Abstract
We previously reported on a novel compound (Compound 1; RUC-1) identified by high-throughput screening that inhibits human αIIbβ3. RUC-1 did not inhibit αVβ3, suggesting that it interacts with αIIb, and flexible ligand/rigid protein molecular docking studies supported this speculation. We have now studied RUC-1's effects on murine and rat platelets, which are less sensitive than human to inhibition by Arg-Gly-Asp (RGD) peptides due to differences in the αIIb sequences contributing to the binding pocket. We found that RUC-1 was much less potent in inhibiting aggregation of murine and rat platelets. Moreover, RUC-1 potently inhibited fibrinogen binding to murine platelets expressing a hybrid αIIbβ3 receptor composed of human αIIb and murine β3, but not a hybrid receptor composed of murine αIIb and human β3. Molecular docking studies of RUC-1 were consistent with the functional data. In vivo studies of RUC-1 administered intraperitoneally at a dose of 26.5 mg/kg demonstrated antithrombotic effects in both ferric chloride carotid artery and laser-induced microvascular injury models in mice with hybrid hαIIb/mβ3 receptors. Collectively, these data support RUC-1's specificity for αIIb, provide new insights into the αIIb binding pocket, and establish RUC-1's antithrombotic effects in vivo.
Introduction
We previously published data on the identification of a novel inhibitor of αIIbβ3 (Compound 1; now referred to as RUC-1).1 We speculated that it interacted exclusively with the αIIb portion of the Arg-Gly-Asp (RGD) binding site based on its specificity for αIIbβ3 compared with αVβ3 and molecular docking studies into the human αIIbβ3 headpiece suggesting that the positively charged piperazinyl nitrogen of RUC-1 interacts with the carboxyl group of D224 in αIIb and that the heterocyclic fused ring of RUC-1 interacts with one or more of the 3 aromatic residues that line the αIIb pocket. RUC-1 also is too short to span between D224 of αIIb and the β3 metal ion-dependent adhesion site (MIDAS) and lacks a carboxyl group to coordinate the MIDAS metal ion, which is an invariant feature of all other small molecule αIIbβ3 antagonists.2-4 In the present study, we further tested whether RUC-1 demonstrates specificity for αIIb by taking advantage of known differences in the abilities of αIIbβ3 antagonists to inhibit αIIbβ3-mediated platelet aggregation in different species. Consistent with these data, we also found that RUC-1 could inhibit thrombus formation in vivo in transgenic mice expressing human (h) αIIb in complex with murine (m) β3, but not wild-type (WT) mice. Estimates of electrostatic and van der Waals interaction energies of RUC-1 docked into the crystal structure of human αIIbβ3 or molecular models of rat αIIbβ3, mouse αIIbβ3, or hybrid human αIIb/mouseβ3 were consistent with the functional data. In aggregate, these data have important implications for understanding the structure of the αIIb binding pocket and the potential antiplatelet effects of αIIb-specific αIIbβ3 antagonists.
Methods
Approvals
Human studies were approved by the Institutional Review Boards at the Children's Hospital of Philadelphia and the Rockefeller University with informed consent obtained in accordance with the Declaration of Helsinki. Animal studies were also approved by the Institutional Animal Care and Use Committees at both institutions.
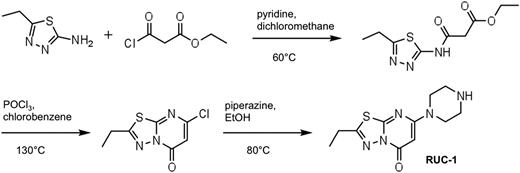
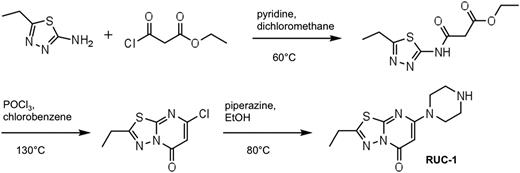
Synthesis of RUC-1 and RUC-1-piperidine
RUC-1 (Figure 1) was synthesized based on a modification of the synthesis of Roma et al5 in 3 steps, starting with ethyl-3-chloro-3-oxopropanoate and 5-ethyl-1,3,4-thiadiazole. The resulting intermediate was cyclized using phosphorus oxychloride. The product was purified by flash silica gel chromatography, and purity was assessed by both nuclear magnetic resonance (NMR) (Bruker DPX 400; Bruker) and matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (PerSeptive DE STR; Applied Biosystems).
RUC-1 synthesis. The initial step produced intermediate “a,” with an 84% yield; the second, cyclization step yielded intermediate “b” with a yield of 18%; and the final step yielded RUC-1 (52% yield).
RUC-1 synthesis. The initial step produced intermediate “a,” with an 84% yield; the second, cyclization step yielded intermediate “b” with a yield of 18%; and the final step yielded RUC-1 (52% yield).
Generation and characterization of murine platelets expressing hybrid human-mouse αIIbβ3
Human αIIb and murine β3 (hαIIb/mβ3) platelets.
The production of mice transgenic for the hαIIb gene locus has been previously described.6 These mice were crossed with mice homozygous for targeted disruption of the mαIIb gene (Itga2b, kindly provided by Dr Frampton, University of Birmingham, Birmingham, United Kingdom).7,8 The resulting mice were a mixture of C57Bl/6 and SV129 backgrounds. The genotypes of mice containing the hαIIb transgene and homozygous for the targeted disruption of mαIIb were confirmed by polymerase chain reaction (PCR), and direct assessment of surface expression of the receptors was performed on washed platelets prepared from platelet-rich plasma (PRP) as previously described.9,10 Fluorescein isothiocyanate (FITC)–conjugated anti–human CD41 (HIP8; eBioscience) antibody was used to detect hαIIb, and phycoerythrin-conjugated anti–mouse CD41 (MWReg30; BD Biosciences, San Jose, CA) antibody was used to detect mαIIb.
Murine αIIb and human β3 (mαIIb/hβ) platelets.
Human normal β3 cDNA was excised from the pcDNA3 mammalian expression vector (a kind gift of Dr Peter Newman, Blood Center of Southeastern Wisconsin, Milwaukee, WI) and ligated into the mouse stem cell virus MigR1 vector containing an internal ribosome entry site (IRES)–green fluorescent protein (GFP) insertion prior to the polyadenylation signal11 (a generous gift of Dr Mark Kahn, University of Pennsylvania, Philadelphia, PA). Virus containing hβ3 cDNA was then generated using Ecopack 2-293 cells (ATCC). Supernatant containing the virus was collected 72 hours after transfection, passed through a 0.45-μm filter, and stored at −70°C until further use.
Fetal liver cell transplantation was performed as per Zou et al12 with minor modifications. Fetal liver cells were harvested from Itgb3−/− embryos on a mixed C57Bl/6 and 129S6/SVEV background13 at E14.5-E16.5, and the cell suspension was enriched for CD34+ hematopoietic progenitors using negative selection (EasySep; StemCell Technologies). Enriched cells were then cultured overnight in media containing 100 ng/mL murine stem cell factor, 10 ng/mL murine interleukin 6, and 20 ng/mL murine interleukin 3 (all from PeproTech). Fetal cell cultures were infected at 0.5 transducing units (TDU) per cell on 2 consecutive days with MigR1-β3 viral supernatant in the presence of stem cell factor, interleukins 3 and 6 (concentrations as above), and 8 μg/mL hexadimethrine bromide (polybrene; Sigma-Aldrich). Infected cells (∼1.5-2 × 106/mouse; ∼60% expressing GFP and hβ3) were then injected intravenously into a lethally irradiated WT (ie, Itgβ3+/+) mouse (900 rads of X-rays in 2 divided doses, 3 hours apart). Platelet studies were performed with blood obtained 5 weeks or more after transplantation.
Platelet aggregation
Blood was drawn via cardiac puncture from anesthetized Sprague Dawley rats (Taconic), WT C57Bl/6 mice (The Jackson Laboratory), and mice expressing hαIIb/mβ3 and diluted 1:1 with a mixture of 4 parts 0.165 mM NaCl, 0.01 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.4 containing 2 mM CaCl2 and 1 mM MgCl2 and 1 part 3.8% sodium citrate Blood from consenting human volunteers was obtained from a peripheral vein using a 19-gauge needle and anticoagulated with 1:10 vol 3.8% sodium citrate. PRP was isolated by centrifugation at 22°C at 350g for 10 minutes (rats), 250g for 2.5 minutes (mice), or 650g for 4 minutes (human). Mouse PRP samples were adjusted to 400 000 platelets/μL with the buffer used for dilution and human PRP was adjusted to 300 000 platelets/μL with platelet-poor plasma. Samples of PRP were either untreated or incubated for 5 minutes at 37°C with 100 μM RUC-1. Platelet aggregation was induced by adding to PRP adenosine diphosphate (ADP) at 30 μM (rats and WT mice), 20 or 30 μM (hαIIb/mβ3 mice), or 5 μM (humans), and light transmission was measured over time in an aggregometer (Kowa AG-10E; Kowa) with stirring. Percent inhibition was calculated by comparing the initial slope of untreated samples to RUC-1-treated samples.
Soluble fibrinogen binding
Whole blood from WT mice, mice expressing hαIIb/mβ3, or mice expressing mαIIb/hβ3 on their platelets was drawn from the retrobulbar venous plexus into an equal volume of 200 μM PPACK (Calbiochem) in 165 mM NaCl. Samples were diluted in HEPES-modified Tyrode buffer [HBMT; 138 mM NaCl, 12 mM NaHCO3, 10 mM HEPES, 2.7 mM KCl, 0.4 mM NaH2PO4, 0.1% glucose, 0.35% bovine serum albumin (BSA), pH 7.4] containing 50 μM PPACK, 2 mM CaCl2, 1 mM MgCl2, and were left untreated or incubated with 20 or 100 μM RUC-1, 1 mM Arg-Gly-Asp-Ser (RGDS), or 10 mM ethylenediaminetetraacetic acid (EDTA). Alexa488-fibrinogen (WT and hαIIb/mβ3 mice; 200 μg/mL; Invitrogen) or Alexa647-fibrinogen (mαIIb/hβ3 mice) was added, and samples were activated with a PAR-4-activating peptide (AYPGKF, 200 μM; synthesized at the State University of New York at Stony Brook) and incubated at 37°C for 30 minutes. Samples were then diluted 1:10 in HBMT containing CaCl2 and MgCl2 as above and analyzed by flow cytometry. Fibrinogen binding was calculated from the geometric mean fluorescence intensity of platelets (gated by forward and side scatter for WT and hαIIb/mβ3 mice and GFP intensity for mαIIb/hβ3 mice). Fibrinogen binding to unactivated samples was defined as background binding, and PAR-4 peptide-induced fibrinogen binding to untreated samples was used to establish maximal (100%) binding. Studies performed on WT platelets to assess whether the dilution step performed prior to analysis resulted in fibrinogen dissociation demonstrated that samples analyzed immediately after dilution in buffer without or with Alexa488-fibrinogen (to maintain the same fibrinogen concentration) had similar net geometric mean fluorescent intensities (133 and 124 units, respectively). When analyzed 15 minutes after dilution, the values were identical (115 units), representing 86 and 93% of the immediate values.
Ferric chloride carotid artery injury model
The protocol for ferric chloride (FeCl3)–induced injury to the carotid artery was adapted from previously published work14 with minor changes. Four C57BL/6 WT mice, 6 WT mice on a mixed C57BL/6 and SV129 background, and 16 hαIIb/mβ3 mice were anesthetized by intraperitoneal injection of pentobarbital (80 mg/kg Nembutol; Ovation Pharmaceuticals). After 10 minutes, mice were injected intraperitoneally with 10 mM RUC-1 (26.5 mg/kg; n = 8) or the vehicle [1% (n = 2) or 10% (n = 6) dimethyl sulfoxide (DMSO) in 0.165 M NaCl]. The carotid artery was then isolated by blunt dissection, and a Doppler flow probe (Model 0.5VB; Transonic Systems) was positioned around the vessel. Approximately 25 minutes after the compound or vehicle control was administered, a 1 × 2 mm2 piece of filter paper (#1; Whatman International) soaked in 20% FeCl3 was placed on the artery for 3 minutes and then removed. The area was then flushed with distilled water, and blood flow through the artery was monitored for 30 minutes. Arterial flow rate data were analyzed as both “percent reduction in flow” (calculated as the area above the line of the plot of observed flow rate versus observation time, divided by the product of the initial flow rate and the total observation time) and “time to occlusion” (defined as the time from the application of the FeCl3-soaked filter paper until arterial blood flow became undetectable for at least 10 minutes).
Carotid arteries from 1 hαIIb/mβ3 mouse treated with 10% DMSO and 1 treated with RUC-1 were fixed in formaldehyde, cross-sectioned, and stained with hematoxylin and eosin. The sections were visualized with a 20× objective using an Olympus BX60 microscope (Olympus), photographed with a Nikon D5-5M camera (Nikon), and captured in Adobe Photoshop 6.0 (Adobe Systems).
Laser microvascular injury and intravital microscopy
The protocols for laser microvascular injury in blood vessels in the cremaster muscle and intravital microscopic evaluation of subsequent thrombus formation have been previously described.15 Briefly, male mice expressing hαIIb/β3 (3 in each group) that were anesthetized with pentobarbitol (11 mg/kg; Abbott Laboratories) had their cremaster arterioles (20 to 40 μm) studied using an Olympus BX61WI microscope (Olympus) with a 40×/0.8 numeric aperture (NA) water-immersion objective lens. Arteriole laser injuries were done using an SRS NL100 Nitrogen Laser system (Photonic Instruments) at 65% energy level. Visual confirmation of the extravasation of small amounts of blood cells was made for each studied blood vessel as an assurance that a consistent injury had been produced. After the surgery to expose the blood vessels was performed, animals were injected intraperitoneally with 10 mM RUC-1 (26.5 mg/kg) or 10% DMSO. Three arterioles were injured in each mouse. Injuries were initiated 25 to 35 minutes after injection of RUC-1 or DMSO, and 5 minutes after the intravenous injection of labeled antibodies that react with murine platelet GPIbβ (DyLight488; Emfret Analytics) and fibrin (Alexa647)16 into the cannulated jugular vein. Data were collected over 2.5 minutes at 5 frames/s (750 frames/study) and then averaged at each time point.
Molecular models of mouse, rat, and hybrid human/mouse αIIbβ3 headpieces
The ligand-binding regions of human αIIbβ3 share a high amino acid sequence homology with mouse and rat αIIbβ3 (greater than 80% identity in αIIb β-propeller and greater than 89% for the β3 βA, plexin/semaphorin/integrin [PSI], and hybrid domains), thus justifying homology modeling with MODELLER 8v217 of the mouse and rat integrin ligand binding regions using the crystallographic structure of the human αIIbβ3 ligand binding region in complex with eptifibatide (PDB ID, 1TY6)2 as a template. Protein hydrogen atoms were added to the crystal structure of the human αIIbβ3 integrin fragment and the animal and hybrid models using HBUILD within the CHARMM package.18 The αIIb subunit of that crystal structure was combined with the modeled mβ3 subunit to obtain the model of the hybrid hαIIb/mβ3 receptor fragments. The 4 resulting initial structures (human, mouse, and rat αIIbβ3, and the hybrid hαIIb/mβ3) were energy minimized in vacuo using a distance-dependence dialectric constant. First, only hydrogen atoms were allowed to vary, and subsequent cycles of conjugate gradient minimization were applied for complete relaxation of the side-chains and the backbones. The resulting minimized structures of mouse, rat, and hybrid αIIbβ3 had root mean squared deviations (RMSD) of 0.9, 0.6, and 0.9 Å, respectively, from the original crystal structure Cα atoms.
Molecular docking of RUC-1 into human αIIbβ3 and molecular models of mouse αIIbβ3, rat αIIbβ3, and hαIIb/mβ3
Docking of RUC-1 into the ligand binding site of the minimized structures of human αIIbβ3 and into the homologous models of mouse αIIbβ3, rat αIIbβ3, and the hαIIb/mβ3 hybrid was independently assessed with 2 different programs: Glide 519 (Grid-based Ligand Docking with Energetics; Schrödinger, New York, NY) and Flexcdock.1,20 For Glide, receptor structures were first analyzed to assign protonation states and then submitted to a series of restrained minimizations using the OPLS-AA forcefield in Impact 5.0 (Schrödinger), followed by the calculation of Coulomb and van der Waals grids. RUC-1 was prepared for docking using LigPrep 2.2 (Schrödinger). First, the protonation state at physiologic pH was determined with Epik 1.6 (Schrödinger) 21 , and then the geometry of the molecule was optimized in Macromodel 9.6. Docking of RUC-1 into the RGD binding site of αIIbβ3 was carried out with standard precision (SP) Glide 5. A composite Emodel score that combines a proprietary GlideScore (Schrödinger) multiligand scoring function, the nonbonded interaction energy, and the excess internal energy of the generated ligand conformations was used to rank the predicted different poses. Both van der Waals and Coulomb interaction energies between the docked RUC-1 and the αIIbβ3 protein were calculated with reduced net ionic charges on groups with formal charges. Docking of RUC-1 into αIIbβ3 using Flexcdock20 was performed as previously described.1 This program differs from Glide in the force field and searching algorithm, and uses a less complex scoring function. Both docking studies were conducted in vacuo, thus not considering solvent effects.
Statistics
Student t test was used to assess grouped fibrinogen binding and carotid artery model data. Kaplan-Meier survival analysis with the Mantel-Haenszel test was used to analyze outcomes from the carotid artery injury model.
Results
Platelet aggregation
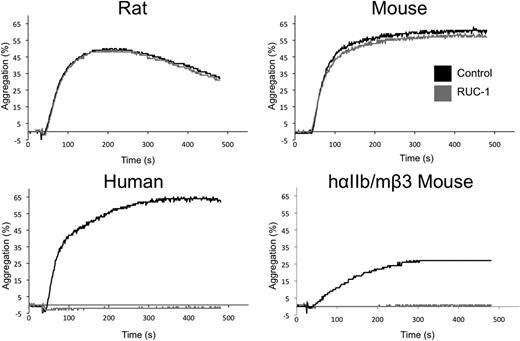
RUC-1 at 100 μM dramatically inhibited aggregation of platelets from humans (97 ± 2% inhibition, n = 3) and mice expressing the hybrid hαIIb/mβ3 receptors (99% inhibition, n = 4), but not WT mice (6 ± 6% inhibition, n = 4) or rats (0 ± 15% inhibition, n = 3; Figure 2).
RUC-1 inhibits aggregation of human platelets and hαIIb/mβ3 murine platelets, but not WT mouse or rat platelets. PRP was isolated from whole blood of WT Sprague-Dawley rats, WT C57Bl/6 mice, healthy human volunteers, or hybrid hαIIb/mβ3 mice, and was treated with either vehicle control or 100 μM RUC-1 for 5 minutes. Aggregation was initiated with ADP and light transmission was monitored through aggregometer cuvettes for 8 minutes. One representative plot from each animal is shown.
RUC-1 inhibits aggregation of human platelets and hαIIb/mβ3 murine platelets, but not WT mouse or rat platelets. PRP was isolated from whole blood of WT Sprague-Dawley rats, WT C57Bl/6 mice, healthy human volunteers, or hybrid hαIIb/mβ3 mice, and was treated with either vehicle control or 100 μM RUC-1 for 5 minutes. Aggregation was initiated with ADP and light transmission was monitored through aggregometer cuvettes for 8 minutes. One representative plot from each animal is shown.
Soluble fibrinogen binding
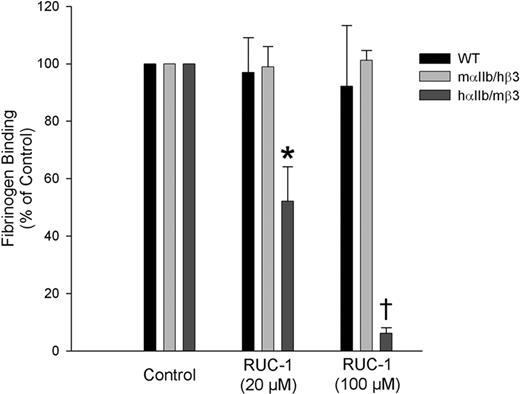
Activated platelets from WT mice bound fibrinogen (Figure 3), and this binding was only minimally inhibited by RUC-1 at 20 or 100 μM. Fibrinogen also bound to activated platelets from mice expressing mαIIb/hβ3, and these receptors were also not inhibited by RUC-1. In sharp contrast, binding of fibrinogen to activated platelets from hαIIb/mβ3 mice was inhibited 48% (± 12%) at a RUC-1 concentration of 20 μM (P = .002) and 94% (± 2%) at 100 μM (P < .001). For comparison, EDTA inhibited the binding of fibrinogen to WT mouse platelets by 97% and hαIIb/mβ3 mouse platelets by 99%. Thus, RUC-1's effects on fibrinogen binding to WT and hαIIb/mβ3 platelets parallels its effects on platelet aggregation.
Binding of soluble fibrinogen to platelets expressing hαIIb/mβ3 is inhibited by RUC-1; WT murine platelets and platelets expressing mαIIb/hβ3 are not inhibited by RUC-1. Whole blood anticoagulated with PPACK from WT mice (n = 4), mice expressing mαIIb/hβ3 (n = 4), or mice expressing hαIIb/mβ3 (n = 4) was diluted in buffer containing 2 mM CaCl2/1 mM MgCl2. Samples were either untreated or treated with 20 or 100 μM RUC-1, and 200 μg/mL fluorescent fibrinogen were added before activating with 200 μM PAR-4 activating peptide. Samples were incubated at 37°C for 30 minutes before diluting and analyzing using flow cytometry. Geometric mean fluorescence intensities of unactivated samples were subtracted as background, and untreated activated samples were used to establish 100% binding (*P = .002, †P < .001).
Binding of soluble fibrinogen to platelets expressing hαIIb/mβ3 is inhibited by RUC-1; WT murine platelets and platelets expressing mαIIb/hβ3 are not inhibited by RUC-1. Whole blood anticoagulated with PPACK from WT mice (n = 4), mice expressing mαIIb/hβ3 (n = 4), or mice expressing hαIIb/mβ3 (n = 4) was diluted in buffer containing 2 mM CaCl2/1 mM MgCl2. Samples were either untreated or treated with 20 or 100 μM RUC-1, and 200 μg/mL fluorescent fibrinogen were added before activating with 200 μM PAR-4 activating peptide. Samples were incubated at 37°C for 30 minutes before diluting and analyzing using flow cytometry. Geometric mean fluorescence intensities of unactivated samples were subtracted as background, and untreated activated samples were used to establish 100% binding (*P = .002, †P < .001).
RUC-1 protects hαIIb/mβ3 mice, but not WT mice, against occlusive carotid artery thrombi
The platelet counts and αIIbβ3 expression levels of mice receiving the DMSO vehicle control solutions or RUC-1 did not differ significantly (data not shown). All 8 hαIIb/mβ3 mice treated with DMSO (1% or 10%) had reductions in carotid artery blood flow after FeCl3 injury, and 7 of 8 developed thrombi large enough to completely occlude blood flow for at least 10 minutes (Figure 4A-B). Nine hαIIb/mβ3 mice were treated with RUC-1. One mouse bled excessively after receiving RUC-1 during isolation of the carotid artery and died. The other 8 mice successfully underwent surgery without excessive bleeding. These mice exhibited much less reduction in blood flow, and none of the mice developed an occlusive thrombus (P < .001; Figure 4B).
RUC-1 protects hαIIb/mβ3 mice, but not WT mice from FeCl3-induced carotid artery thrombotic occlusion. Mice expressing the hybrid hαIIb/mβ3 receptor were injected intraperitoneally with vehicle control [1% (n = 2) or 10% DMSO (n = 6)], or RUC-1 (n = 8; 26.5 mg/kg) approximately 25 minutes before carotid artery injury. Carotid arteries were isolated by blunt dissection and treated with 20% FeCl3 for 3 minutes using a piece of filter paper. The surgical area was flushed with water, and blood flow through the carotid artery was monitored for 30 minutes with a Doppler flow probe. (A) Representative blood flow tracings from 2 mice treated with DMSO and 2 mice treated with RUC-1. Time to occlusion was calculated from the time the filter paper containing the FeCl3 was applied to the artery until the time when the arterial flow rate became undetectable. (B) Kaplan-Meier analysis of time to occlusion data in hαIIb/mβ3 mice receiving either RUC-1 (n = 8) or DMSO vehicle control (n = 8). (C) Kaplan-Meier analysis of time to occlusion data from RUC-1-treated mice comparing WT mice (n = 10) and hαIIb/mβ3 mice (n = 8, data repeated from panel B). (D,E) Hematoxylin and eosin stains of fixed cross-sections of carotid arteries in a mouse treated with DMSO as a control (left) and a mouse treated with RUC-1 (right). An extensive platelet thrombus nearly completely fills the lumen of the control animal's carotid artery, whereas there is minimal platelet thrombus formation in the carotid artery of RUC-1-treated animal. Most of the material in the lumen of the RUC-1-treated animal is trapped erythrocytes. The bar in panel D is 50 μm, and both panels D and E were photographed and reproduced at the same magnification.
RUC-1 protects hαIIb/mβ3 mice, but not WT mice from FeCl3-induced carotid artery thrombotic occlusion. Mice expressing the hybrid hαIIb/mβ3 receptor were injected intraperitoneally with vehicle control [1% (n = 2) or 10% DMSO (n = 6)], or RUC-1 (n = 8; 26.5 mg/kg) approximately 25 minutes before carotid artery injury. Carotid arteries were isolated by blunt dissection and treated with 20% FeCl3 for 3 minutes using a piece of filter paper. The surgical area was flushed with water, and blood flow through the carotid artery was monitored for 30 minutes with a Doppler flow probe. (A) Representative blood flow tracings from 2 mice treated with DMSO and 2 mice treated with RUC-1. Time to occlusion was calculated from the time the filter paper containing the FeCl3 was applied to the artery until the time when the arterial flow rate became undetectable. (B) Kaplan-Meier analysis of time to occlusion data in hαIIb/mβ3 mice receiving either RUC-1 (n = 8) or DMSO vehicle control (n = 8). (C) Kaplan-Meier analysis of time to occlusion data from RUC-1-treated mice comparing WT mice (n = 10) and hαIIb/mβ3 mice (n = 8, data repeated from panel B). (D,E) Hematoxylin and eosin stains of fixed cross-sections of carotid arteries in a mouse treated with DMSO as a control (left) and a mouse treated with RUC-1 (right). An extensive platelet thrombus nearly completely fills the lumen of the control animal's carotid artery, whereas there is minimal platelet thrombus formation in the carotid artery of RUC-1-treated animal. Most of the material in the lumen of the RUC-1-treated animal is trapped erythrocytes. The bar in panel D is 50 μm, and both panels D and E were photographed and reproduced at the same magnification.
Ten WT mice were treated with RUC-1, and they exhibited reductions in blood flow similar to those of the hαIIb/mβ3 mice treated with DMSO, with 9 of 10 developing occlusive thrombi. The other mouse suffered a 50% reduction in blood flow after 6.5 minutes, and several additional decreases in blood flow during the observation period, but did not develop an occlusive thrombus. Kaplan-Meier analysis using time to occlusion indicated that the WT and hαIIb/mβ3 mice treated with RUC-1 constitute 2 distinct groups (P = .001; Figure 4C).
Cross-sections of the hαIIb/mβ3 mouse carotid artery after FeCl3 treatment revealed nearly complete packing of the lumen with platelet-rich thrombus (Figure 4D-E). The deposits of FeCl3 were visible as golden granules on the luminal side of the blood vessel. In sharp contrast, the lumen of the RUC-1 mouse carotid artery contained regions of loosely packed erythrocytes, demonstrating the patency of the artery. Golden FeCl3 granules were also visible on the luminal side of the blood vessel.
RUC-1 decreases thrombus formation in response to microvascular laser injury
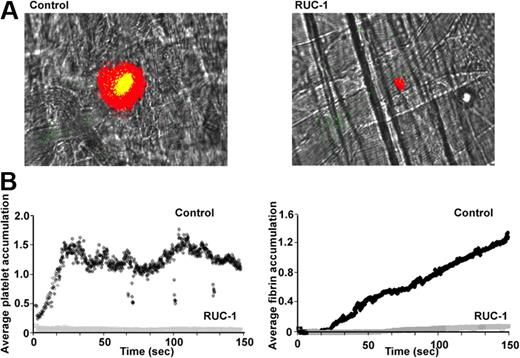
Cremaster arteriole injury studies permitted in situ visualization of thrombus development in real time. There was no difference in thrombus formation in mice that were either untreated or pre-injected with the carrier, 10% DMSO, prior to injury (comparison not shown). Both control groups demonstrated rapid platelet adhesion followed by progressive incorporation of platelets into the thrombi over the first approximately 20 seconds (Figure 5A-B left panels). In addition, after a delay of approximately 35 seconds, both control groups demonstrated progressive incorporation of fibrin into the thrombi (Figure 5B right panel). In sharp contrast, animals pre-injected with RUC-1 prior to injury developed almost no platelet accumulation or fibrin deposition over a comparable time period (Figure 5A right panel, B; supplemental Video 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
RUC-1 protects hαIIb/mβ3 mice from cremaster arteriole thrombus formation. Mice expressing hαIIb/mβ3 were injected with DMSO (control) or RUC-1 (26.5 mg/kg) intraperitoneally 25 to 30 minutes before laser injury. Five minutes before injury, mice were injected intravenously with antibodies to GPIbβ (green) or fibrin (red). (A) Images were obtained 2.5 minutes after surgery. The videos for DMSO and RUC-1 are available as supplemental Video 1 and supplemental Video 2, respectively. (B) Average platelet (left) and fibrin accumulation (right) for the 9 injuries (3 mice per arm with 3 injuries per mouse).
RUC-1 protects hαIIb/mβ3 mice from cremaster arteriole thrombus formation. Mice expressing hαIIb/mβ3 were injected with DMSO (control) or RUC-1 (26.5 mg/kg) intraperitoneally 25 to 30 minutes before laser injury. Five minutes before injury, mice were injected intravenously with antibodies to GPIbβ (green) or fibrin (red). (A) Images were obtained 2.5 minutes after surgery. The videos for DMSO and RUC-1 are available as supplemental Video 1 and supplemental Video 2, respectively. (B) Average platelet (left) and fibrin accumulation (right) for the 9 injuries (3 mice per arm with 3 injuries per mouse).
Sequence analysis and molecular docking studies
The sequences of human, murine, and rat αIIb that make the major contributions to the αIIb portion of the RGD binding pocket are aligned in Figure 6 using human numbering. All 3 species have the D224 that was found to interact with the positively charged regions of the αIIbβ3 antagonists2 and that our docking data indicated interacts with the positively charged piperazinyl nitrogen of RUC-1.1 All 3 also have the aromatic residues F160 and F231 that line the αIIb binding pocket and thus may interact with the heterocyclic fused ring structure of RUC-1. The third aromatic residue that lines the αIIb binding pocket is a Tyr in human (Y190), but a Phe in both mouse and rat. In addition, there are differences in the residues near F160, D224, and F231 in both species that may also produce alterations in the binding affinity for RUC-1.
Alignment of human, mouse, and rat αIIb sequences in the loops that contribute to the αIIb RGD ligand binding pocket. * indicates the 3 aromatic residues that line the pocket and the conserved D224 that interacts with the positively charged regions of the αIIbβ3 antagonists studied by X-ray crystallography.2 The nomenclature indicates the β-propeller blade number, and the loop designation identifies the β-sheets within the blades that are connected. The term loop is used broadly, since an α-helical region exists within the W2-W3 4,1 “loop.”
Alignment of human, mouse, and rat αIIb sequences in the loops that contribute to the αIIb RGD ligand binding pocket. * indicates the 3 aromatic residues that line the pocket and the conserved D224 that interacts with the positively charged regions of the αIIbβ3 antagonists studied by X-ray crystallography.2 The nomenclature indicates the β-propeller blade number, and the loop designation identifies the β-sheets within the blades that are connected. The term loop is used broadly, since an α-helical region exists within the W2-W3 4,1 “loop.”
We docked RUC-1 into models of the human, murine, and rat αIIbβ3 receptors as well as the hαIIb/mβ3 hybrid receptor using 2 different programs (Glide and Flexcdock). Both programs gave similar orientations (or poses) for RUC-1 in each receptor, although there was some variation in the rotation of the piperazine relative to the heterocyclic fused ring. Using both programs, the calculated RUC-1 electrostatic and van der Waals interaction energies were more favorable with the human and hybrid receptors than with either the mouse or rat receptors, with minor differences between the human αIIbβ3 and hαIIb/mβ3 that may reflect the influence of the β3 subunit on the αIIb binding pocket.
Discussion
The data in this study extend our previous observations on RUC-1 and lend additional support for the binding of RUC-1 to the αIIb subunit of αIIbβ3. We tested the inhibitory effect of RUC-1 on murine and rat platelets because previous studies conducted by us found that RGD peptides are less potent at inhibiting murine and rat αIIbβ3 and that the differences in sensitivity are largely or completely due to differences in the αIIb subunit.10,22 Thus, we inferred that the αIIb contribution to the RGD binding pocket differs among these species. In fact, we found that RUC-1 was also much less potent in inhibiting murine and rat αIIbβ3 than human αIIbβ3. To test whether the αIIb subunit was responsible for the differences in sensitivity to RUC-1, we prepared and tested murine platelets expressing hybrid receptors composed of either hαIIb and mβ3 or mαIIb and hβ3. RUC-1 inhibited fibrinogen binding to the mouse platelets expressing hαIIb/mβ3 with a dose response similar to that of inhibiting fibrinogen binding to human platelets, whereas the mouse platelets expressing mαIIb/hβ3, like WT mouse platelets, were not inhibited by RUC-1. Thus, it appears that the nature of the αIIb subunit primarily or exclusively controls RUC-1 sensitivity, which supports our previous data suggesting that RUC-1 binds to αIIb.
The αIIb sequence analysis identified a number of potential differences between human, mouse, and rat that may be responsible for altering the affinity for RUC-1. Additional studies will be required to further elucidate the relative contribution of these differences to the binding of RUC-1. The docking studies paralleled the results of the functional studies, as they indicated that RUC-1's electrostatic and van der Waals interaction energies are more favorable with the human and hybrid hαIIb/mβ3 receptors than the mouse and rat receptors.
Since the platelets of the mouse expressing the hαIIb/mβ3 hybrid receptor demonstrated sensitivity to RUC-1 similar to that of the human platelets, we were able to test the antithrombotic effect of RUC-1 in vivo in these mice. RUC-1 demonstrated marked antithrombotic effects in the FeCl3/carotid artery injury and cremaster arteriole laser injury models compared with a vehicle (DMSO) control. The selectivity of RUC-1 for human αIIb compared with murine αIIb was also observed in this in vivo model, since RUC-1 did not protect the carotid arteries of mice expressing WT murine αIIbβ3 from thrombotic occlusion. The dose of RUC-1 used in these studies (26.5 mg/kg) was chosen on the basis of attempting to achieve a peak concentration of approximately 100 μM assuming distribution into total body water. If RUC-1 is equally effective in preventing platelet-rich thrombosis in humans, this would translate into a parenteral dose of 1.9 g in a 70-kg person. Additional studies will be required to assess RUC-1's dose response and its oral bioavailability, as well as to assess whether it induces less extensive changes in the conformation of αIIbβ3 when given in vivo as was observed in in vitro studies.1
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Howard Hang of Rockefeller University for his advice in designing and performing the synthesis of RUC-1, and Sue Mei Cheah and the Rockefeller University veterinary services for assistance with the rat experiments.
This study was supported in part by grant nos. R01HL19278 (B.S.C.) and P01HL40387 (M.P.) from the National Heart, Lung, and Blood Institute; by grant no. R03MH083257 from the National Institute of Mental Health and the National Institutes of Health (NIH) Roadmap Initiative; a Clinical and Translational Science Award (UL1-RR024143) from the National Center for Research Resources at NIH; and by funds from the Rockefeller University Bridges to Better Medicine Technology Innovation Fund and Stony Brook University.
National Institutes of Health
Authorship
Contribution: R.B. designed and performed research, analyzed data, and wrote the paper; M.A.K. conducted the carotid artery thrombosis model, performed platelet aggregation studies with hαIIb/mβ3 mice, analyzed data, and reviewed the manuscript; J.H. performed the in situ microscopy studies; M.M. performed homology modeling and docking experiments, helped design the synthesis of RUC-1, and helped write the paper; C.A.J. generated the mαIIb/hβ3-expressing mice, performed all animal studies at Rockefeller University, and assisted with data analysis; A.H. synthesized RUC-1; M.J., and J.L. generated the mαIIb/hβ3-expressing mice; R.F. assisted with breeding the hαIIb/mβ3 mice and the FeCl3 carotid injury studies; M.A.T. created and characterized the hαIIb/mβ3 mice; M.F. analyzed data and reviewed the manuscript; M.P. designed the research, analyzed data, and reviewed the manuscript; and B.S.C. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: R.B. and B.S.C. are inventors of RUC-1. Rockefeller University has applied for a patent on RUC-1. B.S.C. is also an inventor of abciximab and in accord with federal law and the policies of the Research Foundation of the State University of New York, he shares in royalties paid to the Foundation for sales of abciximab. The remaining authors declare no competing financial interests.
Correspondence: Barry S. Coller, Rockefeller University, 1230 York Ave, Box 309, New York, NY 10065; e-mail: collerb@rockefeller.edu.


![Figure 4. RUC-1 protects hαIIb/mβ3 mice, but not WT mice from FeCl3-induced carotid artery thrombotic occlusion. Mice expressing the hybrid hαIIb/mβ3 receptor were injected intraperitoneally with vehicle control [1% (n = 2) or 10% DMSO (n = 6)], or RUC-1 (n = 8; 26.5 mg/kg) approximately 25 minutes before carotid artery injury. Carotid arteries were isolated by blunt dissection and treated with 20% FeCl3 for 3 minutes using a piece of filter paper. The surgical area was flushed with water, and blood flow through the carotid artery was monitored for 30 minutes with a Doppler flow probe. (A) Representative blood flow tracings from 2 mice treated with DMSO and 2 mice treated with RUC-1. Time to occlusion was calculated from the time the filter paper containing the FeCl3 was applied to the artery until the time when the arterial flow rate became undetectable. (B) Kaplan-Meier analysis of time to occlusion data in hαIIb/mβ3 mice receiving either RUC-1 (n = 8) or DMSO vehicle control (n = 8). (C) Kaplan-Meier analysis of time to occlusion data from RUC-1-treated mice comparing WT mice (n = 10) and hαIIb/mβ3 mice (n = 8, data repeated from panel B). (D,E) Hematoxylin and eosin stains of fixed cross-sections of carotid arteries in a mouse treated with DMSO as a control (left) and a mouse treated with RUC-1 (right). An extensive platelet thrombus nearly completely fills the lumen of the control animal's carotid artery, whereas there is minimal platelet thrombus formation in the carotid artery of RUC-1-treated animal. Most of the material in the lumen of the RUC-1-treated animal is trapped erythrocytes. The bar in panel D is 50 μm, and both panels D and E were photographed and reproduced at the same magnification.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/1/10.1182_blood-2008-08-169243/4/m_zh89990938330004.jpeg?Expires=1765887901&Signature=MeXALbdjaEnxJMi7-~k3VqBtNNgkvi7gCOer8np22qbp1skfHvjfOhEJ4tBq4yEJPKm3lql9n5PNAb6dZ8AY7bNgqPFtmAcnc7QB~K~3SRsXGDBWvw~L4zWtr4scHKeioAl6vk6zpAvzq2qA5sOqSA~COgMWB~mWw60RbHkFblQBDh~5FwggBky1OIvHHlapl0fFIq5s0Kf88mrppDejPJ5TvivRDMtC0WwShrqQxhEkO~WCBzwzIEEXmPZ-m6Ntxp6pu3TT-MmNOfoMtsw7lD2YTAlbfx~U0Xi~5WjvsLXi0355l4yz6ukktCZfu2WP1KKUq9pfdq27N-VdShjeHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 4. RUC-1 protects hαIIb/mβ3 mice, but not WT mice from FeCl3-induced carotid artery thrombotic occlusion. Mice expressing the hybrid hαIIb/mβ3 receptor were injected intraperitoneally with vehicle control [1% (n = 2) or 10% DMSO (n = 6)], or RUC-1 (n = 8; 26.5 mg/kg) approximately 25 minutes before carotid artery injury. Carotid arteries were isolated by blunt dissection and treated with 20% FeCl3 for 3 minutes using a piece of filter paper. The surgical area was flushed with water, and blood flow through the carotid artery was monitored for 30 minutes with a Doppler flow probe. (A) Representative blood flow tracings from 2 mice treated with DMSO and 2 mice treated with RUC-1. Time to occlusion was calculated from the time the filter paper containing the FeCl3 was applied to the artery until the time when the arterial flow rate became undetectable. (B) Kaplan-Meier analysis of time to occlusion data in hαIIb/mβ3 mice receiving either RUC-1 (n = 8) or DMSO vehicle control (n = 8). (C) Kaplan-Meier analysis of time to occlusion data from RUC-1-treated mice comparing WT mice (n = 10) and hαIIb/mβ3 mice (n = 8, data repeated from panel B). (D,E) Hematoxylin and eosin stains of fixed cross-sections of carotid arteries in a mouse treated with DMSO as a control (left) and a mouse treated with RUC-1 (right). An extensive platelet thrombus nearly completely fills the lumen of the control animal's carotid artery, whereas there is minimal platelet thrombus formation in the carotid artery of RUC-1-treated animal. Most of the material in the lumen of the RUC-1-treated animal is trapped erythrocytes. The bar in panel D is 50 μm, and both panels D and E were photographed and reproduced at the same magnification.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/1/10.1182_blood-2008-08-169243/4/m_zh89990938330004.jpeg?Expires=1765909926&Signature=Kz1vbBuSEgsyCNzE0xWPjlvpsh3tiMos6C96W1sSibr6gYtPw4PNH6qAhhKTg~02r7aXkXzVvFDA~BZAM3hIB5jYGsWHof1N1YmlRoLxpWQBE5jVmNyfTlY6wI5nxgMjomACyDLi07Ph9UeysMrUJ1Z335AhwzvqD0baAxAHdwz6dTohQ1QzU4dI6-vWFY0kIL7IkLhAXbv1Yh0I8Hn1BfL7gl4-~gKn3IopdTC~g3dQkLQ6kfNm-Fdtluqrrk5IaaTuPdFEiFcfNMgDblL8QiEby25tZiXZOkqKDwAnm6yvCD3uoyrnDWOKBEioIhf8huRyPIlzypY4PhduqshR7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

