Abstract
In the pathogenesis of allergic asthma/rhinitis, 2 main types of cells play a role: hematolymphatic cells (mast cells, eosinophils, T cells, B cells) and nonhematolymphatic cells (airway smooth muscle cells, epithelial cells). It is not known which one of the 2 cell types plays the primary role. Here we review the literature on allergic disease transfer and potential cure with allogeneic hematopoietic cell transplantation (HCT), as transferability and curability would support a primary role of hematolymphatic cells and have implications for donor selection for HCT and possible future treatment of severe allergic disease with HCT. A total of 18 nonallergic recipients were reported to develop allergic disease after transplantation; however, conclusive information for transfer was available for only 5 cases. Allergic disease was reported to abate in 3 allergic recipients; however, conclusive information for “cure” was available for only 2 cases. Problems in interpreting the reports include incomplete data on allergic disease in the donor or recipient before transplantation, not knowing the denominator, and the lack of controls. In summary, review of the literature generates the hypothesis that allergic disease is transferable and curable with HCT. A prospective study, including appropriate controls, is needed to evaluate this hypothesis.
Definition, epidemiology, and societal burden of allergic disease
IgE-mediated hypersensitivity to common environmental substances (allergens) is referred to as “allergy” in this review. Presence of allergy is defined as a positive skin prick test (SPT) or higher-than-normal level of allergen-specific IgE in serum measured, for example, by radioallergosorbent test (RAST). Allergy with clinical manifestations (eg, asthma, rhinitis, eczema, or anaphylaxis) constitutes “allergic disease.” It affects more than 700 million people worldwide.1 The prevalence of allergic disease in developed countries is on average approximately 20%
Mortality caused by allergic disease is relatively low. Nevertheless, there are approximately 450 deaths in Canada and 5000 deaths in the United States annually due to asthma, and the incidence may be increasing.2-4 The mortality of severe (oral steroid–dependent) asthma is as high as 2% per year.5
Quality of life is significantly impacted by asthma, rhinitis, and eczema, especially in patients with complications such as pneumonia, sinusitis, or skin infections. This is associated with high direct costs to the health care system and high indirect costs including decreased productivity and missed work. Rhinitis alone accounts for 811 000 missed workdays, 4 230 000 reduced productivity days, and 824 000 school absences in the United States per year.6 The annual direct cost of asthma for the society was estimated in Spain in 1992 to be US$2879 per patient.7
Here we discuss whether, analogous to the transfer/cure of autoimmune diseases,8-10 allergic disease can also be transferred/cured with allogeneic HCT.
Pathogenesis of allergic disease
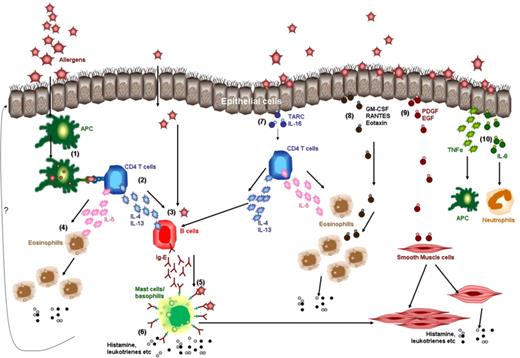
The pathogenesis is still not completely elucidated.11 The hallmark of an allergic disease is, by definition, the enhanced production of IgE antibodies in response to otherwise “harmless” environmental antigens (allergens). Hematolymphatic cells, particularly CD4 T cells, appear to play an important role. Upon inhalation, ingestion, or transcutaneous diffusion of the allergen, dendritic cells and/or allergen-specific B cells present peptides from the allergen to allergen-specific CD4 T cells. These allergen-specific CD4 T cells, which have a Th2 phenotype as they secrete interleukin (IL)–4, IL-5, and IL-13, are responsible for the activation and/or recruitment of the cellular triad of IgE-producing B cells/plasma cells, mast cells, and eosinophils, leading to allergic inflammation. IgE antibodies bind to high-affinity Fcε receptors (FcεRI) present on the surface of mast cells and basophils. Allergen-induced cross-linking of FcεRI triggers the release of vasoactive mediators, chemotactic factors, and other inflammatory mediators (Figure 1). Regulatory T cells suppress allergic disease in murine models of asthma12 and may represent a counterregulatory mechanism also in human allergic disease.13
Hematolymphatic and nonhematolymphatic cells in the pathogenesis of allergic airway disease. The left half of the figure focuses on the role of hematolymphatic cells (1-6) and the right half on the role of nonhematolymphatic cells (eg, airway epithelial cells and smooth muscle cells; 7-10). Dendritic cells (1) present peptides from the allergen to allergen-specific CD4 T cells (2). CD4 T cells secrete cytokines like IL-4, IL-5, and IL-13 (3). IL-4 and IL-13 stimulate the differentiation of B cells into IgE-plasma cells. IL-5 recruits eosinophils and stimulates their differentiation (4). IgE binds to FcεRI on the mast cells or basophils (5). Mast cells, basophils, and eosinophils release vasoactive, chemotactic, and other inflammatory mediators (histamine, leukotrines, etc) (6) resulting in edema, smooth muscle hyperresponsiveness and hypertrophy, basal membrane thickening, and mucus secretion. Perhaps due to the influence of the hematolymphatic cells (?) epithelial cells do the following. Epithelial cells release TARC and IL-16 that activate CD4 T cells (via CCR4 receptor), leading to release of cytokines like IL-4, IL-5, and IL-13 (7). Epithelial cells release GM-CSF and chemokines like RANTES and Eotaxin that lead to recruitment and activation of eosinophils (via CCR3 receptors)(8). Epithelial cells release growth factors like PDGF and EGF that stimulate smooth muscle cells to proliferate and secrete histamine (9). Smooth muscle cells are also activated by mast cells. Epithelial cells release proinflammatory cytokines like TNF-α (stimulates macrophages and dendritic cells) and IL-8 (recruits neutrophils; 10).
Hematolymphatic and nonhematolymphatic cells in the pathogenesis of allergic airway disease. The left half of the figure focuses on the role of hematolymphatic cells (1-6) and the right half on the role of nonhematolymphatic cells (eg, airway epithelial cells and smooth muscle cells; 7-10). Dendritic cells (1) present peptides from the allergen to allergen-specific CD4 T cells (2). CD4 T cells secrete cytokines like IL-4, IL-5, and IL-13 (3). IL-4 and IL-13 stimulate the differentiation of B cells into IgE-plasma cells. IL-5 recruits eosinophils and stimulates their differentiation (4). IgE binds to FcεRI on the mast cells or basophils (5). Mast cells, basophils, and eosinophils release vasoactive, chemotactic, and other inflammatory mediators (histamine, leukotrines, etc) (6) resulting in edema, smooth muscle hyperresponsiveness and hypertrophy, basal membrane thickening, and mucus secretion. Perhaps due to the influence of the hematolymphatic cells (?) epithelial cells do the following. Epithelial cells release TARC and IL-16 that activate CD4 T cells (via CCR4 receptor), leading to release of cytokines like IL-4, IL-5, and IL-13 (7). Epithelial cells release GM-CSF and chemokines like RANTES and Eotaxin that lead to recruitment and activation of eosinophils (via CCR3 receptors)(8). Epithelial cells release growth factors like PDGF and EGF that stimulate smooth muscle cells to proliferate and secrete histamine (9). Smooth muscle cells are also activated by mast cells. Epithelial cells release proinflammatory cytokines like TNF-α (stimulates macrophages and dendritic cells) and IL-8 (recruits neutrophils; 10).
Nonhematolymphatic cells like bronchial smooth muscle cells, myofibroblasts, airway epithelial cells, or skin keratinocytes may also play a role in the pathogenesis of allergic disease.11-14 Epithelial cells produce cytokines (thymic stromal lymphopoietin ‘TSLP’, tumor necrosis factor–α ‘TNF-α’, IL-1, granulocyte monocyte–colony stimulating factor ‘GM-CSF’, tumor growth factor–β ‘TGF-β’), chemokines (regulated upon activation, normal T-cell expressed, and secreted ‘RANTES’, eotaxin, thymus and activation-regulated chemokine ‘TARC’, IL-8) and adhesion molecules (CD54/intercellular adhesion molecule-1 ‘ICAM-1’). These may trigger activation of Th2 cells to secrete IL-4, IL-5, and IL-13; recruitment and differentiation of eosinophils; and other inflammatory responses (Figure 1). Epithelial cells may be stimulated directly by the antigen to produce these molecules without a need for allergen-specific T or B cells.15 Smooth muscle cells of the bronchi are increased in number and hyperresponsive to nonspecific stimuli (eg, methacholine or histamine) in patients with asthma, and produce inflammatory mediators (eg, histamine, leukotrienes). Whether the alterations of the epithelial and smooth muscle cells are primary or secondary to stimuli from the hematolymphatic cells is not known.
HCT in relation to immune disorders
Immune deficiencies that are caused by a defect of hematolymphatic cells are typically cured by allogeneic HCT. Allogeneic HCT can also lead to transfer or cure of autoimmune diseases. The transfer has been reported for thyroiditis/hypothyroidism, polyendocrinopathy, insulin-dependent diabetes, immune thrombocytopenia, inflammatory bowel disease, celiac sprue, sarcoidosis, psoriasis, and myasthenia gravis.8 The cure or long-term remission has been reported for rheumatoid arthritis, Crohn disease, ulcerative colitis, psoriasis, autoimmune hepatitis, multiple sclerosis, hyperthyroidism, systemic lupus erythematosus, and vasculitis.9,10
Transfer of allergy with transplantation
In mice, allergic airway response has been transferred by CD4 T cells from allergen-sensitized to nonsensitized animals.16,17 However, mouse models differ from the human disease.18-20 For example, IL-8 (CXCL8) is produced by nasal epithelial cells of persons with allergic rhinitis21 but is not produced in mice.22 In addition, mast cell infiltration of bronchial smooth muscle, a major correlate of bronchial hyperresponsiveness and allergic asthma in humans,23 is absent in the mouse models of allergic asthma.
In humans, transfer of allergy or allergic disease with T cell–replete HCT has been described in several retrospective case reports (Table 1, patients 1-11)24-31 and one prospective study (Table 1, patients 12-18).32,33
In the retrospective studies, the transfer of allergic disease was described conclusively in 4 patients (patients 1, 4, 5, 6 in Table 1). The most frequent reasons for inconclusiveness for the transfer in the other patients (Table 1 footnotes) were the lack of information on allergic disease in the recipient before transplantation, or the presence of clinical manifestations of allergic disease or elevated allergen-specific IgE in the recipient before transplantation. In the case of patients 9 and 10, the authors reported the transfer of clinical manifestations but did not study allergen-specific IgE. Conversely, in patient 3, the authors reported the transfer of allergen-specific IgE production but not the clinical manifestations.
The only prospective study performed thus far included 11 patients. All had allergic donors; recipients included both those with and those without allergy before transplantation. The study focused on the transfer of allergen-specific IgE production (regardless of clinical manifestations in individual patients). Nevertheless, patient-specific data were reported on 7 donor-recipient pairs (patients 12-18 in Table 1), one of them showing conclusive evidence of allergic disease transfer (patient 12). Short-term follow-up results (up to 1-3 years after transplantation) were reported by Agosti et al32 and long-term follow-up results (14-16 years after transplantation) by Hallstrand et al.33 At 1 to 3 years after transplantation, production of allergen-specific IgE previously seen only in the donor (and not in the recipient) was demonstrated by SPT and/or RAST in 8 of the 11 patients; acquisition by the recipient of new allergen-specific IgE (IgE for allergens that was not elevated in the donor before transplantation) was rare. By 14 to 16 years after transplantation, the production of allergen-specific IgE previously seen only in the donor typically continued; additionally, there was a frequent acquisition by the recipient of new allergen-specific IgE. Among the 11 patients, there were 5 patients who did not have asthma or rhinitis before transplantation and whose donors had asthma or rhinitis. All 5 patients developed asthma or rhinitis by 3 years after transplantation. Three of the 5 patients were followed until 14 to 16 years after transplantation, and at that time all 3 patients had asthma or rhinitis (patients 12, 14, 15). Severity of asthma or rhinitis in these patients appeared to be the same as or worse than in the donor before transplantation.
As hematolymphatic cells may be transferred with solid organ transplantation, it is of interest to also review case reports of allergic disease transfer after solid organ transplantation. This was reported in 3 cases in which the donor died due to anaphylaxis, and his/her organs were used for multiple recipients (Table 2).34-36 The transfer in all 3 cases was restricted to the recipients of liver, perhaps because liver grafting is more likely to result in hematolymphatic cell chimerism than grafting of other solid organs.37 Of the 3 case reports, only the one by Legendre et al36 is conclusive, as recipient pretransplantation allergen-specific IgE was not studied in the other reports.
In summary, transfer of allergic disease was documented conclusively in 5 transplant recipients (patients 1, 4, 5, 6, and 12 in Table 1) and 1 liver transplant recipient (patient 3a in Table 2). However, this does not necessarily mean that allergic disease is transferable with HCT because a control population has not been studied to determine the rate of spontaneous acquisition of allergic disease either in healthy individuals or in nonallergic patients receiving hematopoietic cell transplants from nonallergic donors.
Cure of allergy with HCT
There are only 3 reports of allergic disease remission after HCT from a nonallergic donor (Table 3). The first report (Wahn et al38 ) was on a patient who underwent HCT from his HLA-identical sister for Fanconi anemia. Before transplantation, the patient developed anaphylaxis during a surgical procedure due to latex allergy. Specific IgE was elevated for latex (> 100 kU/L) as well as for multiple aeroallergens. After HCT from a healthy sibling with undetectable latex and aeroallergen-specific IgE, levels of latex and aeroallergen-specific IgE gradually declined, became undetectable at 14 months, and remained undetectable at the end of follow-up at 20 months posttransplantation. Also consistent with allergy remission, total IgE level declined from very high pretransplantation (> 2000 IU/mL) to normal at 1 month (88 IU/mL) and remained normal throughout the end of follow-up at 20 months after transplantation.
In the second report (Koharazawa et al39 ), a patient with chronic myelogenous leukemia and a concomitant 20-year history of eczema underwent HCT from a healthy HLA-matched unrelated donor. The patient was eczema-free from day 70 until the end of follow-up at 4 years after transplantation. Unfortunately, allergen-specific IgE was not reported.
In the third report, Hourihane et al40 described a patient with an unclassified T- and B-cell deficiency who also had eczema, asthma, and peanut-induced angioedema with a high serum level of peanut-specific IgE (23 kU/L). After HCT from a healthy unrelated donor, the patient did not have clinical manifestations of allergic disease, and his total IgE fell from very high (2533 IU/mL) before transplantation to normal (100 IU/mL) by 2 months and remained normal at the last determination at 17 months after transplantation. At the same time, peanut-specific IgE was undetectable by RAST, peanut SPT was negative, and oral challenge with peanuts (8 g) was negative. The patient continued to consume peanuts and peanut butter without problems until the end of follow-up at 24 months after transplantation.
In summary, remission of allergic disease after HCT was conclusively described in 2 cases (patients 1 and 3 in Table 3). However, this does not necessarily mean that allergic disease is curable with HCT because (1) there may be a substantial publication bias in favor of reporting cure, and (2) a control population has not been examined to determine the frequency of spontaneous remission of allergic disease in otherwise healthy allergic individuals or in allergic patients receiving hematopoietic cell transplants from allergic donors.
Mechanism of transfer/cure of allergy with allogeneic HCT
Passive transfer of allergen-specific IgE is unlikely to be responsible for the transfer, as the half-life of serum IgE is short (2-4 days).41 Even if it is presumed that passively transferred donor's serum IgE binds to recipient's mast cells, then also the half-life remains less than 3 weeks. This contrasts with the fact that allergic disease may be ongoing for at least 16 years after transplantation.33
Th2-biased immune reconstitution after HCT could be the mechanism for allergy “transfer.”42,43 Th2 cytokines like IL-4 and IL-10 may be up-regulated in allograft recipients44 ; however, persistent Th2 bias as a routine consequence of HCT is unlikely because allergic disease is not commonly reported after HCT. We found only one case report that describes acquisition of allergy after allogeneic HCT from a nonallergic donor.43
Donor-type nonhematolymphatic cells (eg, airway smooth muscle cells, myofibroblasts, epithelial cells) might exist in the recipient. These cells could originate from circulating nonhematolymphatic cell precursors transferred with the graft or from transferred hematopoietic stem cells differentiating into the nonhematolymphatic cells due to hematopoietic stem cell plasticity.45 However, the percentage of donor-derived airway epithelial cells is low (< 10%) even at more than 10 years after transplantation.46,47 Thus, a substantial contribution of donor nonhematolymphatic cells to the mechanism of allergy transfer is unlikely.
The most likely mechanism of allergy transfer is the transfer of either hematopoietic cell precursors having a tendency to differentiate into allergy-prone hematolymphatic cells, or the transfer of mature allergen-specific B or T cells. The former is supported by the frequent acquisition by the recipients of grafts from allergic donors of new allergen-specific IgE (not detected in the donor).33 The latter is supported by the transfer of ovalbumin-triggered allergic asthma from ovalbumin-allergic to nonallergic mice by CD4 T cells,16,17 and by the fact that in the 5 human cases of conclusive transfer of allergic disease with HCT (Table 1), both the donor and the recipient were allergic to the same allergen(s).
Cure or remission of allergic disease could be due to the exchange of allergy-prone hematolymphatic cells for healthy hematolymphatic cells. In the 3 case reports of allergy “cure” (Table 3), the recipients became complete chimeras (all hematolymphatic cells of donor origin). It would be interesting to know whether allergy cure/remission can also be achieved in the setting of mixed chimerism. If yes, this would suggest that transferred regulatory cells (which may be deficient in allergic patients12 ) from the healthy donor could play a role in the cure/remission. Could the conditioning chemo/radiotherapy account for the cure/remission? Probably not, because cytotoxic therapy (with or without autologous HCT) does not result in human asthma/allergic disease cure.48-51 Consistent with that, in mice cytotoxic therapy does not eliminate antigen-specific IgE.52 Could posttransplantation therapy with immunosuppressive drugs account for the cure/remission? Probably not, because in the 3 reported cases (Table 3), allergic disease did not recur within 8 to 32 months after the discontinuation of immunosuppressive drugs. Moreover, in the 3 reported cases of allergy transfer with liver grafting (Table 2), allergic disease manifested while the patients were on immunosuppressive drugs.
Conclusions
Given the 5 conclusive cases of transfer and 2 conclusive cases of cure, the hypothesis may be proposed that allergic disease is transferable and curable with HCT. However, the likelihoods of transferability and curability are unknown. To determine the likelihoods, large cohorts of nonallergic patients with allergic donors, allergic patients with nonallergic donors, and appropriate controls need to be prospectively studied. The controls should include (1) controls for spontaneous development of allergic disease over the period of follow-up (healthy persons of similar age and genetic background as the hematopoietic cell transplant recipients); (2) controls for spontaneous remission (otherwise healthy persons with allergic disease); and (3) controls for development or remission of allergic disease due to posttransplantation immune dysregulation (cohorts of nonallergic patients with nonallergic donors as controls for the allergic disease development, and cohorts of allergic patients with allergic donors as controls for the allergic disease remission).
Overally, the setting of allogeneic HCT offers an opportunity for unique insights into the pathogenesis of human allergic disease. A large prospective clinical study with appropriate controls and mechanistic studies is needed to elucidate whether the hematolymphatic versus other cells play the primary role in the pathogenesis. This could guide future allergy drug development. The determination of the likelihood of cure will be important for potential development of HCT as a “surgical” (once-in-life) therapy for severe allergic disease in the future, if and when HCT-related morbidity and mortality have become negligible. Determination of transferability will help refine hematopoietic cell transplant donor selection criteria, which should improve the outcome of patients with hematologic malignancies treated with HCT.
The large prospective study to determine the likelihoods of transfer and cure of allergic disease in patients undergoing HCT for hematologic disorders is currently under development by the authors of this review. HCT centers potentially interested in joining the study are encouraged to contact the corresponding author.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
J.S. is a recipient of Canada Research Chair and Alberta Heritage Foundation for Medical Research Clinical Scholar awards.
Authorship
Contribution: F.K. wrote the manuscript; T.S.H., M.G., and W.R.H. critically reviewed the manuscript; and J.S. edited the drafts and the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan Storek, MD, PhD, 3330 Hospital Drive NW, HSC1869, University of Calgary, Calgary, AB, Canada T2N 4N1; e-mail: jstorek@ucalgary.ca.