replicated this effect. Studies in thymectomized mice demonstrated that blockade of the calcineurin/NFAT pathway after bone marrow transplantation led to a failure of peripheral CD8 tolerance. Moreover, CD8 adoptive transfer studies demonstrated that NFAT1 is cell-intrinsically required for peripheral CD8 tolerance. NFAT1 deficiency did not impair CD8 T-cell up-regulation of PD1, which is required for CD8 tolerance in this model. NFAT1 has previously been shown to have a role in CD4 cells for anergy induction and for programming CD4 cells to become regulatory cells. By generating mice lacking NFAT1 in CD4 but not CD8 cells, we demonstrate that NFAT1 is neither required for CD4 tolerance induction nor for their regulatory function on CD8 T cells. Thus, our study reveals a CD8 T cell–intrinsic NFAT1 requirement for CD8 tolerance in vivo.
Introduction
The principle mechanisms of CD8 T-cell tolerance—clonal deletion, anergy, suppression/regulation, and ignorance—have been elucidated in systems that use infectious agents or exogenous proteins as model antigens. Potentially autoreactive T cells, especially those with high-affinity T-cell receptors (TCRs) for major histocompatibility complex (MHC)–self-peptide complexes, are eliminated in the thymus during a central deletion process. However, some T cells with lower affinities for self-antigens can escape to the periphery, where peripheral tolerance mechanisms prevent autoimmunity. For these peripheral checkpoints, 2 main criteria seem to be important: (1) the absence of inflammatory stimuli, which activate antigen-presenting cells with subsequent up-regulation of costimulatory molecules; and (2) the persistence of the nominal antigen.1 If the first criterion is not fulfilled, immunity instead of tolerance will be induced (eg, with Toll-like receptor activation in the context of infections),2 and if the second is not fulfilled, tolerance will fade away over time.3
The nuclear factor of activated T cells (NFAT) transcription factor family includes 5 members, NFAT1 to 5, of which NFAT1 and 2 are most important in peripheral lymphocytes. Activation of calcineurin upon TCR triggering leads to dephosphorylation of NFATs, which then are translocated into the nucleus.4 The immunosuppressive agent cyclosporine A (CsA) interrupts the NFAT pathway by binding to cyclophilin and blocking the activity of the calcineurin phosphatase.5 NFAT1 (also known as NFATp or NFATc2) has been associated with induction as well as suppression of immune responses. These studies have all been in CD4 T cells and show in vitro and in vivo that pairing of NFAT1 (activated by TCR signaling) with activator protein 1 (AP-1; activated by costimulation) induces an immune activation program, whereas NFAT1 in the absence of AP-1 induces anergy.6 In contrast, in most published studies, expression of NFAT2 (also known as NFATc1) was associated with a state of unresponsiveness. NFAT2 is involved in some forms of CD8 anergy7 and very recently was shown to regulate expression of the programmed death receptor 1 (PD-1) in a cell-culture model.8
Induction of donor-specific tolerance is a desirable goal in solid-organ transplantation because it could prevent the severe side effects associated with chronic immunosuppression.9 So far, simultaneous nonmyeloablative bone marrow and kidney transplantation has been the only successful strategy to intentionally induce tolerance in a clinical setting.10 However, to avoid extensive recipient T-cell depletion, approaches to inducing peripheral T-cell tolerance are needed. In the mouse model, a minimal protocol that uses low-dose (3 Gy) total body irradiation (TBI) and costimulatory blockade with anti-CD154 (CD40 ligand) monoclonal antibody (mAb) together with bone marrow transplantation (BMT) has reliably induced stable mixed chimerism and permanent survival of MHC-mismatched skin grafts.11 In this model, alloreactive CD4 and CD8 cells are both independently capable of rejecting BM. The mechanistic study of Fehr et al12 has demonstrated that the tolerance of donor-specific peripheral T cells involves very rapid unresponsiveness followed by clonal deletion.12 In this process PD-1 engagement is essential for CD8 but not CD4 tolerance.13
The authors of studies in large animals and rodents14,15 have suggested that calcineurin inhibition blocks the graft-prolonging effects of anti-CD154. In other studies in monkeys and humans,10,16 investigators have successfully used calcineurin inhibitors in protocols achieving tolerance with combined kidney and BMT. Our previous studies in which we used anti-CD154 and allogeneic BMT have shown that CD4 tolerance can be readily achieved in the presence of calcineurin inhibition.17 However, the possible role of a TCR-mediated signal through the calcineurin/NFAT pathway for CD8 allotolerance is unknown. In view of the aforementioned contradictory data, it was important to determine the role of this pathway in CD8 allotolerance induction. We report here on studies using cyclosporine A and NFAT1-deficient mice for this purpose.
Methods
Mice
Wild-type (WT) female C57BL/6 (B6: H-2b) and B10.A (H-2a) mice were purchased from the Frederick Cancer Research Facility. B6/129 F2 and KbDb double-deficient mice were purchased from The Jackson Laboratory and Taconic, respectively. NFAT1−/−18 and 2C TCR tg mice were bred in our animal facility. Animal protocols were approved by the institutional committee at Massachusetts General Hospital.
BMT
Mice (6-12 weeks old) received 3 Gy TBI from a 137Cs irradiator on day −1. Anti-CD154 (CD40 ligand) mAb (MR1; 2 mg; National Cell Culture Center) was administered intraperitoneally on day 0 before the intravenous injection of 20 to 25 × 106 MHC-mismatched BM cells. In some groups, CD8-depleting (2.43, 1.44 mg) or CD4-depleting (GK1.5, 1.76 mg) mAbs were administered to the recipient intraperitoneally on day −1 to deplete both recipient and donor marrow-derived T cells. Ex vivo T-cell depletion of donor BM was performed where specifically indicated. Where indicated, a 2- to 4-week treatment course with 20 mg/kg/day CsA was given subcutaneously in a single daily injection, beginning on day 0. Injection sites were changed daily to avoid skin irritation.
2C/B6 Syn-chimeras
Adoptive transfer of CD8 T cells
Female KbDb double-deficient B6 animals received 3 Gy TBI/anti-CD154 and allogeneic BMT together with 9 × 106 MACS-purified, recipient-type CD8 T cells (from WT B6 or NFAT1−/− B6 spleens) intravenously. CD8 T cells were isolated by the use of CD8 T-cell isolation kits and magnetic-activated cell sorting (Miltenyi Biotec). When indicated, the KbDb knockout (KO) recipients were lethally irradiated and reconstituted with NFAT1−/− BM before allogeneic BMT and CD8 transfer.
PD-1 expression analysis
For analysis of PD-1 expression on CD8 T cells in vivo, CD45.1 B6 mice received 4 Gy TBI and 107 T cell–depleted, syngeneic, CD45.2 bone marrow cells from either WT or NFAT1−/− mice. After reconstitution of peripheral T cells, these animals received 3 Gy TBI/anti-CD154 and allogeneic B10.A BMT. On day 7 after allogeneic BMT, all animals were killed, and their splenocytes were analyzed by flow cytometry for expression of PD-1, MHC class I (to identify recipient cells), and the congenic marker CD45.2 (to distinguish between WT and NFAT1−/− cells in the same animal).
Flow cytometry
Donor cells in peripheral blood were identified with fluorescein isothiocyanate-conjugated anti-H-2Dd (mAb 34-2-12). The cells were counterstained with PE- or APC-conjugated anti-CD4, -CD8, -B220 (BD PharMingen), and -Mac1 (Caltag) mAbs. Propidium iodide staining was used for dead cell exclusion. A cell lineage was defined as chimeric when 5% or greater was 34-2-12+.
Cell-mediated lympholysis assay
Cell-mediated lympholysis assays were performed as described.11 Responder and irradiated stimulator splenocytes (8 × 105 each) were coincubated for 5 days, after which serial 2-fold dilutions were prepared. Target cells were incubated with concanavalin A (2 μg/mL) for 2 days, then labeled with 51Cr (1 μCi/mL) and incubated for 4 hours with responders. 51Cr release was quantified in a gamma counter. Percent specific lysis = ([experimental release − spontaneous release]/[total release − spontaneous release]) × 100%.
Surgical procedures
Mice were shaved and anesthetized with ketamine/xylazine.
Skin grafts.
Tail skin (0.5-1.0 cm2) from donor and third-party mice was grafted on either side of the back and regularly checked for viability until day 100.
Thymectomy.
At the age of 5 weeks, a ventral median skin incision was made, and both thymic lobes were removed by the suction method with immediate closure of the incision with clips, which were removed 7 days later. Complete thymic removal was confirmed at the end of each experiment by surgical exploration.
Statistics
The χ2 test was used to compare incidences of chimerism, and a Student t test or Mann-Whitney test was used to compare values between groups. P values less than .05 were considered significant. GraphPad Prism Software Version 4.0 was used for calculations.
Results
CsA interferes with CD8 tolerance induced by allo-BMT with anti-CD154
Several studies have shown that blockade of the TCR signaling pathway by CsA in anti–CD154-based protocols is deleterious for allotolerance induction in recipients of BMT,15 skin, and islet allografts.19 Moreover, prolongation of renal allograft survival by anti-CD154 has been impeded by CsA treatment in nonhuman primates.14 However, none of these studies dissected the effect of CsA on CD4 versus CD8 cell tolerance. We previously showed that CsA treatment does not interfere with CD4 tolerance in CD8-depleted recipients of allogeneic BMT with 3 Gy TBI/anti-CD154.17
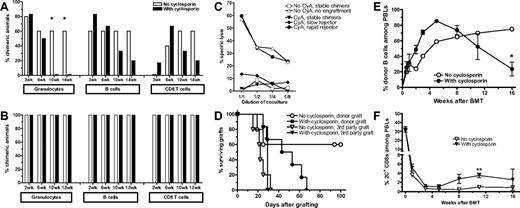
To evaluate the role of TCR-mediated signaling through the calcineurin pathway for CD8 tolerance, B6 mice were conditioned with 3 Gy TBI/anti-CD154 and received an MHC-mismatched B10.A BMT with or without a 2-week course of daily CsA (Figure 1A). The initial rate of chimerism was similar in both groups. However, CsA-treated mice began to lose chimerism 4 weeks after CsA treatment was stopped. Chimerism was lost first in the granulocyte, then in the B-cell, and finally in the T-cell lineages. That this result was attributable to a failure of CD8 tolerance was proven by addition of CD8 depletion to the conditioning regimen (Figure 1B). No difference in the incidence or levels of chimerism was observed in CD8-depleted groups, regardless of CsA treatment. In mice not receiving CD8 depletion, chimerism loss was associated with recovery of donor-specific cytotoxic T-cell responses (Figure 1C) and gradual rejection of donor-type skin grafts placed on day 1 after BMT (Figure 1D). In all groups, third-party skin grafts were rejected with similar kinetics.
CsA treatment inhibits CD8 T-cell tolerance. (A-D) WT B6 mice received 3 Gy TBI and anti-CD154 mAb followed by B10.A BMT. One group of mice was also treated with CsA for 14 days (n = 6-7/group; 1 representative of 3 experiments is shown). In the experiment shown in panel B, recipient CD8 T-cell depletion was performed in addition (n = 7-8/group). (A-B) Incidence of chimerism in different lineages is indicated. Chimerism in CD4 cells (not shown) was comparable with that in CD8 cells. A lineage was defined as chimeric when ≥ 5% donor cells were found in that lineage. Note that T-cell chimerism usually only begins to appear at 2 weeks after BMT and thereafter increases slowly, reflecting the long survival time of peripheral T cells and the slow peripheral repopulation from de novo thymopoiesis. (C) Recipient mice were euthanized at day 90 after BMT, and their splenocytes were used in a cell-mediated lympholysis assay to determine cytotoxicity against donor and third-party targets. CyA indicates cyclosporine A. (D) At day 1 after BMT, skin grafts were placed, and survival was followed until day 100 after grafting. Mean graft survival times were as follows: donor grafts 48 days with CsA vs indefinite without CsA, third-party grafts 29 days with CsA vs 22 days without CsA. (E-F) 2C/B6 syn-chimeric mice received 3 Gy TBI/anti-CD154 and B10.A BMT. One group of mice was also treated with CsA for 28 days (n = 10-11/group). (E) Levels of chimerism in mice that showed engraftment at 2 weeks are depicted over time for the B-cell lineage. (F) The percentage of 2C+CD8+ cells among peripheral blood lymphocytes is depicted over time.
CsA treatment inhibits CD8 T-cell tolerance. (A-D) WT B6 mice received 3 Gy TBI and anti-CD154 mAb followed by B10.A BMT. One group of mice was also treated with CsA for 14 days (n = 6-7/group; 1 representative of 3 experiments is shown). In the experiment shown in panel B, recipient CD8 T-cell depletion was performed in addition (n = 7-8/group). (A-B) Incidence of chimerism in different lineages is indicated. Chimerism in CD4 cells (not shown) was comparable with that in CD8 cells. A lineage was defined as chimeric when ≥ 5% donor cells were found in that lineage. Note that T-cell chimerism usually only begins to appear at 2 weeks after BMT and thereafter increases slowly, reflecting the long survival time of peripheral T cells and the slow peripheral repopulation from de novo thymopoiesis. (C) Recipient mice were euthanized at day 90 after BMT, and their splenocytes were used in a cell-mediated lympholysis assay to determine cytotoxicity against donor and third-party targets. CyA indicates cyclosporine A. (D) At day 1 after BMT, skin grafts were placed, and survival was followed until day 100 after grafting. Mean graft survival times were as follows: donor grafts 48 days with CsA vs indefinite without CsA, third-party grafts 29 days with CsA vs 22 days without CsA. (E-F) 2C/B6 syn-chimeric mice received 3 Gy TBI/anti-CD154 and B10.A BMT. One group of mice was also treated with CsA for 28 days (n = 10-11/group). (E) Levels of chimerism in mice that showed engraftment at 2 weeks are depicted over time for the B-cell lineage. (F) The percentage of 2C+CD8+ cells among peripheral blood lymphocytes is depicted over time.
We have previously demonstrated that tolerance induction of peripheral CD8 cells with anti-CD154 and allogeneic BMT is associated with deletion of donor-reactive peripheral CD8 T cells over a period of approximately 10 days.12 To investigate whether CsA treatment interfered with deletion of peripheral donor-reactive CD8 cells, we used the 2C TCR transgenic model in which CD8 T cells express a directly alloreactive TCR recognizing Ld. In a first step, we generated 2C/B6 syn-chimeric mice (described in Haspot et al13 ) expressing the 2C TCR on a subpopulation of CD8 T cells. These mice then received 3 Gy TBI/anti-CD154 followed by allogeneic BMT from a donor strain expressing the Ld ligand (B10.A), with or without CsA treatment for 28 days. Again, a similar incidence and level of early chimerism were observed in the untreated and CsA-treated groups (Figure 1E). During that initial period, donor-specific TCR transgenic CD8 T-cell deletion also was similar between the 2 groups (Figure 1F). However, after 4 weeks after BMT, the CsA-treated animals again began to slowly lose chimerism. This decrease in chimerism was associated with a gradual increase in the percentage of 2C+ CD8 cells in peripheral blood (Figure 1F). Comparison of Figure 1E and F shows that 2C CD8-cell recovery coincided with chimerism loss in CsA-treated mice.
Taken together, these experiments demonstrated that CsA treatment in recipients of allogeneic BMT with anti-CD154 is detrimental for CD8 but not CD4 tolerance. CsA treatment is associated with incomplete deletion and gradual expansion of donor-reactive CD8 cells, which eventually reject the donor BM graft.
Signaling through NFAT1 is required for CD8 tolerance
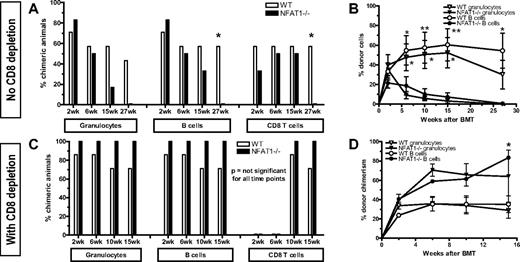
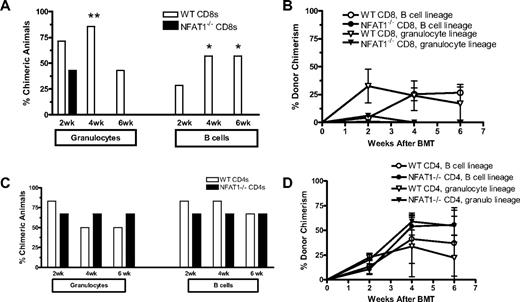
To confirm that the observed effects in CsA-treated mice resulted from specific blockade of the TCR/calcineurin/NFAT pathway rather than from a nonspecific or toxic effect of CsA, we performed similar experiments using NFAT1−/− mice instead of CsA treatment. NFAT1−/− (on mixed B6/129 background) and WT (B6/129 F2) mice received 3 Gy TBI/anti-CD154 treatment followed by fully MHC-mismatched B10.A BMT. NFAT1−/− and WT mice showed a similar incidence and similar levels of chimerism early after BMT (Figure 2A-B). However, by 6 weeks after BMT, chimerism in NFAT1−/− mice had declined markedly and was subsequently lost in all lineages by 27 weeks after BMT. To determine whether this rejection was CD8 T-cell mediated, separate groups of WT and NFAT1−/− mice received CD8 depleting mAb. No differences were observed between the 2 CD8-depleted groups in the incidence of chimerism (Figure 2C-D). These results indicated that the tolerance of CD8 but not CD4 cells was NFAT1 dependent.
CD8 T cells fail to be tolerized in NFAT1−/− mice. WT B6.129/F2 and NFAT1−/− mice received 3 Gy TBI/anti-CD154 and B10.A BMT. For the groups depicted in panels C and D, recipient CD8 T cells were depleted on day −1. Donor cell chimerism in peripheral blood leukocytes was followed over time. (A,C) Incidence of chimerism is indicated for each group in different lineages. Chimerism among CD4 cells (not shown) was comparable with that of CD8 cells. A lineage was defined as chimeric when ≥ 5% donor cells were detected in that lineage. (B,D) Levels of chimerism in mice that showed engraftment at 2 weeks are depicted over time for the granulocyte and B-cell lineages. One representative experiment of 2 is shown (n = 6-7/group).
CD8 T cells fail to be tolerized in NFAT1−/− mice. WT B6.129/F2 and NFAT1−/− mice received 3 Gy TBI/anti-CD154 and B10.A BMT. For the groups depicted in panels C and D, recipient CD8 T cells were depleted on day −1. Donor cell chimerism in peripheral blood leukocytes was followed over time. (A,C) Incidence of chimerism is indicated for each group in different lineages. Chimerism among CD4 cells (not shown) was comparable with that of CD8 cells. A lineage was defined as chimeric when ≥ 5% donor cells were detected in that lineage. (B,D) Levels of chimerism in mice that showed engraftment at 2 weeks are depicted over time for the granulocyte and B-cell lineages. One representative experiment of 2 is shown (n = 6-7/group).
The TCR/calcineurin/NFAT pathway is required for peripheral but not central CD8 T-cell tolerance
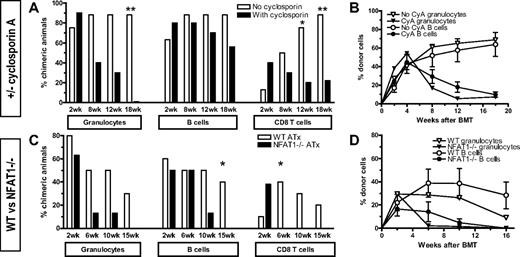
The finding in both experimental settings (CsA treatment, NFAT1−/− recipients) that loss of chimerism and tolerance occurred late and coincided with peripheral recovery of donor-specific CD8 cells (Figure 1C,F) suggested that rejection might be caused by a failure of central rather than peripheral CD8 tolerance. Indeed, CsA has previously been associated with failure of intrathymic tolerance and development of autologous graft-versus-host disease.20 To test whether the lack of NFAT1 signaling resulted in a failure of peripheral tolerance, we performed experiments in mice thymectomized at the age of 4 to 5 weeks. After recovering for 1 month, these mice were used as recipients of 3 Gy TBI/anti-CD154 followed by MHC-mismatched B10.A BMT. In the first experiment, only WT mice were used, and one group received daily injections of CsA for 28 days (Figure 3A-B). Again, initial engraftment was similar in both groups in terms of incidence and levels of chimerism, but delayed loss of chimerism was observed in CsA-treated mice, first in the granulocyte and later in the B- and T-cell lineages. Similar findings were obtained in the experiment comparing thymectomized NFAT1−/− and WT mice (Figure 3C-D). As seen with nonthymectomized recipients, the loss of chimerism was more rapid in NFAT1−/− mice than in CsA-treated mice but occurred in the same sequence in different lineages. We attribute this kinetic difference to the immunosuppressive effects of CsA during the time it is administered (28 days in the experiment in Figure 3A-B). Together, our results demonstrate that NFAT1 is required for the peripheral tolerization of preexisting, mature alloreactive CD8 T cells. Moreover, the ability to achieve long-term tolerance in NFAT1−/− mice initially depleted of peripheral CD8 cells (Figure 2) demonstrates that central tolerance can be achieved independently of NFAT1.
Thymectomy cannot restore CD8 tolerance in CsA-treated and NFAT1-deficient recipients. For each experiment depicted in this figure, 1 group of mice was thymectomized at the age of 4 to 5 weeks. Four weeks later, thymectomized and age-matched control mice received allogeneic BMT with 3 Gy TBI/anti-CD154. (A-B) Thymectomized WT B6 mice were treated with 3 Gy TBI/anti-CD154 mAb MR1 followed by B10.A BMT. One group of mice additionally received daily subcutaneous CsA injections for 28 days, beginning on day 0 (n = 7-10/group; 1 representative of 3 experiments is shown). (C-D) Thymectomized WT B6.129/F2 and NFAT1−/− mice were treated with 3Gy TBI/anti-CD154 mAb and B10.A BMT (n = 7-8/group). (A,C) Incidence of chimerism is indicated for each group in different lineages. Chimerism among CD4 cells was comparable with that among CD8 cells. (B,D) Absolute levels of chimerism in mice that showed engraftment at 2 weeks is depicted over time for the granulocyte and B-cell lineages.
Thymectomy cannot restore CD8 tolerance in CsA-treated and NFAT1-deficient recipients. For each experiment depicted in this figure, 1 group of mice was thymectomized at the age of 4 to 5 weeks. Four weeks later, thymectomized and age-matched control mice received allogeneic BMT with 3 Gy TBI/anti-CD154. (A-B) Thymectomized WT B6 mice were treated with 3 Gy TBI/anti-CD154 mAb MR1 followed by B10.A BMT. One group of mice additionally received daily subcutaneous CsA injections for 28 days, beginning on day 0 (n = 7-10/group; 1 representative of 3 experiments is shown). (C-D) Thymectomized WT B6.129/F2 and NFAT1−/− mice were treated with 3Gy TBI/anti-CD154 mAb and B10.A BMT (n = 7-8/group). (A,C) Incidence of chimerism is indicated for each group in different lineages. Chimerism among CD4 cells was comparable with that among CD8 cells. (B,D) Absolute levels of chimerism in mice that showed engraftment at 2 weeks is depicted over time for the granulocyte and B-cell lineages.
The requirement for NFAT1 is CD8 T cell–intrinsic
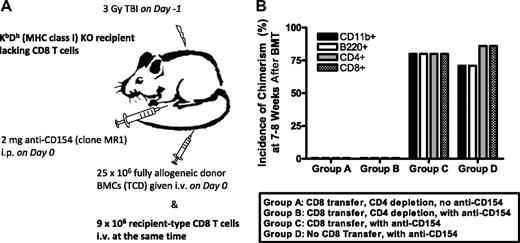
Although the aforementioned studies showed that the TCR/calcineurin/NFAT pathway is required for CD8 tolerance in a thymus-independent manner, they did not distinguish whether NFAT1 activity was required by the CD8 cell itself or whether it might be required CD8-cell extrinsically, perhaps via the regulatory CD4+CD25− T-cell population, which is necessary to tolerize donor-reactive CD8 cells before they are deleted.12 To address the first possibility, we developed an adoptive transfer model in which MHC class I–deficient (KbDb KO) animals lacking CD8 T cells were used as recipients of 3 Gy TBI/anti-CD154, followed by MHC-mismatched B10.A BMT together with adoptive transfer of recipient-type CD8 T cells at the time of BMT (Figure 4A). We first validated the model by showing that adoptively transferred WT CD8 cells are capable of rejecting allogeneic marrow in CD4-depleted KbDb KO mice and that the transferred CD8 cells can be tolerized when CD4 cells are present and anti-CD154 is given (Figure 4B). Thus, this was an appropriate system for evaluation of a possible CD8 T cell–intrinsic requirement for NFAT1.
The requirement for CD8 cell-intrinsic molecules can be determined by the use of a CD8 T-cell adoptive transfer model. (A) Experimental scheme: KbDb double-deficient recipients lacking CD8 T cells were treated with 3 Gy TBI/anti-CD154 mAb followed by infusion of 9 × 106 WT CD8 T cells at the time of T cell–depleted allogeneic BMT. (B) KbDb double-deficient recipients were treated as depicted in panel A. For groups A and B, in vivo CD4 depletion was added to demonstrate that the transferred CD8 T cells are capable of rejection; in contrast, the CD4 cell–replete group C shows that these CD8 cells can be tolerized when CD4 cells are present; group D is a control group that did not receive CD8-cell transfer (n = 6-7/group).
The requirement for CD8 cell-intrinsic molecules can be determined by the use of a CD8 T-cell adoptive transfer model. (A) Experimental scheme: KbDb double-deficient recipients lacking CD8 T cells were treated with 3 Gy TBI/anti-CD154 mAb followed by infusion of 9 × 106 WT CD8 T cells at the time of T cell–depleted allogeneic BMT. (B) KbDb double-deficient recipients were treated as depicted in panel A. For groups A and B, in vivo CD4 depletion was added to demonstrate that the transferred CD8 T cells are capable of rejection; in contrast, the CD4 cell–replete group C shows that these CD8 cells can be tolerized when CD4 cells are present; group D is a control group that did not receive CD8-cell transfer (n = 6-7/group).
Although adoptively transferred recipient-type CD8 T cells persisted similarly in both groups for 2 to 4 weeks after BMT (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), NFAT1−/− CD8 T cells could not be tolerized, as indicated by complete rejection of donor cells 4 weeks after BMT (Figure 5A-B). This rejection occurred even faster than in CsA-treated or in NFAT1−/− mice, possibly because adoptively transferred CD8 T cells did not undergo the full conditioning regimen because they were injected with allogeneic BM 1 day after TBI.
The requirement for NFAT1 for tolerance is CD8 T cell intrinsic. (A,B) KbDb double-deficient recipients were treated with 3 Gy TBI/anti-CD154 mAb followed by infusion of 9 × 106 WT or NFAT1−/− CD8 T cells at the time of T cell–depleted allogeneic BMT (n = 7 animals/group). (A) Incidence of chimerism is depicted at 2, 4, and 6 weeks after BMT for the granulocyte lineage and the B-cell lineage. (B) The level of donor chimerism over time is shown for the granulocyte and the B-cell lineage. (C-D) KbDb double-deficient recipients were lethally irradiated (10.25 Gy) and reconstituted with either WT or NFAT1−/− BM. Two months later, these mice received 3 Gy TBI/anti-CD154 mAb followed by infusion of 9 × 106 WT CD8 T cells at the time of T cell–depleted allogeneic BMT (n = 7 animals/group). (C) Incidence of chimerism is depicted at 2, 4, and 6 weeks After BMT for the granulocyte lineage and the B-cell lineage. (D) The level of donor chimerism over time is shown for the granulocyte and the B-cell lineage.
The requirement for NFAT1 for tolerance is CD8 T cell intrinsic. (A,B) KbDb double-deficient recipients were treated with 3 Gy TBI/anti-CD154 mAb followed by infusion of 9 × 106 WT or NFAT1−/− CD8 T cells at the time of T cell–depleted allogeneic BMT (n = 7 animals/group). (A) Incidence of chimerism is depicted at 2, 4, and 6 weeks after BMT for the granulocyte lineage and the B-cell lineage. (B) The level of donor chimerism over time is shown for the granulocyte and the B-cell lineage. (C-D) KbDb double-deficient recipients were lethally irradiated (10.25 Gy) and reconstituted with either WT or NFAT1−/− BM. Two months later, these mice received 3 Gy TBI/anti-CD154 mAb followed by infusion of 9 × 106 WT CD8 T cells at the time of T cell–depleted allogeneic BMT (n = 7 animals/group). (C) Incidence of chimerism is depicted at 2, 4, and 6 weeks After BMT for the granulocyte lineage and the B-cell lineage. (D) The level of donor chimerism over time is shown for the granulocyte and the B-cell lineage.
To determine whether NFAT1 in any other cell population was required for tolerance induction, we generated mice lacking NFAT1 in all hematopoietic cells except CD8 T cells. To do this, KbDb double-deficient recipients were lethally irradiated (10.25 Gy) and reconstituted with either WT or NFAT1−/− BM. Two months later, these mice received TBI/anti-CD154 mAb followed by infusion of 9 × 106 WT CD8 T cells at the time of allogeneic BMT. No difference was observed in the incidence or levels of chimerism between mice reconstituted with WT or NFAT1−/− BM, indicating that NFAT1 in CD4 cells was not required either for tolerance induction of the CD4 cells themselves or for their tolerogenic effect on CD8 cells (Figure 5C-D).Thus, NFAT1 is intrinsically required for tolerance induction in peripheral CD8 but not CD4 T cells.
PD-1 expression on CD8 T cells is independent of NFAT1
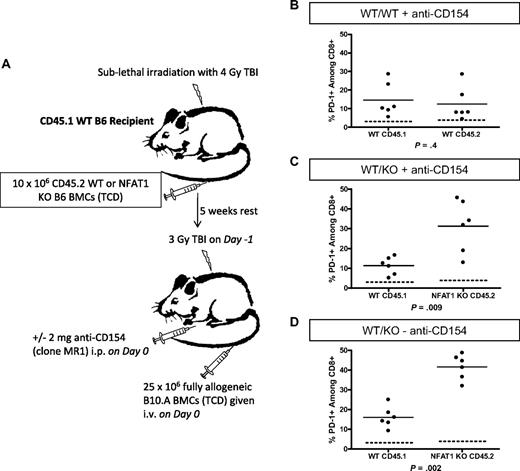
The requirement for NFAT1 for CD8 T-cell tolerance might reflect a role in up-regulation of PD-1, which is essential for CD8 T-cell tolerance in this model.13 To test this hypothesis, animals containing both WT and NFAT1−/− hematopoietic cells were generated as depicted in Figure 6A. These animals were then used as recipients of 3 Gy TBI/anti-CD154 with allogeneic BMT and killed on day 7 for ex vivo analysis of PD-1 expression. As observed previously,13 polyclonal WT CD8 T cells in control WT/WT synchimeric recipients up-regulated PD-1 compared with conditioned control animals (dotted line) that did not receive BM (Figure 6B). Surprisingly, in WT/NFAT1 KO synchimeric recipients of allogeneic BMT, up-regulation of PD-1 was significantly more pronounced on the polyclonal NFAT1 KO CD8 T cells relative to the WT CD8 T cells in the same animal (Figure 6C-D) regardless of whether anti-CD154 was administered. Thus, the role of NFAT1 in promoting CD8-cell tolerance is not related to the requirement for PD1 for CD8-cell tolerance in this model.
NFAT1 is not required for PD-1 up-regulation in vivo. Syngeneic chimeras with a mixture of WT CD45.1 and WT or NFAT1 KO CD45.2 BM were generated as depicted in panel A. On day 7 after allogeneic BMT, splenocytes were analyzed directly ex vivo for PD-1 expression on CD8 T cells. (B-D) The dotted line depicts basal levels of PD-1 expression in conditioned control animals receiving TBI but no BM. PD-1 expression on WT versus NFAT1 KO cells within the same animals was compared with (C) and without (D) administration of anti-CD154.
NFAT1 is not required for PD-1 up-regulation in vivo. Syngeneic chimeras with a mixture of WT CD45.1 and WT or NFAT1 KO CD45.2 BM were generated as depicted in panel A. On day 7 after allogeneic BMT, splenocytes were analyzed directly ex vivo for PD-1 expression on CD8 T cells. (B-D) The dotted line depicts basal levels of PD-1 expression in conditioned control animals receiving TBI but no BM. PD-1 expression on WT versus NFAT1 KO cells within the same animals was compared with (C) and without (D) administration of anti-CD154.
Discussion
This study demonstrates for the first time a critical role for NFAT1 in CD8 tolerance in vivo. The role of NFAT1 as a negative regulator of CD4 T-cell responses became apparent when the phenotype of the NFAT1−/− mouse was analyzed. These animals surprisingly showed increased immune responses to infectious agents and demonstrated an aggravated phenotype in an allergy model in vivo.18 In later studies, it was demonstrated that NFAT1 induces the expression of anergy-associated genes in CD4 cells (recessive tolerance6 ) and that NFAT1, via a physical interaction with FoxP3, plays an important role in the generation and maintenance of Tregs (dominant tolerance21 ). Therefore, NFAT1 may serve as a master switch of transcription programs. Depending on the availability and interaction with other transcription factors, NFAT1 determines the fate of CD4 T cells after TCR triggering: If NFAT1 interacts with the transcription factor AP-1, a proinflammatory program is activated,22 and if NFAT1 interacts with FoxP3, a regulatory program ensues.21 However, our studies show that NFAT1 is not required for CD4-cell tolerance in mice receiving BMT with anti-CD154. Instead, we provide what is, to our knowledge, the first demonstration of a cell-intrinsic role for NFAT1 in CD8-cell tolerance and demonstrate this in an in vivo model.
In fact, inhibitory effects of NFAT1 on CD8 T-cell functions have not been previously described. Teixeira et al23 have demonstrated a crucial role of NFAT1 for interferon-γ (IFN-γ) production in activated CD8 T cells. Although IFN-γ has been reported to promote activation-induced cell death,24 our previous studies have shown that IFN-γ is not required for the tolerization of either CD4 or CD8 T cells in this model.12 The chimerism achieved in mice that have NFAT1 in CD8 but not CD4 T cells rules out the possibility that NFAT1 promotes CD8-cell tolerance in our model through induction of regulatory CD4 T cells. Although we have previously shown that CD4 T cells are required for the establishment of CD8-cell tolerance in this model, the required CD4 cells did not have properties associated with classical natural Tregs.12 Furthermore, similar effects of CsA and NFAT1 deficiency were observed in euthymic and thymectomized mice, arguing for a role of this pathway in peripheral CD8 tolerance rather than intrathymic generation of a requisite regulatory population or in intrathymic CD8 T-cell deletion.
The failure of peripheral CD8 tolerance in CsA-treated and NFAT1−/− mice occurred late, that is, beyond 4 to 6 weeks after BMT, whereas initial engraftment was not affected. We have previously shown that peripheral CD8-cell tolerance in this model involves rapid induction of specific unresponsiveness12,13 followed by deletion. Together with the CD8 T cell–intrinsic NFAT1 requirement and the recovery of donor-reactive TCR transgenic CD8 cells in CsA-treated mice before donor chimerism is lost, our data suggest that NFAT1 is required for the progression of peripheral CD8-cell anergy to complete deletion in mixed chimeras generated under cover of anti-CD154/TBI.
Taken together, our findings demonstrate a critical, cell-intrinsic role for the TCR/calcineurin pathway in peripheral CD8 but not CD4 T-cell tolerance. Moreover, we identify NFAT1 as the critical transcription factor in this tolerogenic pathway. NFAT1 is involved in anergy in CD4 T cells in vitro but is clearly not required for CD4 tolerance in our model. Our demonstration of its role in CD8 T-cell tolerance suggests that an NFAT1-dependent, anergy-like program may be important. However, our previous finding that PD-1, which plays a major role in CD8 exhaustion during chronic viral infection,25 is also required for CD8 T-cell tolerance,13 argues for an exhaustion-like mechanism. Although NFAT2 is required for up-regulation of PD-18 and PD-1 is required for CD8 T-cell tolerance in our model,13 the CD8 T cell–intrinsic requirement for NFAT1 is not explained by a failure to express PD-1. In fact, NFAT1−/− CD8 T cells express significantly greater levels of PD-1 than WT CD8 T cells in the same animals after allogeneic BMT. Because they lack CD8 T cell–intrinsic NFAT1 required to achieve tolerance, the NFAT1−/− CD8 T cells actively reject donor BM regardless of whether anti-CD154 is given. Thus, the greater expression of PD-1 on NFAT1−/− CD8 T cells may reflect their enhanced activation relative to their WT counterparts. Alternatively, the greater expression of PD-1 in NFAT1−/− CD8 T cells may indicate that one of the many functions of NFAT1 is to induce transcription of an inhibitor of PD-1 expression. Importantly, the roles for NFAT1 and PD-1 in CD8 tolerance appear to be independent, and further studies are needed to determine which NFAT1 targets are critical for CD8 tolerance and which transcriptional partners of NFAT1 are involved in this process.
Our findings indicate that caution should be exercised in the use of calcineurin inhibitors in patients undergoing tolerance induction protocols because blocking NFAT1 may have a detrimental effect. However, our studies suggest that this concern does not apply in protocols involving CD8-cell depletion. The results for CD8 but not CD4 cells are consistent with a paradigm proposed several years ago, in which induction of anergy and eventually deletional tolerance is most efficient when a strong antigen-specific signal 1 is combined with blockade of signal 2 and that combined blockade of signal 1 and 2 is deleterious for tolerance rather than helpful, thus “a duel of 2 signals.”26
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Annette Oxenius and Ronjon Chakraverty for their critical review of the manuscript, Orlando Moreno for outstanding animal husbandry and technical assistance, and Gena Coleman for expert assistance with the manuscript.
This work was supported by National Institutes of Health grant RO1HL49915. T.F. was supported by a research fellowship of the Swiss Foundation for Medical and Biological Grants (with support of Novartis Switzerland) and a research fellowship of the Walter and Gertrud Siegenthaler Foundation (Medical Faculty, University of Zurich, Switzerland). C.L. was supported by the National Defense Science and Engineering Graduate Fellowship.
National Institutes of Health
Authorship
Contribution: T.F. and C.L.L. planned experiments; T.F., C.L.L., J.K., T.O., G.Z., T.H., and C.V. performed experiments; T.F., C.L.L., and J.K. analyzed experiments; J.K. and A.R. contributed important intellectual content; A.R. provided NFAT1−/− animals; C.L.L. contributed to the manuscript; T.F. wrote the manuscript; M.S. provided overall direction and oversaw all experimental studies and edited and revised the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Megan Sykes, MD, Transplantation Biology Research Center, Massachusetts General Hospital, MGH East Bldg 149-5102, 13th St, Boston, MA 02129; e-mail: megan.sykes@tbrc.mgh.harvard.edu.
References
Author notes
T.F. and C.L.L. contributed equally to this work.