Abstract
ADAMTS13, a metalloprotease primarily synthesized in liver and endothelial cells, cleaves von Willebrand factor (VWF) at the central A2 domain, thereby reducing the sizes of circulating VWF multimers. Genetic or acquired deficiency of plasma ADAMTS13 activity leads to a potentially fatal syndrome, thrombotic thrombocytopenic purpura (TTP). To date, plasma infusion or exchange is the only proven effective therapy for TTP. In search for a better therapy, an autologous transplantation of hematopoietic progenitor cells transduced ex vivo with a self-inactivating lentiviral vector encoding a full-length murine Adamts13 and an enhanced green fluorescent protein (GFP) reporter gene was performed in Adamts13−/− mice after irradiation. All recipient mice showed detectable ADAMTS13 antigen and proteolytic activity in plasma despite only low levels of bone marrow chimerism. The levels of plasma ADAMTS13 were sufficient to eliminate the ultralarge VWF multimers and offered systemic protection against ferric chloride–induced arterial thrombosis. The data suggest that hematopoietic progenitor cells can be genetically modified ex vivo and transplanted in an autologous model to provide adequate levels of functional ADAMTS13 metalloprotease. This success may provide the basis for development of a novel therapeutic strategy to cure hereditary TTP in humans.
Introduction
ADAMTS13, a reprolysin-like zinc metalloprotease,1,2 is primarily synthesized in hepatic stellate cells,3 endothelial cells,4,5 megakaryocytes, and platelets.6 Plasma concentration of ADAMTS13 ranges from approximately 0.5 to 1.0 μg/mL.7,8 ADAMTS13 cleaves von Willebrand factor (VWF) after the Tyr1605 at the central A2 domain.9 This proteolytic cleavage is essential for preventing an accumulation of unusually large VWF multimers and subsequent platelet aggregation and thrombus formation at the site of vascular injury.10 Deficiency of ADAMTS13, either because of germ-line mutations in the ADAMTS13 gene1 or acquired anti-ADAMTS13 autoantibody formation,11 leads to thrombotic thrombocytopenic purpura (TTP), a potentially fatal thrombotic microangiopathy found in both children and adults
Approximately 5% to 10% of all cases of TTP are caused by a genetic deficiency of ADAMTS13 and are known as hereditary TTP or Upshaw-Schülman syndrome. Patients with this syndrome present as neonates or during early childhood with unexplained jaundice, thrombocytopenia, and microangiopathic hemolytic anemia.12,13 A diagnosis often is not rendered until recurrent episodes are observed. If not treated promptly, central nervous system abnormality, chronic renal insufficiency, and end-stage renal failure may develop in some cases.14,15 With the use of modern diagnostic tools such as measurements of plasma ADAMTS13 activity, inhibitors, and gene sequencing analysis, a definitive diagnosis of hereditary TTP can be made.
To date, the only treatment available for hereditary TTP is intermittent infusions of fresh-frozen plasma.16 The complications associated with administration of plasma, including adverse events with central line placement, bacterial infections, chronic hepatitis C, and allergic reactions to plasma proteins, remain problematic.17 To develop a better therapeutic approach, we explored ex vivo gene therapy in the setting of autologous hematopoietic progenitor cell (HPC) transplantation in a murine model with genetic Adamts13 deficiency. We show that transplantation of ex vivo–transduced HPCs with a lentiviral vector encoding murine Adamts13 can restore ADAMTS13-mediated proteolytic processing of VWF and protection against ferric chloride–induced arterial thrombosis in vivo. The study provides proof in principle of a novel therapeutic strategy to cure hereditary TTP.
Methods
Isolation of murine Adamts13 cDNA
Full-length murine Adamts13 cDNA was isolated by polymerase chain reaction (PCR) using a ready liver cDNA library (Ambion/Applied Biosystems, Austin, TX) as a template and multiple pairs of primers, including m13-1 (5′-atgagccagctttgcctgtggttga-3′) and m13-4 (5′-tgcgttggtcatgttgggag-3′) for the N-terminal fragment (aa1-588) and primer pairs of m13-5 (5′-ctcccaacatgaccaacgca-3′) and m13-8 (5′-ctaggacagagccaggctgtc-3′) for the C-terminal fragment (aa582-1426 and stop). Both N- and C-terminal fragments were then joined to form a full-length murine Adamts13 cDNA by PCR with primers of m13-1 and m13-8. A HotStart Turbo pfu DNA polymerase (Stratagene, La Jolla, CA) was used to reduce the likelihood of amplification errors.2 The amplified cDNA was cloned into pcDNA3.1 TOPO V5-His vector (Invitrogen, Carlsbad, CA) and sequenced with use of a BigDye automatic sequencer (Applied Biosystems, Foster City, CA) at the Nucleic Acid Core Facility at the Children's Hospital of Philadelphia.
Construction of self-inactivated lentiviral vector
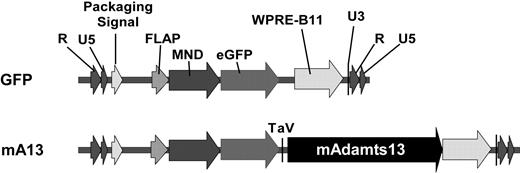
The ZHK construct used in this protocol is a self-inactivating, replication-incompetent HIV-1–based lentiviral vector that has previously been described.18 The transgene cassette was composed of a modified myeloid proliferative sarcoma virus (MND) promoter to drive the expression of enhanced green fluorescent protein (eGFP) and full-length murine Adamts13 isolated in the laboratory. Bicistronic expression was accomplished by inserting the therapeutic gene downstream and in frame with the reporter eGFP cDNA and TaV sequence, a cis-acting hydrolase element derived from the Thosea asigna virus as illustrated in Figure 1.
Schematic representation of lentiviral vectors encoding full-length murine Adamts13 and eGFP reporter genes. Murine full-length ADAMTS13 (encoding amino acid residues 1-1426, mAdamts13) and enhanced green fluorescent protein (eGFP) were cloned into a self-inactivating HIV-1–based vector ZHK to form ZHK-MND-eGFP-Tav3-mAdamts13 (mA13) and ZHK-MND-GFP (GFP). These vectors contain a modified myeloid proliferative sarcoma virus (MND) promoter, an eGFP reporter gene, and Tav sequence. The rev response element (RRE) and the Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), enhancing expression of the transgene, are indicated above the schematic vector structures.
Schematic representation of lentiviral vectors encoding full-length murine Adamts13 and eGFP reporter genes. Murine full-length ADAMTS13 (encoding amino acid residues 1-1426, mAdamts13) and enhanced green fluorescent protein (eGFP) were cloned into a self-inactivating HIV-1–based vector ZHK to form ZHK-MND-eGFP-Tav3-mAdamts13 (mA13) and ZHK-MND-GFP (GFP). These vectors contain a modified myeloid proliferative sarcoma virus (MND) promoter, an eGFP reporter gene, and Tav sequence. The rev response element (RRE) and the Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), enhancing expression of the transgene, are indicated above the schematic vector structures.
Expression of murine Adamts13 cDNA
COS-7 cells (ATCC, Manassas, VA) cultured in DMEM 10% fetal bovine serum in a 6-well plate were transfected with 1 μg plasmid DNA (pcDNA3.1-mAdamts13, hADAMTS13, or pcDNA3.1 (+) vector alone) mixed with 5 μL of LipofectAMINE (Invitrogen) for 5 hours. The transfectant was removed and replaced with Opti-MEM serum-free medium (Invitrogen). After being cultured for 72 hours, the conditioned medium was collected and concentrated 10-fold by Centricon-10. The protein secreted into the conditioned medium was determined by Western blot with anti-V5 IgG as described previously.19 A chimeric protein positope (Invitrogen) was used as a reference.
Kinetic determination of murine recombinant ADAMTS13
The proteolytic activity of recombinant murine ADAMTS13 was determined by a fluorogenic peptide FRETS-vWF73 (Peptides International, Louisville, KY) as previously described.20 The Vmax (slope) was plotted against substrate concentrations [S]. The Michaelis-Menten kinetic constant (Km) was determined by fitting the data into a nonlinear isotherm using SigmaPlot software (Systat Software, San Jose, CA).
Cleavage of human VWF by murine ADAMTS13
Purified plasma VWF (37.5 μg/mL) was incubated at 37°C for 16 hours with approximately 5 to 10 μL of the concentrated conditioned media or citrated murine or normal human plasma in 5 mmol/L Tris-HCl, pH 8.0, containing 1.5 mol/L urea and 5 mmol/L BaCl2. After the reaction was stopped with 10 mmol/L ethylenediamine tetraacetic acid (EDTA), the proteolytic cleavage product of VWF was determined by Western blot under denatured and reduced conditions as previously described.11,19
Preparation of hematopoietic progenitor cells
The Institutional Animal Care and Use Committee at The Children's Hospital of Philadelphia approved the protocol for the study. Bone marrow was harvested from 6- to 8-week-old Adamts13−/− (C57BL6/129Sv) donor mice by flushing the tibias, femurs, and iliac bones with phosphate-buffered saline (PBS; Gibco, Rockville, MD). Passage of the bone marrow through a 70-micron sterile microfilter created a single-cell suspension. The bone marrow mononuclear cells (BMMCs) were separated by density gradient (Histopaque 1077; Sigma-Aldrich, St Louis, MO) at 500g for 30 minutes at room temperature, and washed twice with cold PBS. The number of BMMCs was 5 to 6 × 107 BMMC per donor mouse. The CD48 cells were depleted with the use of anti–CD48-FITC (Biolegend, San Diego, CA) and anti-FITC microbeads (Miltenyi Biotec, Auburn, CA) and column chromatography under a magnetic field (MACS Systems, LD columns, Miltenyi Biotec). This process was repeated twice. The CD48-negative cells were collected, incubated with anti–CD150-PE (Biolegend, San Diego, CA), washed with PBS and then incubated with anti-PE microbeads and applied to a separation unit (MACS Systems, LS columns; Miltenyi Biotec) as per the manufacturer's instructions. After the multistep magnetic sorting processes, CD48−/CD150+ HPCs were purified to greater than 90% of purity in all cases.
Ex vivo transduction of HPCs with lentiviral vector
Vesicular stomatitis virus (VSV)–G pseudotyped vectors were produced by calcium phosphate–mediated transient transfection of HEK293T cells. In brief, cells cultured in DMEM (GIBCO, Invitrogen) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT), 100 U/mL of penicillin and 100 mg/mL streptomycin (GIBCO, Invitrogen) were cotransfected with appropriate amounts of the lentiviral vector plasmid, the packaging plasmid, and the VSV-G expression plasmid. Approximately 16 hours before virus isolation, the media were replaced with similar media minus phenol red. High-titer vectors were prepared by concentrating viral supernatants with ultracentrifugation, then aliquoting and storing them at −80°C. The virus stocks were titered on HEK293T cells by quantifying green fluorescent protein (GFP)–positive cells. The titers used in all transduction protocols were greater than 1010 particles per mL of suspension. A similar vector construct lacking the Adamts13 gene was used as a control.
CD48−/CD150+ HPCs were transduced on a 48-well vitronectin-coated (Takara Bio, Madison, WI) plate in StemPro medium (Invitrogen, Carlsbad, CA). No cytokine-based pretransduction stimulation was used. A multiplicity of infection (MOI) of 100 was used in all cases (for both experimental and control vectors). Cells were exposed to the vectors for a total of 14 to 16 hours, at 37°C and 5% CO2, in the presence of 100 ng/mL of recombinant murine stem cell factor (SCF) and 100 ng/mL of recombinant murine thrombopoietin (TPO; PeproTech, Rocky Hill, NJ). The cells were transduced at density of 105 cells per 100 μL in suspension. For each transduction, a small aliquot was removed and washed twice in cold PBS to remove all viral particles. This aliquot was plated with StemPro medium for a total of 72 hours to determine transduction efficiency. Another aliquot was removed and kept in vector-free culture medium as a negative control. The expression of GFP was directly assessed by flow cytometry. After 14 to 16 hours of transduction, cells were harvested from the wells, washed twice with cold PBS, counted, and concentrated for transplantation.
Transplantation
Adamts13−/− recipient mice were prepared by lethal irradiation at 450 cGy, twice, 3 hours apart at a dose rate of 2.2 cGy/min in a 137Cs-gamma irradiator. After 14-16 hours of culture, transduced HPCs were washed and resuspended in cold PBS, and injected through the tail vein with a 27-gauge needle. Each mouse received 105 transduced HPCs. Along with the transduced cells, each mouse received 2 × 105 syngeneic Adamts13−/− bone marrow mononuclear cells freshly prepared to improve engraftment and to prevent infection. The total volume injected was approximately 0.2 mL per mouse.
Blood sampling
Blood samples (100-200 μL) were collected from retro-orbital sinuses after anesthesia with a heparinized capillary tube at 1, 3, and 5 months of posttransplantation and anticoagulated with 10 to 20 μL of 3.5% sodium citrate. The plasma was collected after centrifugation at 1000g for 5 minutes and kept at −80°C until assay.
Flow cytometry
Citrated blood or bone marrow was diluted 1:100 to 1:200 with heparinized PBS and loaded on a density gradient centrifugation tube. The mononuclear cells were collected after centrifugation at 600g for 25 minutes and subsequently washed with PBS before analysis for GFP-positive cells in a FACSCalibur cytometer (BD Biosciences, San Jose, CA). Approximately 10 000 events were analyzed for each sample.
Immunohistochemistry
Blood smear and tissue sections were prepared from fresh whole blood and paraformaldehyde (4%) fixed and paraffin-embedded tissues, respectively. The blood smear was fixed in cold ethanol/acetic acid (9:1, vol:vol) for 10 minutes. The tissue sections were then deparaffinized in serial concentrations of xylene, followed by rehydration with serial concentrations of ethanol and distilled water. The blood smears and tissue sections were blocked with peroxidase-blocking reagent (Dako, Carpenteria, CA) for 30 minutes at room temperature. The slides were washed with distilled water and PBS containing 0.01% Triton X-100, pH 7.4. The autofluorescence was quenched by cold sodium borohydride in PBS (1 mg/mL; Sigma-Aldrich) for 30 minutes. After a 10-minute block with universal blocking reagent (BioGenex Laboratories, San Ramon, CA), the slides were rinsed with PBS and incubated with rabbit anti-GFP IgG (1:200; Invitrogen) at 4°C, overnight. The slides were rinsed for 10 minutes with PBS and incubated with biotin-labeled goat anti–rabbit IgG (1:200; Vector Laboratories, Burlingame, CA) for 30 minutes. The slides were washed again for 10 minutes with PBS and incubated with streptavidin-peroxidase complexes (Vector Laboratories) for 30 minutes. DAB substrate (Vector Laboratories) was added to determine the bound streptavidin-peroxidase complexes. The nuclei were lightly counterstained with Harris hematoxylin. Digital images were taken with bright field under a Leica microscope (Wetzlar, Germany) with SPOT Advanced Software version 4.6 (Diagnostic Instruments, Sterling Heights, MI).
Assay for murine plasma ADAMTS13 antigen
Polyclonal antibody against ADAMTS13 was prepared by the use of a mixture of recombinant murine full-length ADAMTS13 (0.8 mg) and human CUB domain–deleted ADAMTS13 (delCUB; 0.2 mg) as antigens. A standard 90-day immunization protocol was used by Precision Antibodies (Columbia, MD). The anti-ADAMTS13 IgGs were isolated from antiserum by protein A/G affinity chromatography. The same polyclonal antibody was labeled with EZ-Link NHS-Biotin according to manufacturer's recommendation (Pierce, Rockford, IL). The wells of a high binding microtiter plate (NUNC, Rochester, NY) were coated with 100 μL unlabeled rabbit anti-ADAMTS13 IgG (40 μg/mL) at 25°C for 1 hour. After blocked with 200 μL of 2.5% BSA in PBST, 100 μL of diluted plasma (1:10) with a dilution buffer containing 0.5% BSA, 1.5% ADAMTS13-deficient murine plasma, and 0.05% Tween 20 was added and incubated at 25°C for 2 hours. The plate was washed 3 times with PBS and incubated for 1 hour with 100 μL biotin-labeled rabbit anti-ADAMTS13 IgG (1.4 μg/mL) in the same dilution buffer. After being washed with PBS 3 times, a streptavidin-peroxidase complex (1:200; Vector Laboratories, Burlingame, CA) was added to the plate and incubated for 30 minutes. The bound biotin-streptavidin-peroxidase complexes were determined by TMB (3,3′,5,5′-tetramethylbenzidine) substrate (Vector Laboratories). The reaction was stopped with 50 μL of 1.5 mol/L H2SO4 and the absorbance was determined in SpectraMAX plate reader (Molecular Devices, Sunnyvale, CA) at 450 nm. Pooled murine plasma from wild-type Balb/c mice was used as a reference with an arbitrarily defined concentration of 1 μg/mL.
Assay for murine plasma ADAMTS13 activity
GST-vWF73 (37.5 μg/mL) was incubated with 5 μL murine plasma in 20 mmol/L HEPES, pH 7.5; 50 mmol/L NaCl; and 5 mmol/L CaCl2 for 3 hours. The cleavage product was determined by Western blotting with rabbit anti-GST IgG (1:800) (Molecular Probes, Eugene, OR),21,22 followed by IRDye 800–labeled anti–rabbit IgG (1:12 500; Molecular Probes). The bound fluorescent secondary antibody was quantified by Odyssey Infrared Fluorescent Image System (LI-COR Bioscience, Lincoln, NE). Pooled normal murine plasma (n = 10) from Balb/c mice was used as a standard.
Assay for murine plasma anti-ADAMTS13 IgG
The wells of a high binding microtiter plate (NUNC, Rochester, NY) were coated with 100 μL of purified murine ADAMTS13 (2 μg/mL) in PBS at 25°C for 1 hour. After being blocked with 200 μL of 2.5% BSA in PBST, 100 μL diluted murine plasma (1:10) or rabbit serum containing polyclonal anti–murine ADAMTS13 IgG (∼1:10 000-80 000) with PBS containing 0.5% BSA and 0.05% Tween 20 was added and incubated at 25°C for 1 hour. The plate was washed 3 times with PBS and incubated for 1 hour with 100 μL HRP-conjugated rabbit anti–mouse IgG (1:5000) (DAKO; Carpinteria, CA) or HRP-conjugated donkey anti–rabbit IgG (1:5000); GE Healthcare, Piscataway, NJ). After washed with PBS for 3 times, the bound secondary antibodies were detected by TMB substrate (Vector Laboratories). The reaction was terminated by addition of 50 μL of 1.5 mol/L H2SO4 after 30 seconds of incubation in the positive controls, but 5 minutes in the assays for anti-ADAMTS13 IgG in murine plasma. The absorbance at 450 nm was obtained with the SpectraMAX plate reader (Molecular Devices, Sunnyvale, CA).
Assay for murine plasma VWF multimers
Citrated murine plasma (1.0 μL) was denatured by heating at 60°C for 20 minutes in 70 mmol/L Tris-HCl, pH 6.5 containing 2.4% (wt/vol) SDS, 4% (wt/vol) urea, and 4 mmol/L EDTA.18,23 The denatured VWF was fractionated on 1.0% (wt/vol) SeaKem HGT agarose (Cambrex, East Rutherford, NJ) minigel by electrophoresis at 15 mA for 3.5 hours. After being transferred onto nitrocellulose membrane (Bio-Rad, Hercules, CA) by capillary diffusion with PBS overnight (16 hours) at room temperature,23 the membrane was blocked by 1% (wt/vol) casein in TBST for 30 minutes and incubated with rabbit anti-VWF IgG (DAKO) (1:1500) in TBST (20 mmol/L Tris-HCl, pH 7.5; 150 mmol/L NaCl; and 0.05% Tween 20) containing 1% (wt/vol) casein overnight, followed by the IRDyeCW800-labeled goat anti–rabbit IgG (1:12 500) at 25°C for 1 hour. The fluorescent signal was detected by scanning the membrane on an Odyssey Imaging System as described previously.18
Carotid arterial thrombosis
Mice at approximately 5 months of age were anesthetized with intraperitoneal injection of 10 mg/mL Nembutal (0.1 mL/10 g) and the right carotid artery was exposed by blunt dissection. A Doppler flow probe (Model 0.5VB, Transonic Systems, Ithaca, NY) was placed around the artery. Thrombosis was induced in the exposed carotid artery by applying a piece of filter paper (2 mm) saturated with 10% (vol/vol) ferric chloride to the adventitia for 1 minute. The field was flushed with PBS, and the blood flow was monitored for 30 minutes. The time to initial complete occlusion and the presence or absence of arterial occlusion at 30 minutes was recorded in experimental and control mice.
Statistical analysis
The continuous variables between the controls and experimental groups were analyzed by the Student t test. Values of P less than .05 and .001 are considered to be statistically significant and very significant, respectively.
Results
Characterization of murine Adamts13 cDNA
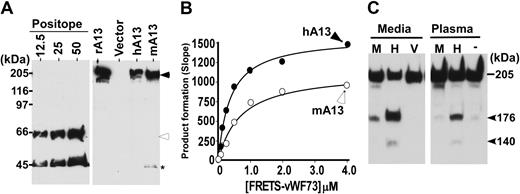
Full-length murine Adamts13 and human ADAMTS13 cDNAs inserted into pcDNA3.1 TOPO V5-His were transiently expressed in COS-7 cells. The conditioned media were collected and concentrated by filtration. Sodium dodecyl sulfate (SDS)–polyacrylamide gel and Western blot with anti-V5 determined the sizes and levels of expressed recombinant proteins. The expressed murine ADAMTS13 migrated at approximately 195 kDa, similar to that of recombinant human ADAMTS13 (Figure 2A). The recombinant murine ADAMTS13 was able to cleave FRETS-vWF73 (Figure 2B) and GST-vWF73 (not shown). Kinetic analysis by FRETS-vWF73 showed that the ratios of kcat/km for cleaving FRETS-vWF73 by recombinant murine ADAMTS13 and recombinant human ADAMTS13 were 1.3 and 3.5, respectively (Figure 2B), suggesting that murine recombinant ADAMTS13 is approximately 2.7-fold less efficient than human recombinant ADAMTS13 in catalyzing human FRETS-vWF73 peptide substrate.
Characterization of murine Adamts13 cDNA. (A) Western blotting determined recombinant murine ADAMTS13. The conditioned medium containing murine (mA13) or human (hA13) ADAMTS13 or vector alone (Vector) was separated under denatured and reduced conditions on 8% SDS polyacrylamide gel electrophoresis (PAGE) and blotted with anti-V5 IgG. The controls include purified V5-tagged reference protein (Positope) (12.5, 25, and 50 ng/lane) and purified recombinant human ADAMTS13 (rAD13) as a positive control. The closed arrowhead indicates full-length murine and human ADAMTS13 proteins (∼195 kDa), whereas the open arrowhead and star indicate the intact and C-terminal fragment of the reference protein. (B) Kinetic analysis of murine (mA13, open circle) and human (hA13, closed circle) ADAMTS13 by FRETS-vWF73 at various concentrations. (C) Proteolytic cleavage of purified human VWF by conditioned medium or plasma containing murine (M) or human (H) ADAMTS13. The medium obtained from vector-transfected cells (V) or assay buffer (−) was used as controls. The proteolytic cleavage products were determined by Western blot with rabbit anti-VWF IgG that preferentially bound C-terminal portion of VWF (176K).
Characterization of murine Adamts13 cDNA. (A) Western blotting determined recombinant murine ADAMTS13. The conditioned medium containing murine (mA13) or human (hA13) ADAMTS13 or vector alone (Vector) was separated under denatured and reduced conditions on 8% SDS polyacrylamide gel electrophoresis (PAGE) and blotted with anti-V5 IgG. The controls include purified V5-tagged reference protein (Positope) (12.5, 25, and 50 ng/lane) and purified recombinant human ADAMTS13 (rAD13) as a positive control. The closed arrowhead indicates full-length murine and human ADAMTS13 proteins (∼195 kDa), whereas the open arrowhead and star indicate the intact and C-terminal fragment of the reference protein. (B) Kinetic analysis of murine (mA13, open circle) and human (hA13, closed circle) ADAMTS13 by FRETS-vWF73 at various concentrations. (C) Proteolytic cleavage of purified human VWF by conditioned medium or plasma containing murine (M) or human (H) ADAMTS13. The medium obtained from vector-transfected cells (V) or assay buffer (−) was used as controls. The proteolytic cleavage products were determined by Western blot with rabbit anti-VWF IgG that preferentially bound C-terminal portion of VWF (176K).
Moreover, the recombinant murine ADAMTS13 at 10 times the murine plasma concentration was able to cleave human multimeric VWF under denatured conditions. The relative cleavage product (176 kDa) generated by murine ADAMTS13 was approximately 10% of that generated by human ADAMTS13 (Figure 2C). No cleavage product was detectable after an incubation of human VWF with 10 μL murine plasma, the amount of human plasma resulting in clearly detectable cleavage product by Western blot (Figure 2C). These results indicate that the murine Adamts13 cDNA isolated from murine liver cDNA library can be expressed and the expressed product is correct in molecular weight and functional in catalyzing both peptidyl and macromolecular substrates.
Ex vivo transduction of HPCs with lentiviral vectors
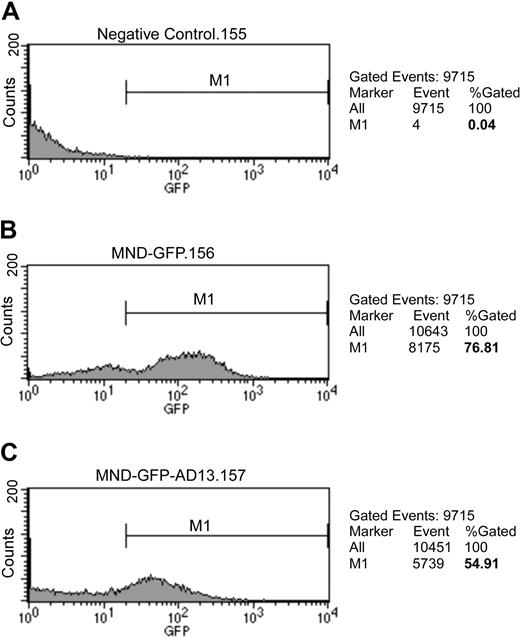
To determine the transduction efficiency, we analyzed the GFP-positive cells by flow cytometry. Purified HPCs were infected with lentiviral vector at MOI of 100 for 16 hours. The vectors were removed, and the HPCs were washed with PBS. After an additional 48 hours in culture, the HPCs were harvested for analysis without fixation. The cultured cells were 76% positive for GFP after transduction with lentiviral vector encoding GFP alone (Figure 3B), but only 55% positive after transduction with vector encoding GFP-Adamts13 (Figure 3C). The difference in GFP positivity rates may relate to the size of the vector constructs.
Ex vivo transduction of HPCs with lentiviral vectors. Isolated HPCs were transduced for 16 hours with lentiviral vector at MOI of 100 and washed with PBS. After additional 72-hour incubation, the cells were analyzed by flow cytometry for GFP-positive cells in nontransduced cells (A) and in cells transduced with vector encoding ZHK-MND-GFP (B) or ZHK-MND-eGFP-Tav3-mAdamts13 (C). The percentage of GFP-positive cells is shown on the right side of each panel.
Ex vivo transduction of HPCs with lentiviral vectors. Isolated HPCs were transduced for 16 hours with lentiviral vector at MOI of 100 and washed with PBS. After additional 72-hour incubation, the cells were analyzed by flow cytometry for GFP-positive cells in nontransduced cells (A) and in cells transduced with vector encoding ZHK-MND-GFP (B) or ZHK-MND-eGFP-Tav3-mAdamts13 (C). The percentage of GFP-positive cells is shown on the right side of each panel.
Engraftment and chimerism
To determine the engraftment and subsequent levels of chimerism from transduced HPCs, the frequency of GFP-positive cells were assessed by flow cytometry in the peripheral blood after depletion of red blood cells. The GFP-positive cells in the mononucleated population of the peripheral blood obtained from mice at 8 and 16 weeks after transplantation with HPCs ex vivo transduced with vector encoding GFP-Adamts13 were 8.5 ± 2.13% (mean ± SD) and 13.0 ± 4.6% (mean ± SD), respectively. In contrast, the GFP-positive rates in the peripheral blood at 8 and 16 weeks of posttransplantation with HPCs transduced with vector encoding GFP alone were 41.8 (± 4.1%; mean ± SD) and 38.8 (± 6.2%; mean ± SD), respectively. The discrepancy in GFP positivity between HPCs transduced with vector encoding GFP alone and GFP-Adamts13 likely relates to the initial difference in transduction efficiency between these 2 vectors.
Localization of transgene-expressing cells in mice
The authors of several studies have demonstrated that, in addition to hematopoietic lineages, differentiated cells originated from transplanted HPCs can be found in various organs. These include mature hepatocytes24 and hepatic stellate cells25 in the liver, skeletal muscle cells,26 and epithelial cells in multiple organs.27 To determine the sites of transgene expression, immunohistochemistry was performed on peripheral blood smears and sections of various organ tissues using a highly specific polyclonal antibody against GFP. Positive GFP staining is indicative of ADAMTS13 expression as the expression of both genes are driven under the same MND promoter as illustrated in Figure 1. Consistent with the flow cytometry results, the multinucleated white blood cells, megakaryocytes/platelets and rare red blood cells were positive for anti-GFP staining (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). The lymphocytes and majority of red blood cells were negative for the staining. The white and red blood cells from nonrecipient mice were also negative (data not shown), suggesting that the positive immune reactivity on the peripheral blood smear observed is highly specific.
Moreover, GFP-positive cells were occasionally identified in the heart (cardiomyocytes), liver (interstitial cells), spleen, and kidneys (tubular epithelial cells) in HPC-recipient mice (Figure S1B). No positive GFP staining was found in the brain, lung, intestine, or pancreas (Figure S1B). The lack of positive immune reactivity with anti-GFP antibody in many organ tissues suggests the homing and differentiation of the ex vivo manipulated and transplanted HPCs into mature cells are extremely rare events. Not only was our immunoperoxidase technique highly specific, but it also was highly sensitive in identifying GFP-positive cells. This was illustrated by observation of numerous GFP-positive cells using the same protocol in the hepatocytes and renal tubular epithelial cells in mice injected in utero with a lentiviral vector encoding GFP (Figure S1Bi,j).
ADAMTS13 antigen and activity in murine plasma
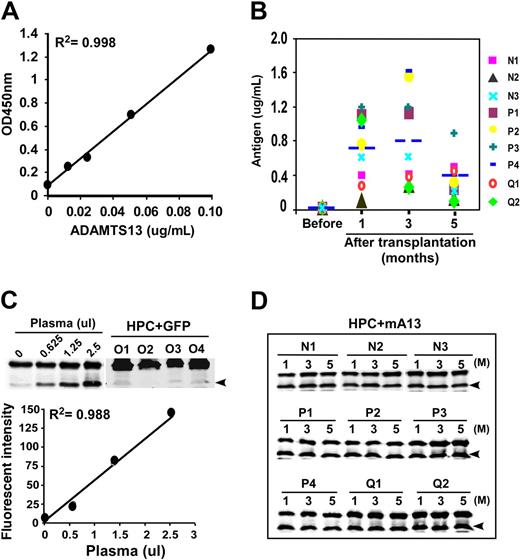
To determine the levels of plasma ADAMTS13 antigen, we established a novel enzyme-linked immunosorbent assay (ELISA) that is sensitive and specific for murine plasma ADAMTS13. All plasma samples from the mice transplanted with HPCs ex vivo transduced with lentiviral vector encoding GFP-Adamts13 exhibited detectable ADAMTS13 antigen. The ADAMTS13 antigen levels at 1, 3, and 5 months were 0.8 (± 0.3) μg/mL, 0.8 (± 0.5) μg/mL, and 0.4 (± 0.3) μg/mL, respectively (Table 1 and Figure 4B). The proteolytic activity was determined by GST-vWF73, but not by FRETS-vWF73 because only 10% proteolytic activity was observed by normal murine plasma compared with human plasma (data not shown), despite recombinant murine ADAMTS13 exhibited only 2.7-fold less efficiently than recombinant human ADAMTS13 to catalyze FRETS-vWF73 derived from human VWF, as shown in Figure 2B.
ADAMTS13 antigen and proteolytic activity in murine plasma. (A) Calibration curve using diluted wild-type murine plasma (0, 1:80, 1:40, 1:20, and 1:10) that corresponds to 0, 0.0125, 0.025, 0.05, and 0.10 μg/mL ADAMTS13 antigen as shown in the x-axis. (B) Plasma levels of ADAMTS13 antigen in various mice at 1, 3, and 5 months after transplantation compared with those in mice without transplantation (Pre). N1 through Q2 represent difference mice. (C) Cleavage of GST-vWF73 by wild-type murine plasma (0, 0.25, 1.25, and 2.5 μL) and by plasmas (5 μL, each) from recipient mice at 1 month of posttransplantation with HPCs transduced with the vector encoding GFP alone (HPC + GFP, lanes O1-O4) as negative controls (top). A representative calibration curve is shown (the fluorescence intensity against μL of murine plasma added; bottom). (D) Cleavage of GST-vWF73 by murine plasma (5 μL) at 1, 3, and 5 months after transplantation of HPCs transduced with the vector encoding GFP-mAdamts13 (N1-N3, P1-P4, and Q1-Q2). Arrowhead indicates the cleavage product of 34.4 kDa.
ADAMTS13 antigen and proteolytic activity in murine plasma. (A) Calibration curve using diluted wild-type murine plasma (0, 1:80, 1:40, 1:20, and 1:10) that corresponds to 0, 0.0125, 0.025, 0.05, and 0.10 μg/mL ADAMTS13 antigen as shown in the x-axis. (B) Plasma levels of ADAMTS13 antigen in various mice at 1, 3, and 5 months after transplantation compared with those in mice without transplantation (Pre). N1 through Q2 represent difference mice. (C) Cleavage of GST-vWF73 by wild-type murine plasma (0, 0.25, 1.25, and 2.5 μL) and by plasmas (5 μL, each) from recipient mice at 1 month of posttransplantation with HPCs transduced with the vector encoding GFP alone (HPC + GFP, lanes O1-O4) as negative controls (top). A representative calibration curve is shown (the fluorescence intensity against μL of murine plasma added; bottom). (D) Cleavage of GST-vWF73 by murine plasma (5 μL) at 1, 3, and 5 months after transplantation of HPCs transduced with the vector encoding GFP-mAdamts13 (N1-N3, P1-P4, and Q1-Q2). Arrowhead indicates the cleavage product of 34.4 kDa.
Proteolytic cleavage of GST-vWF73 by murine plasma after a prolonged incubation (3 hours) was concentration dependent (Figure 4C). The control plasma from mice transplanted with lentiviral vector encoding GFP alone exhibited no detectable ADAMTS13 activity (Figure 4C top panel lanes O1-O4). The plasma levels of ADAMTS13 activity at 1, 3, and 5 months after HPCs transplantation were 24.1% (± 13.7%), 27.5% (± 14.3%), and 26.3% (± 14.3%), respectively (Table 1 and Figure 4D), compared with the activity in pooled murine plasma obtained from wild-type mice (arbitrarily defined as 100%). These data suggest that long-term therapeutic levels of plasma ADAMTS13 protease (> 0.10 μg/mL antigen and 5%-10% activity of normal murine plasma) can be achieved by the autologous transplantation with ex vivo–manipulated HPCs.
Formation of anti-ADAMTS13 IgG antibodies
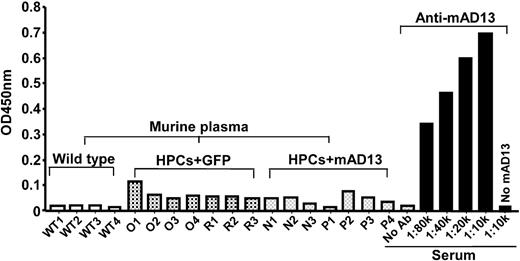
The discrepancy between the levels of plasma antigen and proteolytic activity has promoted us to investigate the formation of anti-ADAMTS13 IgGs in mice receiving HPCs expressing murine ADAMTS13. As shown in Figure 5, no antibody against transduced murine ADAMTS13 was detected in both control and experimental mice (Figure 5). The same amount of immobilized murine ADAMTS13 (0.2 μg/well) was able to bind antimurine/human ADAMTS13 IgG in the rabbit serum, raised against combination of murine recombinant full-length ADAMTS13 and human delCUB (Precision Antibodies) at high dilution of 1:80 000 (Figure 5). These results suggest no antimurine ADAMTS13 IgG formed in the Adamts13−/− mice expressing recombinant murine ADAMTS13 after HPCs transplantation.
Anti-ADAMTS13 IgGs in murine plasma. Murine plasmas (1:10) obtained from wild-type mice (WT1-WT4), Adamts13−/− mice transplanted with HPCs expressing GFP alone (O1-O4 and R1-R3) and GFP-ADAMTS13 (N1-N3 and P1-P4) at 3 months were incubated with immobilized purified murine ADAMTS13 on a microtiter plate. The bound IgG was detected by HRP-conjugated anti–mouse IgG as described in “Assay for murine plasma anti-ADAMTS13 IgG.” The positive control was serum (1:10 000 to 80 000) from a rabbit immunized with purified murine and human ADAMTS13 proteins (Anti-mAD13) according to the standard protocol. Negative control was negative in the wells with no ADAMTS13 immobilized despite of an incubation with rabbit anti-mAD13 serum (1:10 000) or murine plasma (1:10).
Anti-ADAMTS13 IgGs in murine plasma. Murine plasmas (1:10) obtained from wild-type mice (WT1-WT4), Adamts13−/− mice transplanted with HPCs expressing GFP alone (O1-O4 and R1-R3) and GFP-ADAMTS13 (N1-N3 and P1-P4) at 3 months were incubated with immobilized purified murine ADAMTS13 on a microtiter plate. The bound IgG was detected by HRP-conjugated anti–mouse IgG as described in “Assay for murine plasma anti-ADAMTS13 IgG.” The positive control was serum (1:10 000 to 80 000) from a rabbit immunized with purified murine and human ADAMTS13 proteins (Anti-mAD13) according to the standard protocol. Negative control was negative in the wells with no ADAMTS13 immobilized despite of an incubation with rabbit anti-mAD13 serum (1:10 000) or murine plasma (1:10).
Plasma VWF multimers
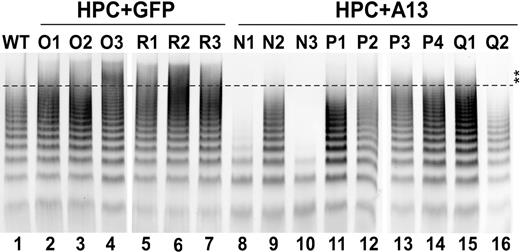
To determine the biologic role of the expressed ADAMTS13 in processing VWF in vivo, plasma VWF multimers obtained from mice 2 months after transplantation were analyzed by a miniagarose gel (1%) electrophoresis plus Western blotting with anti-VWF IgG. The UL-VWF multimers were clearly detectable in 6 of 7 plasma samples obtained from Adamts13-deficient mice transplanted with HPCs transduced with a vector encoding GFP alone (Figure 6 lanes 2-7). However, the UL-VWF multimers were essentially eliminated in all mice expressing GFP-ADAMTS13 (Figure 6 lanes 8-16). Particularly, N1, N3, and Q2 mice exhibited markedly reduced concentration of plasma VWF antigen and sizes of VWF multimers (Figure 6 lanes 8,10,16). The plasma VWF multimer distribution in most of mice expressing recombinant murine ADAMTS13 was quite similar to that in wild-type mice (Figure 6 lane 1). These data suggest that the secreted murine ADAMTS13 from transduced HPCs is able to adequately process newly released UL-VWF in vivo under fluid shear stresses.
Plasma VWF multimer in transplanted mice. Plasma (2.5 μL) from wild-type (lane 1), Adamts13−/− mice expressing GFP (lanes 2-7 or O1-O3 and R1-R3) and full-length murine ADAMTS13 (lanes 8-16 or N1-N3, P1-P4, and Q1-Q2) at 2 months of age were fractionated by 1% mini-agarose gel electrophoresis. The VWF was determined by Western blot with anti-VWF IgG, followed by IRDye800-conjugated anti–rabbit IgG as described in “Assay for murine plasma VWF multimers.” The fluorescent signal was determined by an Odyssey infrared imaging system. Double stars indicate the area of UL-VWF multimers. All mice expressing murine ADAMTS13 exhibited reduced UL-VWF multimers.
Plasma VWF multimer in transplanted mice. Plasma (2.5 μL) from wild-type (lane 1), Adamts13−/− mice expressing GFP (lanes 2-7 or O1-O3 and R1-R3) and full-length murine ADAMTS13 (lanes 8-16 or N1-N3, P1-P4, and Q1-Q2) at 2 months of age were fractionated by 1% mini-agarose gel electrophoresis. The VWF was determined by Western blot with anti-VWF IgG, followed by IRDye800-conjugated anti–rabbit IgG as described in “Assay for murine plasma VWF multimers.” The fluorescent signal was determined by an Odyssey infrared imaging system. Double stars indicate the area of UL-VWF multimers. All mice expressing murine ADAMTS13 exhibited reduced UL-VWF multimers.
Ferric chloride–induced arterial thrombosis
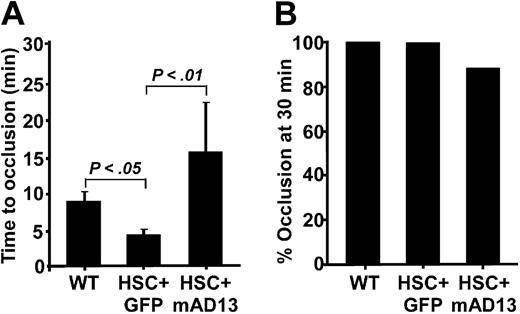
To further determine antithrombotic function of the expressed murine ADAMTS13 in vivo, we performed the ferric chloride–induced carotid arterial thrombosis assay in a single blind fashion (the operator did not know which mouse received transplantation with HPCs expressing ADAMTS13 or GFP alone). We showed that the carotid arterial occlusion times in Adamts13−/− mice transplanted with HPCs transduced with the control vector encoding GFP alone were 5.6 (± 1.6; means ± SD), similar to 4.3 (± 0.8) minutes for Adamts13−/− mice that did not undergo transplantation recently reported,18 but slightly shorter than that in wild-type mice (P < .05; Figure 7A).
Ferric chloride–induced arterial thrombosis. (A) The time to occlude the carotid artery of Adamts13−/− mice 5 months after transplantation with HPCs expressing GFP (HPC + GFP) and full-length murine Adamts13 (HPC + mAD13). (B) The percentage of occluded carotid arteries after 30 minutes of monitoring in all 3 groups is shown. The Student t test was performed to determine the statistical significance between each control and the experimental group. P values less than .01 and .05 indicate statistically significant difference between the 2 compared groups.
Ferric chloride–induced arterial thrombosis. (A) The time to occlude the carotid artery of Adamts13−/− mice 5 months after transplantation with HPCs expressing GFP (HPC + GFP) and full-length murine Adamts13 (HPC + mAD13). (B) The percentage of occluded carotid arteries after 30 minutes of monitoring in all 3 groups is shown. The Student t test was performed to determine the statistical significance between each control and the experimental group. P values less than .01 and .05 indicate statistically significant difference between the 2 compared groups.
However, the arterial occlusion times in mice that received HPCs transduced with vector encoding GFP-Adamts13 were 15.5 (± 6.8) minutes, which were significantly prolonged compared with the GFP control group (P < .01; Figure 7A). One of 8 mice expressing ADAMTS13 did not form an occlusive thrombus for up to 30 minutes, whereas all the mice in the GFP (7/7) and wild-type (10/10) control groups formed occlusive thrombi before 30 min-utes (Figure 7B). These results suggest that the ectopic expression of ADAMTS13 in hematopoietic cells (erythrocytes, multinucleated cells, megakaryocytes, and platelets) may be more efficacious than plasma ADAMTS13 for systemic antiarterial thrombosis.
Discussion
Patients with hereditary TTP require a lifelong plasma therapy as often as every 2 weeks to prevent exacerbations of the disease and reduce mortality.15 Such treatment, although therapeutically effective, is not without complications. There are multiple possible strategies to potentially correct the defect in hereditary TTP, including systemic or localized delivery of a gene transfer vehicle, and engraftment of long-lived cells, or cells with repopulating capacity as platforms for the systemic delivery of ADAMTS13. Such strategies would potentially provide continuous synthesis of ADAMTS13 to prevent the risk of serious microvascular thrombosis. Somatic gene therapy has shown promises,18 but there may be disadvantages such as vector toxicity, immunologic responses to vector or therapeutic protein, and potential germline transmission. Orthotopic liver transplantation or possible stellate cell engraftment may be useful approaches, but drawbacks include lack of donors or cell isolation procedures and requirements for controlling recipient rejection of the graft. In light of these limitations, we developed an ex vivo gene therapy approach targeting long-term repopulating hematopoietic cells in an Adamts13-deficient murine model.28 This approach is a relatively safe procedure and particularly attractive as a corrective therapy for TTP because as low as 5% to 10% of proteolytic activity (or ∼0.10-0.20 μg/mL protein) is sufficient to correct TTP symptoms as shown in humans.16
In this study, we demonstrate that plasma ADAMTS13 proteolytic activity on average can be restored in Adamts13−/− mice to approximately 25% of normal. This level of functional activity of ADAMTS13 corresponds to approximately 0.25 to 0.50 μg/mL of total ADAMTS13 protein in plasma, which is consistent with our ELISA data (Figure 4). Although our ELISA method is highly sensitive and specific for measuring murine plasma ADAMTS13, the antigen levels do not seem to correlate well with the proteolytic activity determined by GST-vWF73. The reason for the discrepancy between the activity and antigen measurement is not fully understood. One possibility is that a prolonged incubation of GST-vWF73 with murine plasma results in overestimating low ADAMTS13 activity, which has been observed previously.8 In addition, a partially degraded ADAMTS13 protein (such as N terminus of ADAMTS13 up to the spacer domain) that does not react with our anti-ADAMTS13 IgG, primarily recognizing the C-terminus of ADAMTS13 (data not shown) remains still active in catalyzing GST-vWF73 peptide under these conditions.21 The possibility of anti-ADAMTS13 IgG formed in mice expressing recombinant murine ADAMTS13 has been ruled out (Figure 5). Nevertheless, this amount of circulating ADAMTS13 antigen is almost 10-fold greater than that of circulating coagulation factor VIII (0.02-0.05 ng/mL)29 and approximately 3-fold of factor IX (> 0.25 ng/mL) observed in the hemophiliac murine models, in which the similar strategy of engrafting genetically engineered hematopoietic stem cells has been used.30
The expressed ADAMTS13 appears to be biologically active in vivo, based upon the substantial reduction of UL-VWF multimers and protection against ferric chloride–induced arterial thrombosis. There are advantages in using hematopoietic cells as vehicles for the systemic delivery of a therapeutic protein: (1) HPCs can readily be isolated from patients through a bone marrow harvest or apheresis and (2) HPCs can be transduced ex vivo with a viral vector and subsequently transplanted back to the patients. Because of these advantages, HPCs are being used as a gene therapy vehicle for the treatment of a variety of diseases.31-33 Particularly relevant as a therapeutic strategy for hereditary TTP is the fact that ADAMTS13 is normally synthesized and secreted from a variety of cells derived from HPCs such as megakaryocytes and platelets6,34 and hepatic stellate cells.3,6,35 We have observed GFP-positive cells in the peripheral blood and multiple organ tissues (Figures S1B), suggesting multilineage engraftments by transplanted HPCs.
The reasons for the disparity in the levels of peripheral blood chimerism between our experimental (10%) and control groups (40%) are not clear. The initial difference in the transduction efficiency of HPCs with a vector encoding GFP-ADAMTS13 or GFP alone (approximately 2-fold greater in the control) may in part account for the disparity in the long-term rate of engraftment of these cells. In addition, the lower percentage of GFP-positive cells may be associated with the increased size of the Adamts13 vector or changes in stability of the mRNA produced. It is not known, however, whether the lower rate of engraftment in HPCs transduced with vector encoding GFP-ADAMTS13 has anything to do with the adverse effect of the ectopically expressed ADAMTS13 in certain cell lineages.
There are several critical hurdles we may have to overcome before this strategy can be applied to treat patients. First, it remains to be determined what is the optimal ex vivo transduction protocol for human HPCs. Studies have shown that a cytokine cocktail containing stem cell factor, thrombopoietin, and Flt-3 ligand may support human HPCs during transduction without affecting long-term cell engraftment.36,37 Second, the best viral vector and promoter that can drive successful gene expression but not cause oncogenesis as a result of insertional mutagenesis and/or transactivation activation of chromosomal genes are yet to be discovered. The lentiviral vectors appear to have the advantage, with their significantly lower propensity for integrating in potentially dangerous regions of human genome than gamma-retroviral vectors. In addition, self-inactivating vectors have better safety profile than those with intact long terminal repeats (LTRs).38
With regard to the promoter, the CMV promoter and murine PGK promoter were among the first to be used, but both were shown to drive relatively low levels of gene expression in NOD/SCID xenochimeras.39,40 The elongation factor-1α (EF-1α) promoter, on the other hand, is among the strongest promoters tested in vitro, 3- to 4-fold stronger than the PGK promoter in a human CD34+ cell line41 and in cultured cord blood cells.41,42 The EF-1α promoter can drive expression of a transgene for 15 weeks in the progeny of CD34+ cells engrafted into NOD/SCID mice.41 Other tissue-specific promoters may be used for stable expression of various soluble proteins and for delivering the protein to the site where it is needed. For example, an erythroid-specific vector system has been tested in expression of various clotting factors (factors VIII and IX), the enzymes deficient in lysosomal storage disease, erythropoietin and antibodies.43 The glycoprotein Ibα promoter44,45 and Tie-2 promoter46 can be used to drive high levels of genes of interest in megakaryocytic lineage and endothelium, respectively. The ectopic expression of ADAMTS13 in megakaryocytes/platelets and endothelial cells may not only be curative for hereditary TTP, but also for acquired TTP with autoantibodies against ADAMTS13. Such a success has been reported for restoring hemostasis to hemophilia A mice with preexisting antibodies.47
Despite low engraftment of transplanted cells, therapeutic plasma levels of ADAMTS13 activity and antigen in mice have been achieved by autologous transplantation of ex vivo–manipulated HPCs with a self-inactivating lentiviral vector encoding a full-length murine Adamts13 under the control of a modified MND promoter. This may pave the way toward development of HPCs-mediated gene transfer strategy in an autologous fashion to cure hereditary TTP in humans. Whether the same levels of success in bone marrow chimerism and plasma ADAMTS13 can be achieved or not in humans in the absence of irradiation remains to be determined in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr David Ginsburg (Department of Human Genetics and Internal Medicine, and Howard Hughes Medical Institute, University of Michigan, Ann Arbor, MI) for providing us with Adamts13−/− mice and Dr Mortimer Poncz for providing us with the Doppler ultrasound arterial flow detection system.
This study was supported by grants from National Institutes of Health (Bethesda, MD; R01-HL079027 and P50-HL081012 to X.L.Z and R01-HL64715 and HL/DK63434 to A.W.F.).
National Institutes of Health
Authorship
Contribution: P.L., D.S., and X.L.Z. designed and performed the experiments, analyzed the data, and wrote the manuscript; W.J.C., M.N., M.E., A.R., N.D., and P.W.Z. performed experiments; F.S. contributed critical reagents and revised manuscript; and P.W.Z. and A.W.F. helped design the experiments, analyze the data, and revise the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: X. Long Zheng, Department of Pathology and Laboratory Medicine, The Children's Hospital of Philadelphia and, The University of Pennsylvania Medical Center, 816G Abramson Research Center, 3615 Civic Center Boulevard, Philadelphia, PA 19104; e-mail: zheng@email.chop.edu.
References
Author notes
*P.L. and D.S. contributed equally to this work.